Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Relationship Between Proteinase with a Disintegrin and a Metalloproteinase Domain-9 (ADAM9), Inflammation, Airway Remodeling, and Emphysema in COPD Patients
Authors Cui L , Li H, Xie M , Xu X, Zhang YM, Wang W , Dou S , Xiao W
Received 13 August 2020
Accepted for publication 9 November 2020
Published 14 December 2020 Volume 2020:15 Pages 3335—3346
DOI https://doi.org/10.2147/COPD.S276171
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Liwei Cui,1 Haijun Li,2 Mengshuang Xie,1 Xia Xu,2 Yingmei Zhang,3 Wei Wang,1 Shuang Dou,1 Wei Xiao1
1Department of Pulmonary Medicine, Qilu Hospital, Shandong University, Jinan, People’s Republic of China; 2Department of Cadre Health Care, Qilu Hospital, Shandong University, Jinan, People’s Republic of China; 3Department of Pulmonary Medicine, Linyi People’s Hospital, Linyi, People’s Republic of China
Correspondence: Wei Xiao
Department of Pulmonary Medicine, Qilu Hospital, Shandong University, 107 Wenhuaxi Road, Jinan 250012, People’s Republic of China
Email [email protected]
Background and Objective: The link between ADAM9 and airway remodeling and emphysema severity in COPD patients has not been elucidated. Here, we investigated the relationship between ADAM9 levels in sputum and airway epithelium and the clinical characteristics of COPD patients.
Methods: A sputum cohort and a lung tissue cohort were included in the study. Pulmonary function and computed tomography data were analyzed in COPD patients, non-COPD smokers, and non-smokers. Soluble ADAM9 and interleukin 8 (IL-8) levels in sputum supernatants as well as surface ADAM9 expression in airway epithelium were detected. Emphysema scores were calculated by the percentage of low attenuation area (%LAA-950), and airway remodeling was measured via airway thickening and loss of airway counts.
Results: Both soluble ADAM9 levels in sputum and relative surface ADAM9 expression in airway epithelium were increased in COPD patients. Sputum ADAM9 levels were negatively correlated with forced expiratory volume in 1 s of predicted (FEV1% of predicted) and positively correlated with sputum IL-8 levels, but not with CT measured emphysema nor airway remodeling. The ADAM9 expression in airway epithelia was positively correlated with %LAA-950 and airway wall thickening parameters (wall area percentage, WA%; the square root of the wall area in a standard airway with a 10 mm internal perimeter, Pi-10), while negatively correlated with airway counts derived from the 4th to 9th bronchial generations.
Conclusion: Airway ADAM9 levels in sputum and airway epithelium were both elevated in COPD patients compared to non-COPD controls. Sputum ADAM9 seemed to be associated with inflammatory responses in COPD, while epithelial ADAM9 was more correlated with emphysema and airway remodeling.
Keywords: chronic obstructive pulmonary disease, proteinase with a disintegrin and a metalloproteinase domain-9, computed tomography, emphysema, airway remodeling
Introduction
In China, chronic obstructive pulmonary disease (COPD) is highly prevalent in the adult population, affecting 8.6% of the general population aged 20 years or older and 13.7% of people aged 40 years or older.1 Cigarette smoking, ambient air pollution and other noxious particles are COPD risk factors, that result in persistent airflow limitation and respiratory symptoms, including cough, sputum production and dyspnea.2
Small airway disease (SAD) and emphysema are two key features of COPD, and both contribute to the development of airflow obstruction and heterogenous clinical phenotypes.3 In COPD, the small airways undergo marked remodeling, characterized by increased airway wall thickness, which stems from epithelial reprogramming, mucus hypersecretion, increased infiltration of inflammatory cells, smooth muscle hyperplasia and fibrosis.4 Recent evidence has revealed that both the narrowing of small airway lumens and the loss of small airways could drive the formation of airflow obstruction, and the loss of small airways is closely related to increased severity of emphysema.5,6 These studies indicate that small airway remodeling is linked to emphysematous lesions in COPD, and similar underlying changes might occur in both disappearing airways and damaged alveoli.
