Back to Journals » Clinical Epidemiology » Volume 12
Remote Ischemic Conditioning in Patients with Acute Coronary Syndromes: A Systematic Review with Meta-Analysis and Trial Sequential Analysis
Authors Sandven I , Eritsland J, Abdelnoor M
Received 18 February 2020
Accepted for publication 14 May 2020
Published 10 June 2020 Volume 2020:12 Pages 595—605
DOI https://doi.org/10.2147/CLEP.S249785
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Irene Petersen
Irene Sandven,1 Jan Eritsland,2 Michael Abdelnoor3,4
1Oslo Centre for Biostatistics and Epidemiology (OCBE), Oslo University Hospital, Oslo, Norway; 2Department of Cardiology, Oslo University Hospital, Oslo, Norway; 3Centre of Clinical Heart Research, Oslo University Hospital, Oslo, Norway; 4Epidemiology and Biological Statistics, Independent Health Research Unit, Oslo, Norway
Correspondence: Irene Sandven Email [email protected]
Objective: To evaluate the efficacy of remote ischemic conditioning (RIC) as compared to no conditioning on clinical endpoints in acute coronary syndromes (ACS) patients undergoing percutaneous coronary intervention (PCI).
Design: Systematic review of randomized clinical trials (RCTs).
Material and Methods: Literature was searched up to September 13, 2019, and we identified a total of 13 RCTs. The efficacy of RIC on incidence of clinical events during follow-up was quantified by the rate ratio (RR) with its 95% confidence interval (CI), and we used fixed and random effects models to synthetize the results. Small-study effect was evaluated, and controlled for by the trim-and-fill method. Heterogeneity between studies was examined by subgroup and meta-regression analyses. The risk of false-positive results in meta-analysis was evaluated by trial sequential analysis (TSA).
Results: Pooled analysis of 13 trials (7183 patients) showed that RIC compared to no conditioning revealed a non-significant risk reduction on endpoint mortality (RR=0.81, 95% CI: 0.56– 1.17) during a median follow-up time of 1 year (range: 0.08– 3.8) with low heterogeneity (I2=16%). Controlling for small-study effect showed no efficacy of RIC (adjusted RR: 1.03, 95% CI: 0.66– 1.59). Pooled effect of RIC on the incidence of myocardial infarction (MI) from 11 trials (6996 patients) was non-significant too (RR=0.85, 95% CI: 0.62– 1.18), with no observed heterogeneity (I2=0%) or small-study effect. A similar lack of efficacy was found in endpoint congestive heart failure (CHF) from 6 trials including 6098 patients (RR=0.71, 95% CI: 0.44– 1.15), with moderate heterogeneity (I2=30%). TSAs showed that the pooled estimates from the cumulative meta-analyses were true negative with adequate power.
Conclusion: Evidence from this updated systematic review demonstrates no beneficial effect of RIC on the incidence of clinical endpoint mortality, MI and CHF during a median follow-up of 1 year in ACS patients undergoing PCI.
Keywords: remote ischemic conditioning, mortality, myocardial infarction, congestive heart failure, meta-analysis, trial sequential analysis
Introduction
Remote ischemic conditioning (RIC) is a non-invasive procedure providing temporal episodes of reversible ischemia through repeated inflations and deflations of a blood pressure limb cuff. RIC has been tried as a supplement to percutaneous intervention (PCI) in patients presenting with acute coronary syndromes (ACS) to protect against ischemia/reperfusion (I/R) injury, which refers to the damage caused by reperfusion of an organ exposed to a period of ischemia. In RIC the brief episodes of reversible ischemia with reperfusion in a vascular bed remote from the ischemic organ (eg the heart) have been shown to reduce I/R injury probably through neuronal or humoral mediators.1 There are three temporal variants of RIC according to when the remote conditioning stimulus is applied; before (pre-conditioning), during (per-conditioning), or immediately after reperfusion (post-conditioning). In animal models, RIC has consistently been shown to reduce infarct size,2 and subsequently a reduction of infarct size by RIC was reported in small-sized “proof-of-concept” clinical studies.3–6 Meta-analyses of clinical trials point towards an association between RIC and lower levels of cardio-specific biomarker release, and suggest a beneficial effect of RIC on major adverse cardiac events such as mortality, myocardial infarction (MI), and congestive heart failure (CHF) combined or separately.7–12 A trend towards reduced clinical events at follow-up after RIC in ST-elevation myocardial infarction (STEMI) patients has been reported in two trials13,14 while no improvement by RIC was reported in one recent large multicenter trial15 providing additional clinical data.
