Back to Journals » Patient Preference and Adherence » Volume 14
Relationship Between Adherence to Opioid Analgesics and Pain Beliefs Among Patients with Cancer Pain at Tertiary Care Hospitals in Malaysia
Authors Kan E, Mustafa S, Chong WW, Premakumar CM , Mohamed Shah N
Received 24 March 2020
Accepted for publication 20 June 2020
Published 12 August 2020 Volume 2020:14 Pages 1411—1419
DOI https://doi.org/10.2147/PPA.S255289
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Elaine Kan,1,2 Suzana Mustafa,1 Wei Wen Chong,2 Chandini Menon Premakumar,2 Noraida Mohamed Shah2
1Pharmacy Department, Kuala Lumpur Hospital, Ministry of Health, Kuala Lumpur, Malaysia; 2Centre of Quality Management of Medicines, Faculty of Pharmacy, Universiti Kebangsaan Malaysia, Kuala Lumpur, Malaysia
Correspondence: Noraida Mohamed Shah
Centre of Quality Management of Medicines, Faculty of Pharmacy, Universiti Kebangsaan Malaysia, Jalan Raja Muda Abdul Aziz, Kuala Lumpur 50300, Malaysia
Tel +60 3 9289 8038
Email [email protected]
Context: Pain is a common and distressing symptom among cancer patients. Opioid analgesics are the mainstay of cancer pain management, and adequate adherence plays an important role in achieving good pain control.
Purpose: To determine the level of adherence to opioid analgesics in patients with cancer pain and to identify factors that may influence the adherence.
Patient and Methods: This was a cross-sectional study conducted from March to June 2018 at two tertiary care hospitals in Malaysia. Study instruments consisted of a set of validated questionnaires; the Medication Compliance Questionnaire, Brief Pain Inventory and Pain Opioid Analgesic Beliefs─Cancer scale.
Results: A total of 134 patients participated in this study. The patients’ adherence scores ranged from 52– 100%. Factors with a moderate, statistically significant negative correlation with adherence were negative effect beliefs (rs= − 0.53, p< 0.001), pain endurance beliefs (rs = − 0.49, p< 0.001) and the use of aqueous morphine (rs = − 0.26, p=0.002). A multiple linear regression model on these predictors resulted in a final model which accounted for 47.0% of the total variance in adherence (R2 = 0.47, F (7, 126) = 15.75, p< 0.001). After controlling for other variables, negative effect beliefs were the strongest contributor to the model (β = − 0.39, p< 0.001) and uniquely explained 12.3% of the total variance.
Conclusion: The overall adherence to opioid analgesics among Malaysian patients with cancer pain was good. Negative effects beliefs regarding cancer pain and opioids strongly predicted adherence.
Keywords: beliefs, cancer pain, compliance, opioid analgesic
Plain Language Summary
Cancer patients often encounter pain, which is distressing and affects their quality of life. Opioid analgesics are the mainstay of cancer pain management and pain control will not be achieved if patients do not adhere to the analgesics prescribed. This study was conducted to determine the level of adherence to opioid analgesics in patients with cancer pain and to identify factors that may influence the adherence. Validated questionnaires were utilized as study instruments on cancer patients receiving opioid analgesics for cancer pain. These questionnaires assessed adherence level towards opioid analgesics, pain characteristics and beliefs about pain and opioid analgesics. Adherence to regular opioid analgesics was found to be good and negative effects beliefs with regards to cancer pain and opioids was the strongest predictor for opioid adherence. Appropriate interventions designed to promote positive effects beliefs is highly warranted to optimize adherence towards the use of regular opioid analgesics for managing cancer pain.
Introduction
Cancer is a major cause of morbidity worldwide and in Malaysia, it is the ninth leading cause of hospitalizations in 2016.1 Studies had found that delays in seeking treatment are responsible for the advanced stage at diagnosis in patients with breast cancer.2–4 A local study attributed the use of complementary and alternative medicines (CAMs) to be significantly associated with delay in presentation and resolution of diagnosis for patients with breast cancer.5 The use of CAMs that has been reported among cancer patients in Malaysia includes nutritional supplements, traditional medicines and spiritual healing.6,7 Therefore, cancer and its complications at advanced stages of the disease such as pain represent a tremendous disease burden in Malaysia.
In a meta-analysis of epidemiological studies (of 117 studies with 63, 533 patients) on cancer pain, pooled pain prevalence rates were 51% in patients with cancer of all stages and 66.4% in advanced or metastatic disease.8 There are currently no epidemiological data on the prevalence of cancer pain in Malaysia. However, a study conducted in a Malaysian palliative care unit reported that 89% of patients with advanced disease experienced pain.9 Pain is one of the most distressing symptoms experienced by cancer patients. Poorly controlled pain impairs function, generates changes in appetite, sleep patterns and mood as well as reduces the quality of life.10
The World Health Organization (WHO) has developed guidelines for the pharmacologic and radiotherapeutic management of cancer pain. This includes the use of three-step analgesic ladder, which explains the need for pain assessment and appropriate management of pain based on pain severity.11 Therefore, adequate adherence to a prescribed analgesic regimen is imperative for cancer pain relief. Adherence is defined as “the extent to which the patient follows medical instructions”.12 Inadequate adherence with an analgesic regimen is said to be one of the main reasons why cancer patients have unrelieved pain.13 Studies have shown that cancer patients are often reluctant to use pain medications, owing to their negative beliefs regarding analgesics and perceptions regarding cancer pain.14 This is unsurprising considering that social and behavioural scientists have proposed that a person’s belief system significantly influences their behaviour, or in this case, medication-taking habits.15 Cancer patients commonly believe that analgesics, especially opioid analgesics, have many side-effects and may lead to drug addiction and tolerance. Some cancer patients also believe that cancer pain cannot be controlled or relieved, and has to be tolerated as much as possible.16 There are several published studies which have investigated the association between sociodemographic factors and cancer pain characteristics with analgesic adherence in cancer patients.17,18 Factors that were linked with analgesic adherence include age, type of cancer, type of pain and other symptoms such as anxiety or sleep disturbance.17
Despite the availability of efficacious pharmacological treatment and updated treatment guidelines for cancer pain,19,20 poorly controlled cancer pain is a prevalent and persistent problem worldwide.8 Currently, no published literature was found on the level of analgesic adherence in patients with cancer pain in Malaysia and its influencing factors. The present study aimed to elucidate this and findings from this study will be essential in the formulation of more effective strategies to manage cancer pain.