The balance of proteinases and anti-proteinases is regulated precisely in healthy individuals, and the loss of balance is a key factor in the pathogenesis of COPD. A large volume of studies have demonstrated that multiple matrix metalloproteinases (MMPs) levels, including MMP-1, −8, −9 and −12, are increased in COPD patients and participated in the pathogenesis of COPD, especially in emphysema development.7,8 Proteinases with a disintegrin and a metalloproteinase domain (ADAMs), belonging to the metzincin superfamily, show similar structural features to MMPs but have several distinct domains, including a metalloproteinase domain, a disintegrin domain and a cysteine-enriched domain.9 Multiple studies have shown that ADAM family members, including ADAM8, ADAM17, ADAM33 might participate in the pathogenesis of COPD.10–13
ADAM9 is normally expressed in epithelial cells, inflammatory cells, and smooth muscle cells in human lung tissues.14 A murine study using an ADAM9 knockout mice model showed alleviated airspace enlargement in response to cigarette smoking exposure, which indicated its importance in emphysema.11 Despite this, the link between ADAM9 expression and airway remodeling in COPD patients remains to be elucidated.
In this study, we compared soluble ADAM9 levels in sputum and surface ADAM9 expression in airway epithelium in COPD patients, non-COPD smokers, and non-smoking controls, and investigated its relationship with airflow obstruction, inflammation, and CT measured airway remodeling and emphysema.
Methods
Study Population
Two subjects’ cohort were recruited in the present study, including a sputum specimen cohort and a tissue specimen cohort. All subjects underwent pulmonary function tests and CT scans prior to recruitment. COPD was diagnosed based on a postbronchodilator FEV1/FVC ratio of less than 0.7 according to the guidelines of the Global Initiative for Chronic Obstructive Lung Disease (GOLD).2
The sputum cohort consisted of non-smoking controls (non-smokers, n=21), non-COPD smokers (smokers, n=21), and stable COPD patients (n=42). The patients had no acute exacerbation within the month prior to recruitment and no long-term antibiotics/systemic steroids use history. The non-COPD smokers had at least a 10 pack-year smoking history and no airflow limitation defined by GOLD criteria. Non-smoking controls were subjects with no smoking history nor airflow limitation. Exclusion criteria consisted of asthma, bronchiectasis, pulmonary abscess, interstitial lung disease, tuberculosis, central lung mass, and systematic disease such as congestive heart failure, autoimmune disease, or infection.
The tissue cohort consisted of non-smoking controls (non-smokers, n=28), smoking controls (smokers, n=34), and stable COPD patients (n=36). Lung tissue specimens were collected from patients who underwent lobectomy or pneumonectomy for peripheral lung masses or nodules. The lung tissues used for analysis were resected ≥5 cm away from the margins of pathological lesions (such as tumors). COPD and smokers were defined using the same criteria as the sputum cohort. Patients with asthma, diffuse bronchiectasis, pulmonary infection, lung interstitial abnormalities, cancer metastasis, and secondary pulmonary changes from rheumatic diseases, etc., were excluded from the study.
All the subjects in the study were recruited from Qilu Hospital, Shandong University, Jinan, China. The study was conducted in accordance with the declaration of Helsinki. The project was approved by the ethics committee of Qilu Hospital, Shandong University (No. KYLL-2018-316). Written informed consent was obtained from each participant prior to recruitment.
Thoracic CT Acquisition
Thoracic CT scans were conducted through 64-slice spiral CT scanners (Philips Brilliance, Amsterdam, the Netherlands). Scans were obtained at the full inspiratory phase with no intravenous contrast. The tube voltage was 120 kV, and the current ranged from 20 to 500 mA, with a rotation time of 0.5 s. Exposure time was set at 0.5 s, and consecutive images were reconstructed with a 1.0-mm slice thickness. Imaging data were retrieved for further analysis.