Objective
Clinical trials assessing RIC-induced effect on clinical endpoints have been few. Therefore, we conducted an updated systematic review followed by meta-analysis and trial sequential analysis (TSA) to evaluate the efficacy of RIC as compared to no conditioning on clinical endpoints in patients with ACS undergoing PCI, and to help clarify the need for additional trials.
Materials and Methods
The review protocol has been registered at https://www.crd.york.ac.uk/PROSPERO/, ID: CRD42020147789.
Search
With guidance from a qualified medical librarian, we searched Ovid Medline and Embase until September 13, 2019 with no date or language restrictions. The population was limited to adult humans above 18 years, and the design to randomized clinical trials (RCTs). In Ovid Medline, the search was conducted using Medical Subject Headings (MeSH) and text words including, but not restricted to: remote and (ischemic or myocardial infarction) and (conditioning or preconditioning or postconditioning) and (randomized controlled trial or controlled clinical trial). An additional search in Cochrane Database of Systematic Reviews was performed, and we searched for ongoing systematic reviews using Prospero. Unpublished clinical trials were searched for by consulting the Clinicaltrials.gov website. Reference lists of published meta-analyses were screened for any relevant studies not included in the original search.
Study Selection
Due to a therapeutic research question we included only RCTs comparing RIC with no conditioning in adult patients with ACS according to the specified research question presented in Table 1. Two investigators (IS and MA) independently evaluated the publications to assess whether they met the predefined inclusion criteria. We resolved any disagreement through discussion with a third author (JE).
 |
Table 1 Specification of the Research Question Applying a PICO (Population, Intervention, Comparison and Outcome) Model |
Quality Assessment of the Trials
Two reviewers (IS, MA) independently assessed the quality of included studies following the Cochrane Collaboration’s tool assessing risk of bias in RCTs;16 randomization and concealment of treatments allocation (selection bias), blinding of investigator to outcome (detection bias), dropout (attrition bias), adequacy of analysis according to intention to treat (other bias), and a beforehand power analysis for clinical endpoints.
Data Abstraction
Data regarding publication status (first author, publication year, and country where the study took place), patient-related characteristics of the total cohort (mean age, frequency of male gender, hypertension, diabetes mellitus, and smokers), outcomes (total mortality, MI, and CHF), results (number of events, total number of patients in intervention and comparison group, and follow-up time), and study quality (risk of bias assessment) were extracted in duplicate on a standardized form according to the á priori protocol. Disagreements were resolved by discussion among the review authors and subsequent consensus.
Summary Measures
The pooled effect of intervention on each clinical endpoint was quantified by the rate ratio (RR) with its 95% confidence interval (CI), considering the person-year model to control for variability in the duration of follow-up. Both fixed and random effects model was considered, but in the presence of heterogeneity between trials the random effects model was preferred according to the DerSimonian and Laird method.17 Using this model the estimate of the pooled effect measure and its CI incorporate the additional variability due to inter-study variance (τ2).
