Back to Journals » Journal of Asthma and Allergy » Volume 15
Relationship Between Soluble Urokinase Plasminogen Activator Receptor (suPAR) and Disease Outcome in Adult-Onset Asthma
Authors Niemelä T, Kankaanranta H , Vähätalo I , Loponen J, Tuomisto LE, Niemelä O, Hämäläinen M, Moilanen E, Ilmarinen P
Received 14 January 2022
Accepted for publication 17 April 2022
Published 10 May 2022 Volume 2022:15 Pages 579—593
DOI https://doi.org/10.2147/JAA.S356083
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Taito Niemelä,1 Hannu Kankaanranta,1– 3 Iida Vähätalo,2 Juho Loponen,1,2 Leena E Tuomisto,2 Onni Niemelä,1,4 Mari Hämäläinen,5 Eeva Moilanen,5 Pinja Ilmarinen1,2
1Faculty of Medicine and Health Technology, Tampere University, Tampere, Finland; 2Department of Respiratory Medicine, Seinäjoki Central Hospital, Seinäjoki, Finland; 3Krefting Research Centre, Institute of Medicine, University of Gothenburg, Gothenburg, Sweden; 4Department of Laboratory Medicine, Seinäjoki Central Hospital, Seinäjoki, Finland; 5The Immunopharmacology Research Group, Faculty of Medicine and Health Technology, Tampere University and Tampere University Hospital, Tampere, Finland
Correspondence: Pinja Ilmarinen, Department of Respiratory Medicine, Seinäjoki Central Hospital, Seinäjoki, Finland, Tel +35 850 420 0596, Email [email protected]
Background: Soluble urokinase plasminogen activator receptor (suPAR) has emerged as a novel biomarker for various inflammatory conditions and has been proposed to associate with the severity of asthma. However, the relationship between suPAR and clinical asthma features is poorly understood.
Objective: To examine associations of serum suPAR levels with clinical characteristics of asthma and to define the phenotype with high suPAR levels in patients with adult-onset asthma.
Methods: Serum suPAR levels were measured with ELISA from patients with adult-onset asthma participating in the 12-year follow-up visit in the Seinäjoki Adult Asthma Study.
Results: In total, 201 patients were divided into quartiles according to suPAR values. High suPAR patients had more severe asthma symptoms and poorer asthma control. They also had higher levels of interleukin 8 (IL-8), interleukin 6 (IL-6), matrix metalloproteinase 9 (MMP-9), and blood neutrophil counts than those with low suPAR levels. The use of high-dose inhaled and oral corticosteroids was more common in patients with elevated suPAR. Such patients also had visited healthcare more frequently during the follow-up period, had more comorbidities, and were physically less active than those with low suPAR levels. The above-mentioned results remained similar after excluding the patients with co-existing COPD; only association to hospitalizations was lost. In multivariable binary regression analyses, the highest suPAR quartile was associated with higher cumulative dispensed oral corticosteroid use, more severe symptoms, and uncontrolled asthma.
Conclusion: High suPAR levels occur in uncontrolled adult-onset asthma patients characterized by neutrophilic inflammation, high corticosteroid use, frequent healthcare visits, and multimorbidity with unhealthy lifestyle. This biomarker could be useful in determining asthma phenotypes and target new asthma treatments.
Keywords: asthma, adult-onset, neutrophil, phenotypes, suPAR, uncontrolled
Introduction
Asthma is a common obstructive pulmonary disease affecting all age groups.1 Recent studies on asthma phenotypes have emphasized a clear distinction between childhood asthma and adult-onset disease.2–4 Patients with onset of asthma in childhood are predominantly males, often with familial atopy, well responsive to glucocorticoids, and generally defined by T-helper 2 (Th2) cell-predominant T2-inflammation.4,5 In contrast, adult-onset asthma patients are usually non-atopic females with poor responsiveness to corticosteroids. While childhood-onset asthma constitutes a significant portion of asthma patients, adult-onset asthma has been shown to be even the dominant form of asthma in many Western countries.6,7 Although many characteristics of adult-onset asthma have been under intensive research in recent years, the underlying inflammatory mechanisms and characteristics of possible clinically significant disease biomarkers are still poorly understood.
Urokinase plasminogen activator receptor (uPAR) is a glycosylphosphatidylinositol (GPI)‐anchored membrane protein8 and is found in various immunoactive cells such as macrophages and T-lymphocytes and endothelial cells, smooth muscle cells, and tumor cells.9 While uPAR has physiological functions in the dissolution of fibrin clots, cell migration, inflammatory response, and cell apoptosis, it has its pathological functions in extracellular matrix degradation and tumor cell migration through urokinase plasminogen activator (uPA) binding.10
Soluble urokinase plasminogen activator receptor (suPAR), originates from cleavage of membrane-bound uPAR and functions primarily as a scavenger for uPAR ligands such as uPA and vitronectin9 but is thought to have an ability to interact with G-protein coupled receptors, indicating a role in cell signaling pathways.11 suPAR is found in urine, sputum, blood, plasma, serum, and cerebrospinal fluid and is increased along with activation of the immune system functioning as a chemotactic agent and thus reflecting the immunological activity.9 High suPAR levels are connected to various pathological conditions, both acute and chronic, such as type 2 diabetes, cardiovascular diseases, pneumonia, cirrhosis, and cancer predicting mortality and poor disease outcomes in such conditions.9,12–16
Both suPAR and uPAR have been previously suggested to be associated with asthma as uPAR is thought to play a role in bronchial epithelial remodeling in asthmatic lungs.17,18 In asthma patients, suPAR levels have been found to be elevated in sputum, serum, and lung biopsy specimens compared to healthy controls.17,19,20 Recently, there has been a growing interest towards defining the clinical significance of elevated suPAR levels in asthma patients. A recent study suggests that suPAR is a marker that also predicts mortality and readmissions in asthma.21 In a patient cohort recruited from the hospital setting and with unknown age of asthma onset, high suPAR levels were suggested to be associated with severe and non-atopic disease phenotype.18
However, more information on the characteristics of asthma patients with high suPAR levels and the possible clinical value of suPAR as a biomarker in asthma is clearly needed. While recent data suggests that suPAR may be useful in the assessment of asthmatic epithelium status,17 no previous studies have investigated the clinical significance of serum suPAR in patients with adult-onset asthma. Therefore, we examined here the association of suPAR with clinical features of asthma in a cohort of real-life unselected patients with adult-onset asthma to characterize a phenotype of asthma with elevated serum suPAR levels.