Quantitative Analysis of Emphysema Severity and Airway Remodeling
The imaging data were quantitatively analyzed via 3D slicer software (www.slicer.org). Emphysema was defined as the percentage of voxels below −950 Hounsfield units (Hu) to total lung voxels (%LAA-950).5,6,15
Airway wall parameters were quantified using the following parameters: wall thickness (WT), wall area percentage (WA%), and the square root of wall area with a 10 mm internal perimeter (Pi10). The right apical bronchus (RB1), the right basal posterior bronchus (RB10), the left apical bronchus (LB1), and the left basal posterior bronchus (LB10) were identified as the target airways. The upper middle 1/3 from the bronchial origin to the bifurcation was chosen as the site for measuring WT and WA. WA% was quantified as WA/total bronchial area×100.16 The mean WT and WA% were calculated as the average of four target bronchi of the third, fourth, and fifth airway generation separately. Pi10 was defined as the square root of WA of a hypothetical airway with a 10 mm internal perimeter, and this hypothetical airway was constructed from the calculated WA above.17
Airway counts were quantified via a visual inspection under the conditions of a window width of 1000 Hu and a level of −500 Hu. Considering the perpendicular direction of the airway, the right superior lobal bronchus (RB1) was identified as the initial site of airway counting and was designated as the 3rd airway generation. Slice-by-slice examination was performed to identify the bifurcation of the RB1. The daughter bronchi were calculated and recorded based on Weibel’s model of airway anatomy as previously described.6,15,18 The expected number of daughter bronchi was calculated as 2^(n-2) where n represents the airway generation. For a more accurate calculation of airway counts by CT, airways before the 10th generation were calculated in the study. Here, airway counts of the 4th to 9th and 6th to 9th airways were summed to evaluate the loss of airway counts.
Sputum ADAM9 and IL-8 Analysis
Sputum was induced by hypertonic saline, following the safe measurement protocol.19 Sputum samples were then collected and processed within 2 h once harvested. Dithiothreitol was added in a volume four times the weight of the sputum samples. The samples were then dissolved gently, filtered through sterile nylon mesh and centrifuged at 800 × g and 4°C for 10 min. The supernatants were then obtained and stored at −80°C. Soluble ADAM9 and IL-8 were measured using enzyme-linked immunosorbent assay (ELISA) kits (Cusabio, Wuhan, China) with detection sensitivity of 15.6 pg/mL and 7.110 pg/mL, seperately.
Immunohistochemistry Analysis for ADAM9 Expression
Tissue sections with a 4-μm thickness were made from paraffin-embedded lung specimens. The sections were then processed through dewaxing and antigen retrieval by citrate buffer (pH 6.0) for 15 min with the temperature in the range of 92°C–98°C. The sections were incubated with the primary antibody anti-ADAM9 IgG of a rabbit (Abcam, Cambridge, MA). Visualization was then performed with an EnVision detection system and a DAB color development kit (ZhongShan Golden Bridge Biotechnology, Beijing, China). The mean staining density of ADAM9 in the airway epithelium was quantified using Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA).
Statistical Analysis
Descriptive data are presented as the mean ± standard deviation (SD). SPSS 20.0 (IBM, Armonk, NY, USA) was used for analysis. Comparison between continuous variables in normal distribution was performed via Student’s t-tests, while variables that were not normally distributed were compared by Mann–Whitney U-tests. Categorical variables were analyzed by Chi-square tests. A correlation analysis for continuous variables in normal distribution was performed by Pearson’s correlation analysis, otherwise by Spearman’s rank correlation coefficient. P < 0.05 was considered statistically significant.
Results
Demographic and Clinical Characteristics
Demographic and clinical characteristics of the subjects from the sputum cohort and the tissue cohort are shown in Tables 1 and 2, separately. In the sputum cohort, no significant difference was shown between COPD patients and the non-COPD smokers in terms of age, gender, smoking history, and BMI. The non-smokers were slightly younger than the smokers and COPD patients in this study. There were no obvious differences in gender composition and BMI between non-COPD smokers and non-smokers. Subjects with GOLD III–IV accounted for 14/42 of the total COPD patients in this cohort.
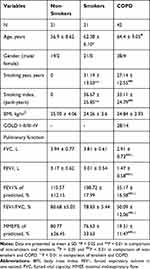 |
Table 1 Clinical Characteristics of Subjects in the Sputum Cohort |
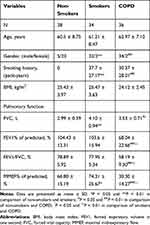 |
Table 2 Clinical Characteristics of Subjects in the Lung Tissue Cohort |
In the tissue cohort, there were no significant differences in terms of age, gender ratio, smoking history and BMI between the smokers and COPD patients (P > 0.05). The non-smokers included more female subjects than the smokers and COPD patients (P < 0.01), and no obvious differences in age and BMI were found between the smokers and the non-smokers. Considering thoracotomy surgery tolerance, the majority of COPD patients enrolled in the tissue cohort (29/36) suffered from mild-to-moderate airflow obstruction.