Sources of Heterogeneity, Evaluation and Quantification
Statistical heterogeneity among studies was assessed with Cochran’s Q test. The magnitude of heterogeneity was evaluated by the I2 statistics which describes the proportion of total variation due to heterogeneity rather than chance.18 When heterogeneity was present we used subgroup analyses and meta-regression to investigate possible sources of heterogeneity. We stratified our data according to type of ACS (STEMI versus non-STEMI or Stable/Unstable Angina), timing of RIC (pre-, per- or post-conditioning), and the following study characteristics; concealment of randomisation, blinding of the investigator on the outcome, analysis according to intention to treat strategy, and presence of á priori power analysis for the study. To further explore potential sources of heterogeneity we considered a random effect meta-regression analysis, where the outcome variable is the observed RR from every trial indicating treatment effect and the different study-level and patient-level characteristics (covariates). Sources of heterogeneity were considered as important if the covariate decreased the between- study variance. The estimate of τ2 in the presence of a covariate in comparison to that when the covariate is omitted, allows the proportion of the heterogeneity variance explained by the covariate to be calculated.19 Because we had less than ten trials for endpoint CHF, meta-regression was not performed.20
Evaluation of Small-Study Effect
In order to assess potential small-study effect we used the funnel plot, which is a good visual evaluation of sampling bias. To further assess potential bias we used two well-established tests of small-study effect; Begg and Mazumdar rank correlation,21 and Egger’s test of asymmetry.22 For power considerations a test of funnel plot asymmetry was not applied for endpoint CHF.23
Correction for Small-Study Effect
In the presence of small-study effect, we considered the trim-and-fill method24 to adjust for small-study effect. This method is a kind of sensitivity analysis to assess the potential impact of missing studies.
Trial Sequential Analysis (TSA)
Evaluation of the meta-analyses by TSA was performed to eliminate false-positive results, and to help clarify the need for additional trials. This method was developed by Pogue and Yusuf25,26 and advocated the use of Lan-DeMets trial sequence monitoring boundaries (TSMB) for cumulative meta-analysis. More recently the method was used accounting for bias and observed heterogeneity in a retrospective cumulative meta-analysis.27 We used TSA as it is implemented in Stata and with the ldbounds package in R-statistical software, and considered three types of information sizes calculated by the program: 1) Accrued Information Size (AIS), which is the total number of patients (N) in the meta-analysis. Type-I error, Type-II error and relative risk reduction (RRR) are entered by user, and we estimated power for the given RRR and sample size. 2) Low Bias Information Size (LBIS), where RRR is calculated from only those trials with low bias. Required sample is calculated for this RRR, Type-I error and Type-II error. 3) Low Bias Heterogeneity adjusted Information Size (LBHIS), which is calculated similar to LBIS and then adjusted for heterogeneity. We determined LBHIS when heterogeneity was over 30%. We set Type-I error at 5%, Type-II error at 20%, and for AIS the RRR at 10%. The fixed model was applied when there was low heterogeneity (I2 < 30%) and the random model when I2 ≥ 30%. We estimated the TSMB for AIS, LBIS and LBHIS to detect potentially spurious level.28
Software for Meta-Analysis
Statistical analyses were performed with STATA 15.0,29 and R–Package–Meta.30 TSA was performed combining Stata commands with R packages.28
We followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines for research reporting.31
Results
Trial Selection
After identifying 1220 references, 1184 were excluded due to irrelevant content and duplicate publications leaving 36 potentially eligible. Records were excluded mainly because they did not contain the right population (ACS patients), intervention (RIC versus no conditioning) or clinical endpoints. Finally, 12 studies3–6,13–15,32–36 met our inclusion criteria, whereof one with two intervention arms,34 thus 13 trials were considered in the meta-analysis (Figure 1). No additional publications were identified in the forward citation searching or hand searching of reference lists.
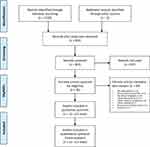 |
Figure 1 PRISMA flow diagram of study selection from the literature searches for the systematic review of randomized clinical trials (RCTs) investigating the efficacy of remote ischemic conditioning (RIC) in acute coronary syndromes (ACS) patients undergoing percutaneous coronary intervention (PCI). Note: Copied from Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi:10.7326/0003-4819-151-4-200908180-00135.31 |
Trial Characteristics
The 13 trials included a total of 7183 ACS patients undergoing PCI (Table 2); the subtypes of ACS were STEMI in 9 trials and NSTEMI or S/UA in 4 trials. Median follow-up was 1 year (range: 0.08–3.8). The patients median age was 62.4 years (range: 51.6–67), 75.7% were men (range: 35.4–94.6), 52.1% with hypertension (range: 22.6–78.5), 27.9% with diabetes mellitus (range: 8.6–49.2), and 53.3% were smokers (range: 32.4–70). The trials were on populations from 3 geographical regions; Europe (6 trials), Asia (4 trials), and North America (3 trials).