Methods
Study Design and Patients
This study is part of the Seinäjoki Adult Asthma Study (SAAS), a prospective 12-year follow-up study with 257 patients with new-onset asthma diagnosed at adult age (≥15 years). The patients gave written informed consent to the study protocol, which was approved by the Ethics Committee of Tampere University Hospital, Tampere, Finland, and is in accordance with the declaration of Helsinki.22 The details of the SAAS study protocol with specific inclusion and exclusion criteria have been published elsewhere23 and are provided in the additional files (Supplementary Table 1). In short, new-onset adult asthma was diagnosed by a respiratory physician during the period 1999–2002. The diagnosis was based on typical asthma symptoms such as dyspnea on exertion and wheezing and was confirmed by objective lung function measurements showing variable or reversible obstruction. Patients with comorbidities, smoking history, or other lung diseases in addition to asthma were not excluded.23 After diagnosis, the patients were treated according to the principles of the Finnish Asthma Programme.24 The total cohort consisted of 257 patients of which 203 patients returned to the follow-up visit 12 years (mean 12.2 years, range 10.8–13.9) after diagnosis. Due to missing information on suPAR, two patients were excluded from the study (Supplementary figure 1). Structured questionnaires were used to evaluate asthma control and status, medication, comorbidities, and lifestyle.
Lung Function, Inflammatory Parameters, Comorbidities, Lifestyle Factors, and Other Clinical Measurements
Information on determining lung function can be found in the supplementary materials “Lung function, inflammatory parameters, lifestyle factors, and other clinical measurements” section. Asthma symptoms were measured with Airways Questionnaire 20 (AQ20)25 and Asthma Control Test (ACT).26 Asthma control evaluation was completed in accordance with the Global Initiative for Asthma (GINA) 2010 report.27 The definition of comorbidities and their classification was based on a previous study.28 Comorbidities were self-reported or estimated from self-reported medication and uncertain cases were confirmed from patient records. The conditions included as comorbidities have been previously reported.29 Detailed information on the determination of lifestyle factors and other clinical measurements can be found in the supplementary materials.
Use of Oral and Inhaled Corticosteroids, Adherence, and Hospitalizations
Prescribed inhaled corticosteroids (ICS) doses were attained from medical records and were converted to budesonide equivalents and annual prescribed ICS medication for each patient was calculated as previously described.30 Data on all dispensed oral corticosteroids (OCS) and ICS was obtained from the Finnish Social Insurance Institution. More detailed information on the assessment of OCS and adherence to ICS are presented in the supplementary materials. Hospitalizations were gathered from medical records during the 12-year follow-up.
Biomarker Analyses and Inflammatory Parameters
Plasma suPAR and MMP-9 levels were measured only during the 12-year follow-up visit with commercially available enzyme-linked immunosorbent assay kits according to the manufacturers’ instructions (suPARnostic®, ViroGates, Birkeroed, Denmark, and R&D Systems Europe Ltd, Abingdon, UK). The detection limits and inter-assay coefficient of variations were 0.8 pg/mL and 2.2% (suPAR) and 7.8 pg/mL and 2.9% (MMP-9), respectively. Determination of other inflammatory parameters is described in the supplementary materials.
Statistical Analysis
Continuous data are expressed as median (interquartile range) or mean ± standard deviation. Comparison between four groups was carried out by using one-way ANOVA with Tukey’s post hoc test, Kruskal–Wallis test, or Chi-squared test. Multivariable binary logistic regression was performed to determine the association between suPAR quartiles, and the outcome variable purchased OCS>1200mg prednisolone adjusting for gender, age>50 years, BMI categories (<25, 25–29.99, ≥30), pack-years≥10 and comorbidity count (>1). Multinomial logistic regression was used to evaluate the association between suPAR (1st to 3rd quartiles vs, 4th quartile) and asthma control, adjusting for gender, age>50 years, BMI≥30, pack-years≥10, and comorbidity count (>1). A negative binomial regression analysis was performed to analyze factors associated with higher AQ20 score. We calculated the incidence rate ratios (IRRs) with 95% CI for higher AQ20 scores. Owing to overdispersion, we used negative binomial regression and adjusted it for age, sex, pack-years, suPAR, multimorbidity, and BMI. The natural logarithm of the length of follow-up was set as an offset variable. In addition, sensitivity analysis was performed by excluding patients fulfilling the criteria of co-existing COPD. Statistical analyses were performed using SPSS software, version 26 (IBM SPSS Statistics, Armonk, NY). P-value <0.05 was regarded as statistically significant.