Sputum ADAM9 Levels and Airway Epithelial ADAM9 Expression Were Increased in COPD Patients
The sputum ADAM9 levels in sputum specimens were compared between non-smokers, smokers, and COPD patients (Table 3, Figure 1A). After adjustment for age, gender, and BMI, the ADAM9 levels were significantly higher in the sputum specimens of COPD patients than the non-smokers (P = 0.036) and the smokers (P = 0.039), while no significant difference was found between the non-smokers and the smokers (P > 0.05).
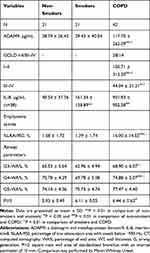 |
Table 3 Sputum ADAM9, IL-8 Levels and CT Measurement of Subjects in the Sputum Cohort |
In the tissue cohort, ADAM9 protein could be found in almost of all the subjects’ airways. Immunostaining of surface ADAM9 in the airway was mainly localized to the apical side of epithelium, and could also be found in the basal side of the pseudostratified epithelium (which might be related to the basal cells of the epithelium), mesenchymal cells and some inflammatory cells as well, especially in the COPD patients (Figure 2A). Significantly higher expressions of ADAM9 were observed in the airway epithelium of COPD patients than in smokers and non-smokers, and the smokers furtherly showed higher positivity of ADAM9 than the non-smokers (P < 0.05 for all; Table 4, Figure 2B).
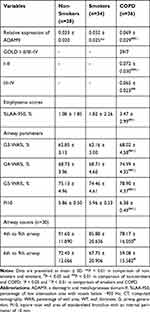 |
Table 4 Relative Expression of Airway Epithelial ADAM9 and CT Measured Emphysema Scores and Airway Remodeling in Lung Tissue Cohort |
Increased Sputum ADAM9 Levels Correlate with Airflow Obstruction, Sputum IL-8 Levels, but Not with Emphysema Severity nor Airway Remodeling
Since sputum ADAM9 levels were increased in the COPD patients, correlations of ADAM9 levels and subjects’ clinical characteristics were analyzed. This revealed a negative correlation between log10 sputum ADAM9 concentration and FVC (r = −0.346, P = 0.001; Figure 1B), FEV1% of predicted (r = −0.395, P < 0.001; Figure 1C), FEV1/FVC (r = −0.344, P = 0.001; Figure 1D) and MMEF% of predicted (r = −0.403, P < 0.001; Figure 1E). ADAM9 levels in sputum were positively correlated with sputum inflammatory cytokine IL-8 levels (r = 0.558, P < 0.001; Figure 1F). However, no obvious correlation between log10 sputum ADAM9 concentration and CT measured airway remodeling (WA% of 3rd-5th airway generations and Pi10) and emphysema severity (%LAA-950) was shown (P > 0.05, Table 5).
 |
Table 5 Correlation Analysis Between Sputum ADAM9 Levels and Airway Epithelial ADAM9 Expression and Subjects’ Characteristics |
Increased Airway Epithelial ADAM9 Expression Correlates with Subjects’ Clinical Characteristics, CT-Measured Emphysema Scores and Airway Remodeling
In the lung tissue cohort, the relative expression of ADAM9 in the airway epithelium showed a positive correlation with subjects’ smoking exposure (r = 0.281, P = 0.006; Figure 3A) and a negative correlation with airflow obstruction parameters, including FEV1% of predicted (r = −0.271, P = 0.013; Figure 3B), FEV1/FVC (r = −0.455, P < 0.001; Figure 3C) and MMEF% of predicted (r = −0.256, P = 0.046; Figure 3D).
In the present study, the COPD patients showed increased WA% as well as Pi10 than non-smokers and smokers (P < 0.05 for all; Table 4). Moreover, lower airway counts (4th to 9th) from RB1 were measured in the COPD patients when compared to non-smokers (78.17 ± 16.05 vs 91.60 ± 11.89, P < 0.05).