RIC interventions were quite similar among all trials with 3–5 cycles of 5-minutes inflation and 5-minutes deflation of a blood pressure cuff to 200 mmHg sited on an upper arm or lower limb/thigh. RIC was performed as pre-conditioning in 6 trials,4–6,13–15 as per-conditioning in 4 trials,3,34,35 and as post-conditioning in 3 trials.32,33,36
Clinical outcomes were considered as primary endpoints in 2 trials only,13,15 and reported as secondary/additional endpoints in the rest of them. All trials reported on mortality, 11 on MI,3,5,6,13-15,32–34,36 and 6 on CHF.4,5,13-15,32
Quality Assessment
Randomization and adequate concealment were considered adequate in 7 trials, blinding of the endpoint assessor to treatment allocation in all trials for endpoint mortality while for other endpoints misclassification might exist. No dropout was reported during follow-up in 9 trials, and intention-to-treat strategy was followed in 8 trials (Table 3). In summary, 5 of the included trials represented overall low risk of bias.3,13-15,36 Power estimation on clinical endpoint was present in two trials only.13,15
Synthesis of Results from Individual Trials
Mortality was reported in 13 trials (7183 patients) and the pooled effect of RIC compared to no conditioning was RR=0.81 (95% CI: 0.56–1.17) with low heterogeneity (I2=16%), indicating no statistically significant effect (Figure 2A). The funnel plot visually showed the possibility of small-study effect (Figure 3A) and asymmetry was indicated by the Egger’s test (p=0.032), but not by the Begg’s test (p=0.7603). The trim-and-fill simulation method suggested 5 studies as missing, and the adjusted point estimate was altered towards the null-effect (adjusted RR=1.03, 95% CI: 0.66–1.59). In stratification analysis the only significant subgroup difference found was between trials adequately powered for clinical outcomes and those not (p=0.0457), and in meta-regression this study-related characteristic was the only covariate significantly associated with intervention effect (p=0.0096).
TSA supported lack of evidence for an effect of RIC compared with no conditioning for endpoint mortality. Cumulative fixed-effect meta-analysis with Lan-DeMets bounds showed a true negative result for AIS of 7183 patients. The power of the meta-analysis for a 10% RRR and the conventional Type-I error of 5% was 99%. The LBIS required is lower ( 803patients) for a RRR of 18% and a power of 80% without changing the conclusion; RIC does not reduce the incidence of mortality in PCI treated ACS patients. Our meta-analysis is satisfactory powered for outcome mortality (Table 4A).
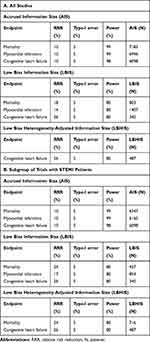 |
Table 4 Trial Sequential Analysis (TSA) for Power Estimation |
Myocardial Infarction (MI) during follow-up was reported in 11 trials (6996 patients) and the pooled effect of RIC compared to no conditioning was statistically non-significant (RR=0.85, 95% CI: 0.62–1.18) with no heterogeneity (I2=0%) (Figure 2B). The funnel plot visually indicated no small-study effect (Figure 3B), confirmed by the Egger’s test (p=0.740) and the Begg’s test (p=0.6971). Stratification analysis and meta-regression were not indicated since there was no observed heterogeneity between the trials.