Results
Patient Characteristics
The main clinical characteristics of the patients at 12-year follow-up visit are presented in Table 1. Most patients were overweight, female, and non-atopic. Approximately half of the patients were current or ex-smokers and 76% used inhaled corticosteroids daily. The median age was 59 years and 39% of the patients had at least two comorbidities.
 |
Table 1 Main Characteristics of the Patients at 12-Year Follow-Up |
suPAR Quartiles
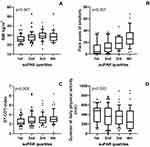
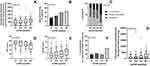
The patients were subsequently divided into quartiles according to the level of serum suPAR. The patients with high suPAR values (3rd or 4th quartile) were older, had higher BMI, higher amount of smoked pack-years, and were less often atopic compared to those with low suPAR values (1st or 2nd quartiles) (Figure 1A and B, Table 2). Patients in the 4th suPAR quartile had higher doses of ICS during the 12-year follow-up period and were more likely to use daily add-on medication at the 12-year follow-up visit compared to those in the 1st suPAR quartile (Figure 2A and B, Table 2).
 |
Table 2 Background and Medication of the Patients at the 12-Year Follow-Up Visit, as Classified into Quartiles According to Serum suPAR Levels |
The 4th quartile had more asthma symptoms based on ACT and AQ20, poorer asthma control, and lower lung function at the 12-year follow-up visit than the lower quartiles (Figure 2C–E, Table 3). Furthermore, patients in the 4th suPAR quartile had used more oral corticosteroids (OCS), had more healthcare visits, and were more frequently hospitalized for respiratory-related reasons during the 12-year follow-up period compared to the lower suPAR quartiles (Figure 2F and G, Table 4).
 |
Table 3 Lung Function, Asthma Symptoms, and Inflammation Markers of the Patients at the 12-Year Follow-Up Visit, as Classified into Quartiles According to Serum suPAR Levels |
 |
Table 4 Disease Burden, Comorbidities, and Lifestyle Factors of the Patients at the 12-Year Follow-Up Visit, as Classified into Quartiles According to Serum suPAR Levels |
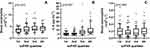
Higher suPAR levels were associated with higher blood neutrophil count and higher serum levels of IL-6, IL-8, hsCRP, and MMP-9 (Figure 3A and B, Table 3, Supplementary Table 5). suPAR showed no association with blood eosinophil count, FeNO, or IgE levels (Figure 3C, Table 3, Supplementary Table 5).
The 4th quartile had also more comorbidities; they were more likely to have COPD, diabetes mellitus, coronary artery disease, hypertension, hypercholesterolemia, metabolic syndrome, and depression or psychiatric disease at 12-year follow-up visit than patients in the lowest quartile (Table 4). The higher number of comorbidities in the 4th suPAR quartile led us to examine the association between suPAR levels and various lifestyle factors. We found that patients in the 4th quartile consumed more alcohol, had a higher GT-CDT index (a biomarker of alcohol consumption), had higher screen time outside of work, and were less physically active (Figures 1C and D, Table 4).
As patients in the 4th suPAR quartile had more often co-existing COPD, we considered it as a possible confounding factor and excluded patients with co-existing COPD from the analysis. After the exclusion, the main results remained otherwise similar: High-suPAR-patients had significantly lower ACT-score, higher amount of purchased ICS and OCS, and more comorbidities. Additionally, high suPAR levels were associated with higher blood neutrophil count and higher serum levels of IL-6, IL-8, and hsCRP despite the exclusion of patients with coexisting COPD. However, differences regarding lung function between suPAR quartiles were lost. Further, the trends between higher suPAR and uncontrolled asthma, atopy, daily add-on medication use, hospitalizations, and lower physical activity remained, even though the statistically significant associations were lost (Table 5, Supplementary Tables 2–4).
 |
Table 5 Results After Excluding Patients with Co-Existing COPD at 12-Year Follow-Up Visit, as Classified into Quartiles According to Serum suPAR Levels |
It is possible that mild asthma not requiring ICS or OCS therapy might contribute to the results. We, therefore, examined patients’ true dispensed medication during the last two years of the 12-year follow-up and excluded those who had no dispensed ICS from the analysis. There was a total of 37 (18.2%) with no dispensed ICS in the last two years of follow-up suggesting they have mild asthma. After excluding these patients, the main results remained similar; high suPAR levels were associated with all examined non-T2-markers, ACT score, LABA-use, smoking history, hospitalizations, and comorbidities (Supplementary Table 6). Further, the trends between higher suPAR and BMI, Uncontrolled asthma (GINA), average dispensed ICS daily dose during 12 years, purchased OCS during the 12-years remained (Supplementary Table 6).
Regression Analysis
Next, we examined the association between the suPAR quartiles and several asthma outcomes (dispensed oral prednisolone >1200mg during the follow-up period and level of asthma control at follow-up visit) after adjusting for possible confounding factors by using multivariable logistic regression analysis. The association between the 4th suPAR quartile and >1200mg dispensed prednisolone remained when adjusted for BMI, age, sex, pack-years, and multimorbidity (OR 2.76, CI 1.06–7.16; p=0.037) (Supplementary Table 7). Likewise, using negative logistic regression analysis, when adjusted for BMI, age, sex, pack-years, and multimorbidity, the association between the 4th suPAR quartile and higher AQ20 score remained (IRR 1.15, CI 1.02–1.31; p=0.025) (Supplementary Table 8).