There was a positive correlation between surface ADAM9 expression in airway epithelium and emphysema scores %LAA-950 (r = 0.392, P = 0.001; Figure 4A). As for airway remodeling, airway epithelium ADAM9 expression showed a positive correlation with airway wall thickening, including WA% of the 3th, 4th and 5th bronchus (r = 0.317, 0.418, 0.297, P < 0.05 for all, Figure 4B), and Pi-10 (r = 0.243, P = 0.044, Figure 4C). Furthermore, airway counts of the 4th to 9th and 6th to 9th airways were all inversely correlated with airway epithelium ADAM9 expression (r = −0.508, −0.512, P < 0.01 for all, Table 5, Figure 4D).
Discussion
In the present study, our results demonstrated that both sputum ADAM9 levels and airway epithelial ADAM9 expression were significantly increased in COPD patients from two distinct cohorts. In the sputum cohort, the elevated ADAM9 levels showed a significant correlation with inflammatory cytokine IL-8 concentration and decreased airflow parameters, including FEV1% of predicted and FEV1/FVC ratio. However, no obvious correlation was shown between sputum ADAM9 levels and CT measured emphysema severity nor airway wall thickening. In the lung tissue cohort, in addition to airflow obstruction parameters, the increased ADAM9 expression in airway epithelium was positively correlated with subjects’ cigarette smoking history and emphysema severity. Furthermore, the expression of ADAM9 was also correlated with airway wall thickening and airway counts of the 4th to 9th bronchi.
ADAMs, together with secretory type ADAMs with thrombospondin motifs (ADAMTS), are proteinases belonging to the metzincin superfamily which also include MMPs.9 Ostridge et al20 investigated the roles of MMPs in airway remodeling and showed that MMP-3, −7, −9, −10 and −12 were closely associated with SAD defined by the ratio of mean lung density on expiration and inspiration (MLD E/I) but not with markers of large airway thickness, indicating that MMPs potentially participated in the destruction of the matrices surrounding small airways during the pathogenesis of COPD. This was further verified in a study by Eurlings et al in which matrix remodeling in the small airway wall showed similar changes, including decreased elastin and increased hyaluronan and collagen deposition in alveolar walls.21
Proteinases in the ADAM family share structural and functional similarity with MMPs but exhibit key distinct features. The specific metalloproteinase domain and disintegrin domain endow ADAMs with not only catalytic activity but also adhesive activity, allowing ADAMs to modulate cellular fates such as proliferation, differentiation, and cell death.22–24 In COPD, several ADAMs could serve as “molecular scissors”. These proteinases could cleave ectodomain of multiple cytokines, cytokine receptors, growth factors and adhesion proteins.25,26 For example, ADAM17, also known as TNF-α converting enzyme (TACE), can cleave ectodomains of substrates such as IL-6 receptors, TNF-α, amphiregulin (AREG) and heparin-binding EGF-like growth factor (HB-EGF), which can activate downstream pathways related to inflammation responses, collagen deposition and myofibroblast proliferation.12 To explore the role of ADAM9 in the pathogenesis of COPD, Wang et al revealed that ADAM9 knockout in mice could alleviate cigarette smoke-induced emphysema by degrading elastin as well as promoting apoptosis of alveolar septal cells.11 Despite the formation of emphysema, the mechanisms of how ADAM9 affects airway remodeling, including epithelial proliferation, differentiation and airway fibrosis largely remain to be investigated.11,27
In the present study, sputum ADAM9 levels were obviously elevated in COPD patients and were negatively correlated with the severity of airflow limitation. The main sources of ADAM9 in the sputum supernatants are soluble forms of ADAM9 released from polymorphonuclear neutrophils (PMNs), epithelial cells and several other cell types.25,28 The roles of soluble forms of ADAMs in disease remain largely unknown. Fry and Toker reported that soluble form ADAM9 could promote breast cancer cell migration while membrane-bound ADAM9 inhibited cell migration via enhancing cellular adhesion.29 This indicated that soluble ADAM9 might play different roles in diseases when compared with membrane-bound ADAM9. Considering the relationship between COPD and inflammation, we next investigated the link between sputum ADAM9 and inflammatory cytokines. IL-8 is one of the most studied chemokines secreted by monocytes, macrophages, epithelial cells, and endothelial cells.30 In COPD, IL-8 could participate the pathogenesis of COPD via promoting inflammatory infiltration in response to cigarette exposure, and the loss of epithelial contacts.31 Our results revealed that sputum ADAM9 levels were closely correlated with IL-8 levels but not with emphysema severity nor airway wall thickening. A possible explanation for this is that soluble ADAM9 levels in sputum largely relied on the inflammatory cells, which also secreted cytokines such as IL-8. Furthermore, soluble ADAM9 might affect tissue remodeling processes such as emphysema-like destruction and airway wall thickening in an indirect manner, for example, increasing the permeability of epithelial barrier, thereby amplifying tissue damage.