TSA supported lack of evidence for an effect of RIC compared with no conditioning on endpoint MI. Cumulative fixed-effect meta-analysis with Lan-DeMets bounds showed a true negative result for AIS of 6996 patients. The power of the meta-analysis for a 10% RRR and Type-I error of 5% was 99%. The LBIS required is 1407 without changing the conclusion for a RRR of 14% and a power of 80%; RIC does not reduce the incidence of MI in PCI treated ACS patients. Our meta-analysis is satisfactory powered for endpoint MI (Table 4A).
Congestive Heart Failure (CHF) was reported in 6 trials (6098 patients) and the pooled effect of RIC compared to no conditioning was statistically non-significant (RR=0.71, 95% CI: 0.44–1.15) with moderate heterogeneity (I2=30%) (Figure 2C). The funnel plot visually indicated small-study effect (Figure 3C), but asymmetry was not investigated further because of too few trials. Stratification analysis and meta-regression were not performed due to less than 10 trials. CHF was defined in 3 trials.13–15 The incidence of CHF in the largest trial with 5115 patients15 and the other 5 trials4,5,13,14,32 was close, and included in each others’ 95% CIs.
TSA supported lack of evidence for an effect of RIC compared with no conditioning on endpoint CHF. Cumulative fixed-effect meta-analysis with Lan-DeMets bounds showed a true negative result for AIS of 6098 patients. The power of the meta-analysis for a 10% RRR and Type-I error of 5% was 98%. The LBHIS required is 487 patients without changing the conclusion for a RRR of 26%, Type-I error of 5% and power of 80%; RIC does not reduce the incidence of CHF in PCI treated ACS patients. Our meta-analysis is satisfactory powered for endpoint CHF (Table 4A).
When stratifying on the placement of the blood pressure cuff, upper arm or lower limb/thigh, there was no difference in pooled effect as far the 3 clinical endpoints considered.
Subgroup of STEMI Trials
Our results were robust in the subgroup of STEMI trials. For endpoint mortality from 9 trials (6347 STEMI patients) the pooled effect estimate was larger but still non-significant (RR=0.66, 95% CI: 0.39–1.11), and a true negative result indicated with sufficient power (Table 4B). For endpoint MI from 7 trials (6160 STEMI patients) the pooled effect estimate was unchanged (RR=0.84, 95% CI: 0.59–1.19) and a true negative result indicated with sufficient power (Table 4B). For endpoint CHF there was no change as the patient population was STEMI in all 6 trials.
Discussion
Summary of Evidence
Evidence from this updated systematic review suggests no protective effect of RIC on the incidence of clinical adverse events during follow-up in patients with ACS undergoing PCI. TSAs performed on meta-analyses conducted demonstrate true negative intervention effects on the endpoints mortality, MI and CHF with adequate power.
Strengths and Limitations
We performed a comprehensive literature search to prevent missing relevant trials.37 Trial selection and data extraction were done by two authors to minimize transcription errors, and the components used for quality assessment are validated and reported to be associated with bias.38 We have respected the important principles for meta-analysis methodology regarding eligibility criteria for the individual trials and analysis methods, pinpointing small-study effect and correcting for it when necessary.19,20 In addition to standard methods in meta-analysis, TSA was performed to avoid false-positive conclusions.27,28 Few trials provided data on development of CHF during follow-up, they used different definitions, and the probability of misclassification of this endpoint cannot be excluded. In the large trial of Hausenloy et al15 a blinded independent validation committee reviewed all events according to standard operation procedures. In this case the nature of the misclassification is non-differential which dilutes the efficacy of the intervention,39 and the true effect of RIC on CHF might be stronger than the one estimated by this study. The major limitation of our study was the small-study effect and underpowered trials to evaluate clinical endpoints.
The Problem of Small-Study Effect for Endpoint Mortality
The majority of trials included in our meta-analysis were small and power deficient for investigating clinical outcomes, except Hausenloy et al15 and Gaspar et al.13 In this situation we found no treatment effect when stratifying on trials adequately powered versus power deficient. The impact of underpowered trials on meta-analysis results has been investigated,40 indicating better intervention effects in underpowered than in adequately powered trials. Another reason might be that smaller trials are conducted and analyzed with less methodological rigor than larger trials, and overestimation of the intervention effects has been highlighted in lower quality trials as compared to trials of higher quality.41 Controlling for small-study effect by the trim-and-fill method confirmed the non-efficacy of RIC for endpoint mortality.