When analyzing the association between suPAR and asthma control, we divided suPAR quartiles into two groups (1st-3rd quartiles vs 4th quartile) to increase statistical power. The association between the 4th suPAR quartile and uncontrolled asthma remained when adjusted for BMI, age, sex, pack-years, and multimorbidity (OR 2.67 CI 1.02–6.96; p=0.045) but the association between the 4th suPAR quartile and partially controlled asthma showed no significance after the same adjustments (Supplementary Table 9).
The results remained unaffected in all three models after adjustment for single comorbidities (type 2 diabetes, coronary artery disease, hypertension, hypercholesterolemia, and metabolic syndrome) (data not shown).
Discussion
In this study, we showed that adult-onset asthma patients with high suPAR levels have more often uncontrolled asthma, elevated levels of neutrophilic count, more severe asthma symptoms, more frequent hospitalizations, and higher usage of inhaled and oral corticosteroids than those with lower suPAR levels. Patients with high suPAR are also characterized by unhealthy lifestyles and associated morbidity. Results remained similar after excluding patients with co-existing COPD and patients with no dispensed ICS during the last two years of the follow-up period. Our results suggest the existence of a high suPAR phenotype of asthma characterized by poor asthma control, neutrophilic inflammation, and unhealthy lifestyle.
In the present series of patients with adult-onset asthma, high suPAR levels were associated with uncontrolled asthma, which was defined according to GINA,27 and more severe asthma symptoms, defined by lower ACT score and higher AQ20 score. To our knowledge, our study is the first to show an association between GINA-defined uncontrolled asthma and elevated suPAR. We also found impaired lung function in patients with high suPAR levels. Our results are supported by findings of a previous study, where high suPAR values were associated with poor lung function and severe asthma in two cohorts with limited generalizability.18 In our study of consecutive patients representing well the general population of adult-onset asthma patients, we consistently found higher numbers of patients with severe asthma in those with high serum suPAR levels. In a previous cross-sectional study on asthmatic pregnancy, increased suPAR values were also found to be connected to the impaired asthma control status of non-pregnant patients. Although generalization of the results to a larger population is limited, the study importantly did not show a significant difference in suPAR levels between healthy pregnant patients and pregnant well-controlled asthma patients,32 even though difference has been found between healthy controls and asthma patients in general.17,19,20 The results above combined with ours imply that mainly uncontrolled, but not well controlled, asthma may be related to elevated suPAR. Even after the exclusion of patients with co-existing COPD, the trends remained and high suPAR was associated with uncontrolled asthma in regression analyses adjusted for BMI, age, sex, pack-years, and multimorbidity, endorsing the association between asthma control and suPAR.
Adult-onset asthma may be primarily characterized by non-T2 inflammation. One non-T2 endotype of asthma is neutrophilic and may be mediated by Th1, Th17, and ILC1 cells through toll-like receptor (TLR) activation. While neutrophils and non-T2 cell counts are thought to increase naturally with age, they likely play a pivotal role in adult-onset asthma pathogenesis.33 In non-T2-asthma, activation of TLR is thought to increase Th1 and Th17 cell responses leading to an increase in the production of cytokines such as IL-8, TNF-α, and various MMPs such as MMP-9 and consequently activating neutrophilic inflammation and further release of cytokines.34 MMP-9 and IL-8 are neutrophil-related mediators produced by eg, bronchial epithelial cells and macrophages and are related to increased asthma severity35 and uncontrolled asthma, respectively.36 Stimulation of TLRs or cytokine receptors on immunologically active cells also induces uPAR gene expression which leads to suPAR released into blood circulation. As suPAR plays a role in inflammation by impairing neutrophil efferocytosis and chemotaxis and might sustain the inflammatory response and maintain chronic inflammation in patients, suPAR may play a role in the chronic neutrophilic inflammation typical to non-T2 asthma through TLR activation.34 In our study, we found an association between high suPAR levels and elevated blood neutrophils, IL-8, and MMP-9, further solidifying the connection between suPAR and non-T2 mediators. Supporting our findings, neutrophils have been observed to be the main source of suPAR in critically ill patients37 and elevated uPAR has been suggested to induce MMP-9-mediated wound repair in asthmatic patients.17 IL-8 has been shown to stimulate human peripheral blood mononuclear cells and neutrophils to rapidly increase surface expression of uPAR and induce the release of suPAR.34 It could be speculated that stimulation of TLRs and cytokine receptors induce Th1-cells and Th17-cells and neutrophils to release various non-T2-mediators including suPAR, IL-8, and MMP-9 that sustain neutrophilic inflammation by various interactions. Our results link IL-8 to high suPAR in asthmatic patients, a result not previously reported. Easily available markers for non-T2 asthma are not yet available and currently, non-T2 asthma is defined by low blood eosinophil level or non-T2 neutrophilic asthma by measuring lung neutrophil infiltration with lung biopsies or sputum samples.34 Although suPAR is not in routine clinical use yet, it could be a possible surrogate for currently available methods as it can be easily measured from blood serum and urine,9 though the possible practical clinical use of suPAR measured from urine has not yet been studied.