Expression of ADAM9 in airway epithelium was found to be positively correlated with smoking exposure and airflow obstruction in this study. The airway epithelium is the initial site exposed to cigarette smoke and other noxious particles, and epithelial reprogramming could then result in airway wall thickening, peripheral airway destruction, and emphysema development. Contrary to sputum ADAM9, epithelial ADAM9 expression was positively correlated with emphysema severity. This might be partially explained by the ADAM9-mediated increased death of alveolar septal cells, which further promoted alveolar destruction and emphysema formation. These results coincided with the murine study by Wang et al in which cigarette smoking-exposed ADAM9−/- mice exhibited reduced apoptosis of septal cells and alleviated airspace enlargement.11
In terms of airway remodeling, airway epithelial ADAM9 expression was correlated with both airway wall thickening and the loss of airway counts. This indicated that epithelial ADAM9 might affect airway remodeling in a more direct manner. The similarity between emphysema and airway remodeling has received much attention recently. A study by Gosselink et al utilized laser capture microdissection (LCM) technique and suggested that small airways and the surrounding parenchyma shared similar genes related to wound repair.32 Another study focused on matrix remodeling in COPD showed that similar increased elastin degradation and increased collagen and hyaluronan deposition in both alveolar and small airway walls in COPD patients.21 These studies suggested that similar structural destruction and ECM deposition might occur in both distal airways and alveoli in COPD. In the present study, both airway counts and emphysema scores were positively correlated with epithelial ADAM9 expression, implying that membrane-bound ADAM9 might exert similar effects on the survival of airway and alveolar epithelial cells. The excessive death of structural cells would upset the balance of tissue repair and ultimately lead to parenchymal destruction.
The results of this study also showed positive correlation between epithelial ADAM9 expression and airway wall thickening, including WA% and Pi10. Different from the loss of distal airway counts, the CT measured airway wall thickening mainly stemmed from epithelial changes, mucus hypersecretion, infiltration of inflammatory cells, smooth muscle hyperplasia and fibrosis.4 This indicated that ADAM9 expressed in airway epithelial cells could also participate in the remodeling of proximal airway through epithelial disorder, inflammation infiltration, or ECM deposition, which could be different from relative peripheral airways.
There were several limitations in the present study. Firstly, as a pilot study, we mainly investigated the correlation between airway ADAM9 levels and airway remodeling and emphysema. The exact mechanisms of how ADAM9 affects airway epithelial abnormalities and/or disordered mesenchymal cells still need to be studied furtherly. Since airway basal cells are responsible for maintaining airway epithelial integrity and are regarded as the original site of COPD-related disorders, the possible influence of ADAM9 protein on airway basal cell proliferation and differentiation remains to be studied.33,34 Secondly, RB1 was chosen as the target bronchus for measuring airway number for its perpendicular orientation to scanning planes, and only a set of subjects with no pulmonary lesions in the right superior lobe was selected here. A larger cohort of subjects need to be involved to explore the link between ADAM9 and the remodeling of more distal airways.
Conclusions
Airway ADAM9 levels in sputum and airway epithelium were both elevated in COPD patients compared to non-COPD controls. Sputum ADAM9 was been associated with inflammatory responses in COPD, while epithelial ADAM9 was closely correlated with emphysema and airway remodeling. This suggests that different roles of soluble and membrane-bound ADAM9 in the pathogenesis of COPD.