Evaluation of the Risk of False-Positive Result in Our Present Meta-Analyses
Often meta-analyses give a false-positive result, especially when updated with the publication of a new trial.27,28 When we evaluated the power of our meta-analyses for the 3 endpoints considered, the TSAs indicated presence of excellent statistical power for a 10% risk reduction and a 5% Type-I error as we included in the cumulative meta-analyses 7183, 6996, and 6098 patients, respectively. The newest trial15 assessed to be of high quality contributed with 5115 patients in the meta-analyses, and decisive for the conclusion of no effect of RIC on endpoint mortality, MI and CHF.
The Potential of False-Positive Results in Previous Meta-Analyses
Previous meta-analyses have reported cumulative pooled beneficial treatment effects of RIC on clinical endpoint mortality,8,10,12 MI,8,11,12 CHF,8,11 and different composite endpoints.7–9,11,12 Unfortunately none of them considered a power analysis of the cumulative effect in the meta-analyses, and their estimates might be false positive presumably due to small-study effect.
Discrepancies in Efficacy of RIC in ACS Between Surrogate Endpoints (SEPs) and Clinical Outcomes
Eleven of the 13 trials included in our study had SEPs as primary outcomes. Myocardial damage was commonly measured by biomarkers of infarct size (eg troponin and creatine kinase) and other outcomes such as resolutions of ST-segment elevation and change in left ventricular ejection fraction (LVEF), and their results pointed to possible effect of RIC. Unfortunately, experience in clinical research underlines the failure of SEPs to be a valid measure of clinically important outcomes in different fields of clinical research.42 SEPs can be used in Phase 2 trials to determine whether an intervention is biologically active and whether to perform large trials with clinically important outcomes. RCTs with SEPs are comparable to experiments in animal model and must be used with great caution if at all in making clinical decisions. In our situation there was concordance between animal models and efficacy on SEPs as both of them considered measures of infarct size as an endpoint. However, trials adequately powered for meaningful clinical outcomes as incidence of mortality, MI and CHF are essential for evidence-based decisions in everyday clinical practice. The need for RCTs investigating clinical endpoints was highlighted in a recent meta-analysis reporting marginal efficacy of RIC during PCI in STEMI patients on SEPs such as infarct size and change in LVEF.43
Implication for Research
The findings from the recent large high-quality RCT15 and the present systematic review suggest that further trials are not necessary to be conclusive regarding the efficacy of RIC on major clinical events as mortality, MI and CHF.
Implication for Practice
According to criteria for grading the quality of evidence and the strengths of recommendations44,45 this systematic review of RCTs provides high-level evidence of a non-preventive effect of RIC against mortality, MI and CHF in ACS patients undergoing PCI. There seem to be no benefits on clinical endpoints during a median follow-up of 1 year (range: 0.08–3.8), and the use of RIC as a supplement to PCI can presently not be recommended.
Conclusion
This systematic review with meta-analysis and TSA suggests that there is no beneficial effect of RIC as a supplement to PCI in the treatment of ACS patients with respect to the incidence of mortality, MI and CHF during a median follow-up time of 1 year.