Also, higher IL-6 and hsCRP levels were associated with suPAR quartiles. While both IL-6 and hsCRP are biomarkers for systemic inflammation, they have been previously associated with neutrophilic inflammation and impaired asthma control.38–40 Of particular interest is IL-6 as it has been linked to worse asthma outcomes in adult asthma patients29 and may stimulate Th17-mediated neutrophil-predominant severe asthma,41 which supports our findings regarding the connection between suPAR and non-T2 asthma. IgE and blood eosinophils were not associated with suPAR, further emphasizing a role for suPAR in non-T2 inflammation. In accordance with this view, a previous study found an association of suPAR with non-atopic asthma, but not with atopic asthma.18 Results were similar even after the exclusion of COPD and statistically significant association with high suPAR and IL-6, IL-8, MMP-9, hsCRP, and high blood neutrophil count remained.
We found that both elevated OCS and ICS use are associated with high suPAR levels. One previous study has explored the connection between suPAR and steroid use in asthmatic patients and found conflicting results.18 In that previous study, where no distinction was, however, made between oral and inhaled corticosteroids. Neutrophilic non-T2 asthma is often less sensitive to corticosteroids and may therefore be represented by high ICS or OCS usage.33 Furthermore, greater use of corticosteroids is a characteristic defining uncontrolled asthma.42 However, it could be speculated that high use of corticosteroids elevates suPAR levels, as it has been proposed to increase neutrophilia.43 Nevertheless, since our data show a clear association between suPAR and other elements of uncontrolled asthma such as more frequent hospital visits, hospitalizations, severe asthma symptoms, and add-on medication use, we presume that uncontrolled asthma associated with high suPAR is the factor accounting for higher corticosteroid use. As our data suggest, both neutrophilia and uncontrolled asthma were related to high serum suPAR and therefore we believe these two characteristics to be behind the association between corticosteroid use and suPAR. However, we cannot completely exclude the possibility that high use of corticosteroids affects suPAR levels. Altogether, we were the first to show the positive association between oral and inhaled corticosteroid use and suPAR levels and envision that high corticosteroid use is a clinical characteristic of the high suPAR phenotype of asthma.
Apart from elevated corticosteroid use, a greater burden of asthma in high suPAR patients is represented by more frequent healthcare visits and hospitalizations as well as longer hospitalization periods than in those with low levels of serum suPAR. Our findings are supported by a recent retrospective cohort study focusing on readmission and mortality in a population consisting of acutely admitted asthma patients.21 Håkansson et al found that high serum suPAR levels were associated with 365-day readmission and mortality rates.21 Furthermore, it was speculated that systemic inflammation in uncontrolled asthma might be responsible for high suPAR in hospitalized patients.21 We endorse this hypothesis since uncontrolled asthma and inflammation markers are both elevated in high suPAR patients in our study and add the missing link that the asthmatic inflammation related to high suPAR is presumably neutrophilic. The exclusion of patients with co-existing COPD reduced the association between suPAR and hospitalizations. This could be due to a low number of hospitalized patients in our general population with adult-onset asthma since the trends remained after the exclusion. Previous studies along with findings in our study indicate that high suPAR asthma patients are more frequently admitted to hospitals due to neutrophilic inflammation and uncontrolled asthma.
Multimorbid patients are often excluded from asthma studies, but we also included those patients with detailed information on comorbidities. In our study, several comorbidities and several other than asthma-related medications in use correlated positively with suPAR levels. Previous studies have found similar results when comparing suPAR levels to comorbidities using the Charlson score.13,44 Particularly cancer, liver disease, diabetes, and cardiovascular diseases were related to increased suPAR levels,14,15,45 and suPAR has even been proposed as a biomarker for cardiovascular diseases.45 Our study showed similarly the association between high suPAR and cardiovascular diseases (coronary artery disease, hypertension, and hypercholesterolemia) and type 2 diabetes mellitus.45 While patients with liver diseases or cancer were rare in our cohort, we observed associations between high suPAR levels and metabolic syndrome, depression, and psychiatric diseases as suggested also by previous studies.46,47 When multimorbidity or single comorbidities were included in our regression models, the associations between increased suPAR and all three asthma outcome variables were retained. Thus, our study suggests that high suPAR patients with adult-onset asthma are generally multimorbid, a possible new characteristic of the high suPAR phenotype of asthma.
Since comorbidities, such as liver problems, associated with high suPAR are often lifestyle-driven,13 we also examined the association between lifestyle factors and serum suPAR. Alcohol marker GT-CDT index showed a weak positive correlation (ρ=0.256) to suPAR and the 4th suPAR quartile had the highest percentage of heavy alcohol consumption (31% vs 10% in the 1st quartile) and the highest pack-years of smoking (median of 26 vs 4 in the 1st quartile). Furthermore, high suPAR patients were physically less active and generally had waist circumference higher than the low-risk limits. The trend between physical activity and suPAR remained even after excluding patients with co-existing COPD. Our findings are supported by data from a population-based follow-up study focusing solely on lifestyle factors and suPAR.12 Similar trends regarding alcohol consumption, smoking status, and physical activity were found suggesting suPAR as a biomarker for lifestyle changes. Interestingly, they also associated unhealthy diet (defined by dietary quality score) with increased suPAR levels, a factor we were not able to study. Thus, it is likely that unfavorable lifestyle factors with lifestyle-driven comorbidities are a typical characteristic of the high suPAR phenotype.