Abbreviations
COPD, chronic obstructive pulmonary disease; MMP, matrix metalloproteinase; ADAM, proteinase with a disintegrin and a metalloproteinase domain; ADAMTS, ADAMs with thrombospondin motifs; IL, interleukin; BMI, body mass index; %LAA-910, percentage of low attenuation area with voxels below −910 Hounsfield units; %LAA-950, percentage of low attenuation area with voxels below −950 Hounsfield units; CT, computed tomography; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; G, bronchial generation; Pi10, square root wall area of standardized bronchus with an internal perimeter of 10 mm; WA%, percentage of wall area; WT, wall thickness; ECM, extracellular matrix; TACE, TNF-αconverting enzyme; HB-EGF, heparin-binding EGF-like growth factor; AREG, amphiregulin.
Ethics Approval and Consent to Participate
The work was approved by the ethics committee of Qilu Hospital, Shandong University, Jinan, China (KYLL-2018-316). Written informed consent was obtained from all participants.
Acknowledgments
The authors thank all the participants in the study for their willingness to contribute to our research.
Funding
This study was supported by grants from the National Key Research and Development Program in China (2016YFC0903603). The funding body has no role in study design, data collection, analysis, and interpretation, and in writing the manuscript.
Disclosure
The authors report no potential conflicts of interest for this work.
References
1. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (The China pulmonary health [CPH] study): A national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/S0140-6736(18)30841-9
2. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for prevention, diagnosis and management of chronic obstructive pulmonary disease (version 2020). Available from: https://goldcopd.org/gold-reports/.
3. Tho NV, Ryujin Y, Ogawa E, et al. Relative contributions of emphysema and airway remodeling to airflow limitation in COPD: consistent results from two cohorts. Respirology. 2015;20(4):594–601. doi:10.1111/resp.12505
4. Higham A, Quinn AM, Cançado JED, et al. The pathology of small airways disease in COPD: historical aspects and future directions. Respir Res. 2019;20(1):49.
5. McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med. 2011;365(17):1567–1575. doi:10.1056/NEJMoa1106955
6. Diaz AA, Valim C, Yamashiro T, et al. Airway count and emphysema assessed by chest CT imaging predicts clinical outcome in smokers. Chest. 2010;138(4):880–887. doi:10.1378/chest.10-0542
7. Churg A, Zhou S, Wright JL. Series “matrix metalloproteinases in lung health and disease”: matrix metalloproteinases in COPD. Eur Respir J. 2012;39(1):197–209. doi:10.1183/09031936.00121611
8. Gharib SA, Manicone AM, Parks WC. Matrix metalloproteinases in emphysema. Matrix Biol. 2018;73:34–51. doi:10.1016/j.matbio.2018.01.018
9. Paulissen G, Rocks N, Gueders MM, et al. Role of ADAM and ADAMTS metalloproteinases in airway diseases. Respir Res. 2009;10(1):127. doi:10.1186/1465-9921-10-127
10. Wang XY, Li W, Huang K, et al. Genetic variants in ADAM33 are associated with airway inflammation and lung function in COPD. BMC Pulm Med. 2014;4:173.
11. Sadeghnejad A, Ohar JA, Zheng SL, et al. Adam33 polymorphisms are associated with COPD and lung function in long-term tobacco smokers. Respir Res. 2009;10(1):21. doi:10.1186/1465-9921-10-21
12. Stolarczyk M, Amatngalim GD, Yu X, et al. ADAM17 and EGFR regulate IL-6 receptor and amphiregulin mRNA expression and release in cigarette smoke-exposed primary bronchial epithelial cells from patients with chronic obstructive pulmonary disease (COPD). Physiol Rep. 2016;4(16):e12878. doi:10.14814/phy2.12878
13. Polverino F, Rojas-Quintero J, Wang X, et al. A disintegrin and metalloproteinase domain-8: a novel protective proteinase in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(10):1254–1267. doi:10.1164/rccm.201707-1331OC
14. Dijkstra A, Postma DS, Noordhoek JA, et al. Expression of ADAMs (“a disintegrin and metalloprotease”) in the human lung. Virchows Arch. 2009;454(4):441–449. doi:10.1007/s00428-009-0748-4
15. Kirby M, Tanabe N, Tan WC, et al. Total airway count on computed tomography and the risk of chronic obstructive pulmonary disease progression. findings from a population-based study. Am J Respir Crit Care Med. 2018;197(1):56–65. doi:10.1164/rccm.201704-0692OC
16. Bodduluri S, Bhatt SP, Hoffman EA, et al. Biomechanical CT metrics are associated with patient outcomes in COPD. Thorax. 2017;72:409–414. doi:10.1136/thoraxjnl-2016-209544
17. Oelsner EC, Smith BM, Hoffman EA, et al. Prognostic significance of large airway dimensions on computed tomography in the general population. The multi-ethnic study of atherosclerosis (MESA) lung study. Ann Am Thorac Soc. 2018;15:718–727. doi:10.1513/AnnalsATS.201710-820OC
18. Weibel ER, Gomez DM. Architecture of the human lung. use of quantitative methods establishes fundamental relations between size and number of lung structures. Science. 1962;137(3530):577–585. doi:10.1126/science.137.3530.577
19. Guiot J, Demarche S, Henket M, et al. Methodology for sputum induction and laboratory processing. J Vis Exp. 2017;130:56612.