Author Contributions
All review authors contributed to conception and design, acquisition of data, or analysis and interpretation of data, drafting or revising the article critically for important intellectual content, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Heusch G, Bøtker HE, Przyklenk K, Redington A, Yellon D. Remote Ischemic Conditioning. J Am Coll Cardiol. 2015;65(2):177–195. doi:10.1016/j.jacc.2014.10.031
2. Bromage DI, Pickard JMJ, Rossello X, et al. Remote ischemic conditioning reduces infarct size in animal in vivo models of ischaemia-reperfusion injury: a systematic review and meta-analysis. Cardiovasc Res. 2017;113(3):288–297. doi:10.1093/cvr/cvw219
3. Crimi G, Pica S, Raineri C, et al. Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction. A randomized controlled trial. JACC Cardiovasc Interv. 2013;6(10):1055–1063. doi:10.1016/j.jcin.2013.05.011
4. Yamanaka T, Kawai Y, Miyoshi T, et al. Remote ischemic preconditioning reduces contrast-induced acute kidney injury in patients with ST-elevation myocardial infarction: a randomized controlled trial. Int J Cardiol. 2015;178:136–141. doi:10.1016/j.ijcard.2014.10.135
5. Liu Z, Zhao L, Hong D, Gao J. Remote ischaemic preconditioning reduces myocardial ischaemic reperfusion injury in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Acta Cardiol. 2016;71(5):596–603. doi:10.1080/AC.71.5.3167504
6. Zhou FZ, Song W, Yin LH, et al. Effects of remote ischemic preconditioning on myocardial injury and endothelial function and prognosis after percutaneous coronary intervention in patients with acute coronary syndrome. Eur Rev Med Pharmacol Sci. 2017;21(20):4642–4648.
7. Gong R, Wu YQ. Remote ischemic conditioning during primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction: a systematic review and meta-analysis. J Cardiothorac Surg. 2019;14(1):14. doi:10.1186/s13019-019-0834-x
8. Liu H, Fu L, Sun X, Peng W, Chen Z, Li Y. Remote ischemic conditioning improves myocardial parameters and clinical outcomes during primary percutaneous coronary intervention: a meta-analysis of randomized controlled trials. Oncotarget. 2017;9(9):8653–8664. doi:10.18632/oncotarget.23818
9. Elbadawi A, Ha LD, Abuzaid AS, Crimi G, Azzouz MS. Meta-analysis of randomized trials on remote ischemic conditioning during primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2017;119(6):832–838. doi:10.1016/j.amjcard.2016.11.036
10. Man C, Gong D, Zhou Y, Fan Y. Meta-analysis of remote ischemic conditioning in patients with acute myocardial infarction. Sci Rep. 2017;
11. McLeod SL, Iansavichene A, Cheskes S. Remote ischemic preconditioning to reduce reperfusion injury during acute ST-Segment-Elevation Myocardial Infarction: A systematic review and meta-analysis. J Am Heart Assoc. 2017;6(5):
12. Le Page S, Bejan-Angoulvant T, Angoulvant D, Prunier F. Remote ischemic conditioning and cardioprotection: a systematic review and meta-analysis of randomized clinical trials. Basic Res Cardiol. 2015;110(2):11. doi:10.1007/s00395-015-0467-8
13. Gaspar A, Lourenço AP, Pereira MA, et al. Randomized controlled trial of remote ischaemic conditioning in ST‑elevation myocardial infarction as adjuvant to primary angioplasty (RIC‑STEMI). Basic Res Cardiol. 2018;113(3):14. doi:10.1007/s00395-018-0672-3
14. Sloth AD, Schmidt MR, Munk K, et al. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J. 2014;35(3):168–175. doi:10.1093/eurheartj/eht369
15. Hausenloy DJ, Kharbanda RK, Møller UK, et al. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): a single-blind randomized controlled trial. Lancet. 2019;395(10207):1415–1424. doi:10.1016/S0140-6736(19)32039-2
16. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ. 2011;343(oct18 2):d5928. doi:10.1136/bmj.d5928
17. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;Sep;7(3):177–188. doi:10.1016/0197-2456(86)90046-2
18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ. 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557
19. Egger M, Smith GD, Altman DG, editors. Systematic Reviews in Health Care: Meta-Analysis in Context. London: BMJ Publishing Group; 1995.