Our study has several important strengths. Firstly, the most prominent difference compared to previous studies concerning asthma and suPAR is that we have follow-up information from a long period of time. Secondly, although the number of participants in our study could have been higher, our study provides a wide set of real-life detailed data on participant clinical characteristics, various comorbidities, lifestyle factors, medication and hospitalizations, and different inflammatory markers. Thirdly, patients in our study are well representative of the general population of adult-onset asthma.48 In addition, asthma was objectively diagnosed by lung function measurements and asthma symptoms. There are however few limitations to our study. Notably, we did not have lung biopsy or sputum samples taken from the patients. Further, at this time we cannot conclude whether suPAR measured in serum represents uPA bound, free, or both. Lastly, while we had comprehensive data on comorbidities, we cannot exclude that unnoticed comorbidities influenced serum suPAR levels.
Conclusions
In conclusion, we show that serum suPAR is a promising asthma phenotype-related biomarker in adult-onset asthma patients. High suPAR levels characterize patients with uncontrolled asthma, high respiratory symptoms, high use of medication, non-T2 inflammation, frequent healthcare visits and hospitalizations, multimorbidity, and unhealthy lifestyle. Though further research is needed regarding the cellular mechanisms underlying these observations, suPAR measurements could be used as a biomarker to help distinct phenotypes of asthma and open new insights into the treatment of asthma.
Trial Registration
Clinical Trials.gov identifier NCT02733016 (April 11, 2016).
Abbreviations
ACT, Asthma Control Test; AQ20, Airways Questionnaire 20; BMI, body-mass index; CDT, carbohydrate-deficient transferrin; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FeNO, fraction of exhaled nitric oxide; GINA, Global Initiative for Asthma; GT, γ-glutamyltransferase; hsCRP, high-sensitivity C-reactive protein; IgE, immunoglobulin E; ICS, inhaled corticosteroid; IL, interleukin; MMP 9, matrix metalloproteinase 9; OCS, oral corticosteroid; OR, odds ratio; SAAS, Seinäjoki Adult Asthma Study; suPAR, soluble urokinase plasminogen activator receptor; TLR, Toll-like receptor; Th2, T-helper 2; uPA, urokinase plasminogen activator; uPAR, urokinase plasminogen activator receptor.
Ethics Approval and Informed Consent
The patients gave written informed consent to the study protocol, which was approved by the Ethics Committee of Tampere University Hospital, Tampere, Finland (R12122). Institutional permissions (TU1114 and LET) were likewise attained.
Consent for Publication
All authors gave written consent for publication.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Information File). According to ethical permission and patient data-protection laws of Finland, single patient data cannot be made available.
Acknowledgments
Aino Sepponen, RN (Department of Respiratory Medicine, Seinäjoki Central Hospital, Seinäjoki, Finland) is gratefully acknowledged for her help through all the stages of this work. Ms. Meiju Kukkonen is warmly acknowledged for the excellent technical assistance.
Author Contributions
T.N, P.I, and H.K. designed the study. T.N. and P.I. wrote the report with input from the other authors. T.N. and I.V. performed the statistical analyses. O.N, M.H, and E.M. were responsible for laboratory analyses. I.V. assembled adherence, prescribed and dispensed data and J.L. contributed to data on physical activity. L.E.T. and H.K. conceived Seinäjoki Adult Asthma Study. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Tampere Tuberculosis Foundation and the Finnish Anti- Tuberculosis Foundation, the Competitive State Research Financing of the Expert Responsibility Area of Tampere University Hospital (Tampere, Finland), the Medical Research Fund of Seinäjoki Central Hospital (Seinäjoki, Finland), the Research Foundation of the Pulmonary Diseases (Helsinki, Finland), the Ida Montini Foundation (Kerava, Finland), the Pirkanmaa Regional Fund of the Finnish Cultural Foundation (Helsinki, Finland), and the Allergy Research Foundation (Helsinki, Finland). None of the sponsors had any involvement in the planning, execution, drafting, or write-up of this study.
Disclosure
The authors declare no conflict of interest related to this study. Dr. Kankaanranta reports grants, personal fees and non-financial support from AstraZeneca, personal fees from Chiesi Pharma AB, GlaxoSmithKline, MSD, Novartis, Mundipharma, Sanofi, SanofiGenzyme, and personal fees and non-financial support from Boehringer-Ingelheim, and Orion Pharma, outside the submitted work. Dr. Vähätalo reports personal fees from Astra Zeneca, outside the submitted work. Dr. Loponen reports personal fees from Astra Zeneca and grants from Tampere Tuberculosis Foundation and Finnish Anti-Tuberculosis Association Foundation, outside the submitted work. Dr. Tuomisto reports non-financial support and personal fees from Chiesi, Boehringer-Ingelheim, personal fees from Astra Zeneca and GlaxoSmithKline, and non-financial support from TEVA, outside the submitted work. Eeva Moilanen reports grants from Tampere University Hospital (VTR grant) during the conduct of the study. Dr. Ilmarinen is an employee of and reports lecture fees from GlaxoSmithKline, reports grants and personal fees from Astra Zeneca, and personal fees from Mundipharma, GlaxoSmithKline, and Novartis, outside the submitted work.