20. Ostridge K, Williams N, Kim V, et al. Relationship between pulmonary matrix metalloproteinases and quantitative CT markers of small airways disease and emphysema in COPD. Thorax. 2016;71(2):126–132. doi:10.1136/thoraxjnl-2015-207428
21. Eurlings IM, Dentener MA, Cleutjens JP, et al. Similar matrix alterations in alveolar and small airway walls of COPD patients. BMC Pulm Med. 2014;26(14):90. doi:10.1186/1471-2466-14-90
22. Zhang P, Shen M, Fernandez-Patron C, et al. ADAMs family and relatives in cardiovascular physiology and pathology. J Mol Cell Cardiol. 2016;93:186–199. doi:10.1016/j.yjmcc.2015.10.031
23. Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Mol Aspects Med. 2008;29(5):258–289. doi:10.1016/j.mam.2008.08.001
24. Oria VO, Lopatta P, Schilling O. The pleiotropic roles of ADAM9 in the biology of solid tumors. Cell Mol Life Sci. 2018;75(13):2291–2301. doi:10.1007/s00018-018-2796-x
25. Dreymueller D, Uhlig S, Ludwig A. ADAM-family metalloproteinases in lung inflammation: potential therapeutic targets. Am J Physiol Lung Cell Mol Physiol. 2015;308(4):325–343. doi:10.1152/ajplung.00294.2014
26. Stolarczyk M, Scholte BJ. The EGFR-ADAM17 axis in chronic obstructive pulmonary disease and cystic fibrosis lung pathology. Mediators Inflamm. 2018;2018:1067134. doi:10.1155/2018/1067134
27. Russell DW, Gaggar A. ADAM9: A damaging player in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(12):1465–1466. doi:10.1164/rccm.201805-1012ED
28. Roychaudhuri R, Hergrueter AH, Polverino F, et al. ADAM9 is a novel product of polymorphonuclear neutrophils: regulation of expression and contributions to extracellular matrix protein degradation during acute lung injury. J Immunol. 2014;193(5):2469–2482. doi:10.4049/jimmunol.1303370
29. Fry JL, Toker A. Secreted and membrane-bound isoforms of protease ADAM9 have opposing effects on breast cancer cell migration. Cancer Res. 2010;70(20):8187–8198. doi:10.1158/0008-5472.CAN-09-4231
30. Ha H, Debnath B, Neamati N. Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics. 2017;7(6):1543–1588. doi:10.7150/thno.15625
31. Thatcher TH, McHugh NA, Egan RW, et al. Role of CXCR2 in cigarette smoke-induced lung inflammation. Am J Physiol Lung Cell Mol Physiol. 2005;289:322–328. doi:10.1152/ajplung.00039.2005
32. Gosselink JV, Hayashi S, Elliott WM, et al. Differential expression of tissue repair genes in the pathogenesis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181(12):1329–1335. doi:10.1164/rccm.200812-1902OC
33. Shaykhiev R, Crystal RG. Early events in the pathogenesis of chronic obstructive pulmonary disease. smoking-induced reprogramming of airway epithelial basal progenitor cells. Ann Am Thorac Soc. 2014;11 Suppl 5(Suppl5):S252–8. doi:10.1513/AnnalsATS.201402-049AW
34. Crystal RG. Airway basal cells. The “smoking gun” of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190(12):1355–1362. doi:10.1164/rccm.201408-1492PP
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