20. Borenstein M, Hegges LV, Higgins JPT, Rothstein H. Introduction to Meta-Analysis.
21. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi:10.2307/2533446
22. Egger M, Davy Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi:10.1136/bmj.315.7109.629
23. Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343(jul22 1):d4002. doi:10.1136/bmj.d4002
24. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi:10.1111/j.0006-341X.2000.00455.x
25. Pogue JM, Yusuf S. Cumulative evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta-analysis. Control Clin Trials. 1997;18(6):580–593. doi:10.1016/S0197-2456(97)00051-2
26. Pogue JM, Yusuf S. Overcoming the limitations of current meta-analysis of randomized controlled trials. Lancet. 1998;351(9095):47–52. doi:10.1016/S0140-6736(97)08461-4
27. Wetterslew J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol. 2008;61(1):64–75. doi:10.1016/j.jclinepi.2007.03.013
28. Miladinovic B, Hozo I, Djulbegovic B. Trial sequential boundaries for cumulative meta-analysis. Stata J. 2013;13(1):77–91. doi:10.1177/1536867X1301300106
29. StataCorp LLC. College Station, TX, USA. Retrieved from: http://www.stata.com.
30. Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7(3):40–45.
31. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi:10.7326/0003-4819-151-4-200908180-00135
32. Qian YX, Dai KS, Zhao LL, Yang XJ. Effects of remote ischemic post-conditioning on platelet activation of AMI patients. Exp Ther Med. 2018;16(2):1273–1277. doi:10.3892/etm.2018.6280
33. Elbadawi A, Awad O, Raymond R, Badran H, Mostafa AE, Saad M. Impact of remote ischemic postconditioning during primary percutaneous coronary intervention on left ventricular remodeling after anterior wall ST-segment elevation myocardial infarction: A single-center experience. Int J Angiol. 2017;26(4):241–248. doi:10.1055/s-0037-1601870
34. Lavi S, Abu-Romeh N, Wall S, Alemayehus M, Lavi R. Long-term outcome following remote ischemic postconditioning during percutaneous coronary interventions-the RIP-PCI trial long-term follow-up. Clin Cardiol. 2017;40(5):268–274. doi:10.1002/clc.22668
35. Verouhis D, Sörensson P, Gourine A, et al. Effect of remote ischemic conditioning on infarct size in patients with anterior ST-elevation myocardial infarction. Am Heart J. 2016;181:66–73. doi:10.1016/j.ahj.2016.08.004
36. Carrasco-Chinchilla F, Muñoz-García AJ, Domínguez-Franco A, et al. Remote ischaemic postconditioning: does it protect against ischaemic damage in percutaneous coronary revascularisation? Randomised placebo-controlled clinical trial. Heart. 2013;99(19):1431–1437. doi:10.1136/heartjnl-2013-304172
37. Egger M, Juni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess. 2003;7(1):1–76.
38. Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273(5):408–412. doi:10.1001/jama.1995.03520290060030
39. Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic Research. Principles and Quantitative Methods. USA: Van Nostrand Reinhold Company Inc; 1982.
40. Turner RM, Bird SM, Higgins JP. The impact of study size on meta-analyses: examination of underpowered studies in Cochrane reviews. PLoS One. 2013;8(3):e59202. doi:10.1371/journal.pone.0059202
41. Hempel S, Miles J, Suttorp MJ et al. Methods research report. Detection of associations between trial quality and effect sizes. agency for healthcare research and quality; January 2012. Retrieved from: www.ahrq.gov.
42. Fleming TR, DeMets DL. Surrogate endpoints in clinical trials: are we being misled? Ann Intern Med. 1996;125(7):605–613. doi:10.7326/0003-4819-125-7-199610010-00011
43. Haller PM, Vargas KG, Haller MC, et al. Remote ischaemic conditioning for myocardial infarction or elective PCI: systematic review and meta-analyses of randomised trials. Eur Heart J Acute Cardiovasc Care. 2020;9(1_suppl):82–92. doi:10.1177/2048872618784150
44. Howick J, Chalmers I, Glasziou P, et al. The 2011 oxford CEBM evidence levels of evidence: introductory document. oxford centre for evidence-based medicine. Retrieved from: http://www.cebm.net/index.aspx?o=5653.
45. Atkins D, Best D, Briss PA, et al. GRADE working group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490–1494.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