References
1. Global Strategy for Asthma Management and Prevention; 2019. Available from: www.ginasthma.org/.
2. Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi:10.1038/nm.2678
3. de Nijs SB, Venekamp LN, Bel EH. Adult-onset asthma: is it really different? Eur Respir Rev. 2013;22:44–52. doi:10.1183/09059180.00007112
4. Ilmarinen P, Tuomisto LE, Kankaanranta H. Phenotypes, risk factors, and mechanisms of adult-onset asthma. Mediators Inflamm. 2015;2015:514868. doi:10.1155/2015/514868
5. Bisgaard H, Bønnelykke K. Long-term studies of the natural history of asthma in childhood. J Allergy Clin Immunol. 2010;126:187–189. doi:10.1016/j.jaci.2010.07.011
6. Kankaanranta H, Tuomisto LE, Ilmarinen P. Age-specific incidence of new asthma diagnoses in Finland. J Allergy Clin Immunol Pract. 2017;5(189–191.e3):189–191.e3. doi:10.1016/j.jaip.2016.08.015
7. Sood A, Qualls C, Schuyler M, et al. Adult-onset asthma becomes the dominant phenotype among women by age 40 years. The Longitudinal CARDIA Study. Ann Am Thorac Soc. 2013;10:188–197. doi:10.1513/AnnalsATS.201212-115OC
8. Ploug M, Behrendt N, Løber D, Danø K. Protein structure and membrane anchorage of the cellular receptor for urokinase-type plasminogen activator. Semin Thromb Hemost. 1991;17:183–193. doi:10.1055/s-2007-1002608
9. Thunø M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27:157–172. doi:10.1155/2009/504294
10. Mahmood N, Mihalcioiu C, Rabbani SA. Multifaceted role of the urokinase-type Plasminogen Activator (uPA) and its receptor (uPAR): diagnostic, prognostic, and therapeutic applications. Front Oncol. 2018;8:24. doi:10.3389/fonc.2018.00024
11. Fazioli F, Resnati M, Sidenius N, Higashimoto Y, Appella E, Blasi F. A urokinase-sensitive region of the human urokinase receptor is responsible for its chemotactic activity. EMBO J. 1997;16:7279–7286. doi:10.1093/emboj/16.24.7279
12. Haupt TH, Rasmussen LJH, Kallemose T, et al. Healthy lifestyles reduce suPAR and mortality in a Danish general population study. Immun Ageing I A. 2019;16:1–8. doi:10.1186/s12979-018-0141-8
13. Haupt TH, Petersen J, Ellekilde G, et al. Plasma suPAR levels are associated with mortality, admission time, and Charlson Comorbidity Index in the acutely admitted medical patient: a prospective observational study. Crit Care. 2012;16:R130. doi:10.1186/cc11434
14. Eugen-Olsen J, Andersen O, Linneberg A, et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med. 2010;268:296–308. doi:10.1111/j.1365-2796.2010.02252.x
15. Garnæs E, Mortensen C, Hobolth L, Andersen O, Nehlin J, Møller S. Kinetics of the soluble urokinase plasminogen activator receptor (suPAR) in cirrhosis. PLoS One. 2019;14:e0220697. doi:10.1371/journal.pone.0220697
16. Eugen-Olsen J, Giamarellos-Bourboulis EJ. suPAR: the unspecific marker for disease presence, severity and prognosis. Int J Antimicrob Agents. 2015;46(Suppl 1):33. doi:10.1016/j.ijantimicag.2015.10.011
17. Stewart CE, Nijmeh HS, Brightling CE, Sayers I. uPAR regulates bronchial epithelial repair in vitro and is elevated in asthmatic epithelium. Thorax. 2012;67:477–487. doi:10.1136/thoraxjnl-2011-200508
18. Portelli MA, Moseley C, Stewart CE, et al. Airway and peripheral urokinase plasminogen activator receptor is elevated in asthma, and identifies a severe, nonatopic subset of patients. Allergy. 2017;72:473–482. doi:10.1111/all.13046
19. Portelli MA, Siedlinski M, Stewart CE, et al. Genome-wide protein QTL mapping identifies human plasma kallikrein as a post-translational regulator of serum uPAR levels. FASEB J. 2014;28:923–934. doi:10.1096/fj.13-240879
20. Xiao W, Hsu YP, Ishizaka A, Kirikae T, Moss RB. Sputum cathelicidin, urokinase plasminogen activation system components, and cytokines discriminate cystic fibrosis, COPD, and asthma inflammation. Chest. 2005;128:2316–2326. doi:10.1378/chest.128.4.2316
21. Håkansson KEJ, Rasmussen LJH, Godtfredsen NS, et al. The biomarkers suPAR and blood eosinophils are associated with hospital readmissions and mortality in asthma - a retrospective cohort study. Respir Res. 2019;20:254–258. doi:10.1186/s12931-019-1234-4
22. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
23. Kankaanranta H, Ilmarinen P, Kankaanranta T, Tuomisto LE. Seinäjoki Adult Asthma Study (SAAS): a protocol for a 12-year real-life follow-up study of new-onset asthma diagnosed at adult age and treated in primary and specialised care. npj Prim Care Respir Med. 2015;25:15042. doi:10.1038/npjpcrm.2015.42
24. Haahtela T, Tuomisto LE, Pietinalho A, et al. A 10 year asthma programme in Finland: major change for the better. Thorax. 2006;61:663–670. doi:10.1136/thx.2005.055699
25. Barley EA, Quirk FH, Jones PW. Asthma health status measurement in clinical practice: validity of a new short and simple instrument. Respir Med. 1998;92:1207–1214. doi:10.1016/S0954-6111(98)90423-1
26. Schatz M, Sorkness CA, Li JT, et al. Asthma control test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117:549–556. doi:10.1016/j.jaci.2006.01.011
27. Global Strategy for Asthma Management and Prevention; 2010. Available from: www.ginasthma.org/.
28. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi:10.1016/S0140-6736(12)60240-2
29. Ilmarinen P, Tuomisto LE, Niemelä O, et al. Comorbidities and elevated IL-6 associate with negative outcome in adult-onset asthma. Eur Respir J. 2016;48:1052–1062. doi:10.1183/13993003.02198-2015
30. Vähätalo I, Ilmarinen P, Tuomisto LE, Niemelä O, Kankaanranta H. Inhaled corticosteroids and asthma control in adult-onset asthma: 12-year follow-up study. Respir Med. 2018;137:70–76. doi:10.1016/j.rmed.2018.02.025
31. Global Strategy for Asthma Management and Prevention; 2002. Available from: www.ginasthma.org/.
32. Ivancsó I, Toldi G, Bohács A, et al. Relationship of circulating soluble urokinase plasminogen activator receptor (suPAR) levels to disease control in asthma and asthmatic pregnancy. PLoS One. 2013;8:e60697. doi:10.1371/journal.pone.0060697
33. Sze E, Bhalla A, Nair P. Mechanisms and therapeutic strategies for non-T2 asthma. Allergy. 2020;75:311–325. doi:10.1111/all.13985
34. Rasmussen LJH, Petersen JEV, Eugen-Olsen J. Soluble Urokinase Plasminogen Activator Receptor (suPAR) as a biomarker of systemic chronic inflammation. Front Immunol. 2021;12. doi:10.3389/fimmu.2021.780641
35. Cundall M, Sun Y, Miranda C, Trudeau JB, Barnes S, Wenzel SE. Neutrophil-derived matrix metalloproteinase-9 is increased in severe asthma and poorly inhibited by glucocorticoids. J Allergy Clin Immunol. 2003;112:1064–1071. doi:10.1016/j.jaci.2003.08.013
36. Hosoki K, Ying S, Corrigan C, et al. Analysis of a panel of 48 cytokines in BAL fluids specifically identifies IL-8 levels as the only cytokine that distinguishes controlled asthma from uncontrolled asthma, and correlates inversely with FEV1. PLoS One. 2015;10:e0126035. doi:10.1371/journal.pone.0126035
37. Gussen H, Hohlstein P, Bartneck M, et al. Neutrophils are a main source of circulating suPAR predicting outcome in critical illness. J Intensive Care. 2019;7:1. doi:10.1186/s40560-019-0381-5
38. Wood LG, Baines KJ, Fu J, Scott HA, Gibson PG. The neutrophilic inflammatory phenotype is associated with systemic inflammation in asthma. Chest. 2012;142:86–93. doi:10.1378/chest.11-1838
39. Fu JJ, Baines KJ, Wood LG, Gibson PG. Systemic inflammation is associated with differential gene expression and airway neutrophilia in asthma. OMICS. 2013;17:187–199. doi:10.1089/omi.2012.0104
40. Gangestad SW, Gangestad SW. Rethinking IL-6 and CRP: why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. 2018;70:61–75. doi:10.1016/j.bbi.2018.02.013
41. Rincon M, Irvin CG. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int J Biol Sci. 2012;8:1281–1290. doi:10.7150/ijbs.4874
42. Tuomisto LE, Ilmarinen P, Niemelä O, Haanpää J, Kankaanranta T, Kankaanranta H. A 12-year prognosis of adult-onset asthma: seinäjoki adult asthma study. Respir Med. 2016;117:223–229. doi:10.1016/j.rmed.2016.06.017
43. Zhang X, Moilanen E, Adcock IM, Lindsay MA, Kankaanranta H. Divergent effect of mometasone on human eosinophil and neutrophil apoptosis. Life Sci. 2002;71:1523–1534. doi:10.1016/S0024-3205(02)01921-5
44. Rasmussen LJ, Ladelund S, Haupt TH, et al. Soluble urokinase plasminogen activator receptor (suPAR) in acute care: a strong marker of disease presence and severity, readmission and mortality. A Retrospective Cohort Study Emerg Med J. 2016;33:769–775.
45. Hodges GW, Bang CN, Wachtell K, Eugen-Olsen J, Jeppesen JL. suPAR: a new biomarker for cardiovascular disease? Can J Cardiol. 2015;31:1293–1302. doi:10.1016/j.cjca.2015.03.023
46. Langkilde A, Petersen J, Klausen HH, Henriksen JH, Eugen-Olsen J, Andersen O. Inflammation in HIV-infected patients: impact of HIV, lifestyle, body composition, and demography - a cross sectional cohort study. PLoS One. 2012;7:e51698. doi:10.1371/journal.pone.0051698
47. Haastrup E, Grau K, Eugen-Olsen J, Thorball C, Kessing LV, Ullum H. Soluble urokinase plasminogen activator receptor as a marker for use of antidepressants. PLoS One. 2014;9:e110555. doi:10.1371/journal.pone.0110555
48. Ilmarinen P, Tuomisto LE, Niemelä O, Kankaanranta H. Prevalence of patients eligible for anti-IL-5 treatment in a cohort of adult-onset asthma. J Allergy Clin Immunol Pract. 2019;7(165–174.e4):165–174.e4. doi:10.1016/j.jaip.2018.05.032
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.