Methods
This was a cross-sectional study conducted at the oncology clinics and wards of two tertiary care hospitals in Kuala Lumpur, Malaysia from March to June 2018. Patients were enrolled in this study if they met the following inclusion criteria: 1) aged ≥18 years old and aware of their cancer diagnosis, 2) prescribed with around-the-clock (ATC) opioid analgesics for cancer pain for at least two months, 3) could read and understand English or Malay language and 4) conscious and able to sign the informed consent form. This study was approved by the Research Ethics Committee of Universiti Kebangsaan Malaysia (Approval Reference: UKM PPI/111/8/JEP-2018-119) and the Medical Research and Ethics Committee of the Ministry of Health Malaysia (Approval Reference: NMRR-18-58-39851). Patients were identified by the researcher through screening of patients’ medical records and were selected using convenience sampling. Those who fulfilled the inclusion/exclusion criteria were invited to take part in this study. Details of the study were explained using an appropriate patient information sheet and those who agreed to participate were requested to sign a consent form.
A set of validated questionnaires consisting of a sociodemographic questionnaire, the Medication Compliance Questionnaire, Brief Pain Inventory─Short Form and Pain and Opioid Analgesic Beliefs Scale─Cancer were utilized in this study. These questionnaires were prepared in English and Malay language. Permission to use and/or translate the questionnaires was obtained from questionnaire developers. A data collection form was used to record information from patients’ medical records on disease information such as cancer diagnosis, treatment status, staging, presence of metastasis (if any) and Eastern Cooperative Oncology Group (ECOG) performance status score.
In this study, Medication Compliance Questionnaire (MCQ), a self-reported questionnaire was used to assess the level of adherence to opioid analgesics. The 10-item questionnaire consists of two domains ─ i) drug-taking behaviour (Questions 1─7) and ii) drug-stopping behaviour (Questions 8─10). The Cronbach’s alpha coefficients were previously reported to be 0.67 and 0.84 for the respective domains.21 It was noted that the internal consistency based on the Cronbach’s alpha coefficient did not meet the 0.70 threshold to be considered as reliable for drug-taking behavior domain and is a limitation. However, this questionnaire was chosen as it is available in English and Malay languages and has been validated among Malaysian patients taking antihypertensive drugs.21 The MCQ has also been used to assess adherence to oral chemotherapy among cancer patients in Malaysia.22 Possible scores on the Likert-like scale ranged from 1 (“never”) to 5 (“very frequent”). All negatively worded scores were reversed and all scores were converted to a 0─100 scale. The final adherence score is a mean of the 10-items which is reported on a percentage scale ranging from 0─100%. Higher scores indicate better adherence to opioid analgesics.
The Brief Pain Inventory (BPI) ─ Short Form is a well-established instrument used in numerous studies on cancer pain worldwide.23 The BPI comprises two domains ─ i) four questions related to pain intensity and ii) seven questions related to pain interference on feelings and function. Pain intensity is scored as the mean of four pain variables ─ pain at its “worst”, “least”, “average” and “right now”; each with a Likert-type scale ranging from 0 (“no pain”) to 10 (“pain as bad as you can imagine”). Pain interference was scored as the mean of the seven interference items ─ general activity, mood, walking ability, normal work, relations with other people, sleeping and enjoyment of life. Each item was rated on a Likert-type scale ranging from 0 (“does not interfere”) to 10 (“completely interferes”). A pain intensity composite score (average of the four items) and a pain interference composite score (average of the seven items) were calculated. The Malay BPI is comparable with the original version of the BPI in terms of its psychometric properties. The Cronbach’s alpha coefficient was 0.811 for pain intensity and 0.884 for pain interference, indicating good internal consistency of the scale.24
The Pain and Opioid Analgesic Beliefs Scale─Cancer (POABS-CA) questionnaire was only available in the English language; therefore, a standard forward-translation and back-translation method was used to translate the English version to Malay language. POABS-CA is a 10-item questionnaire to assess beliefs about pain and opioid analgesics. Each item is rated on a 5-point Likert-type scale ranging from 0 (“strongly disagree”) to 4 (“strongly agree”). It measures two belief subscales ─ i) negative effect belief (Questions 1─5, and 8─9) and ii) pain endurance belief (Questions 6─7, and 10). Subscale composite scores were calculated from a mean of the items in a given subscale. The higher the scores in each subscale, the more negative beliefs the patient has about cancer pain and opioids. The Cronbach’s alpha coefficients were reported to be 0.84 for the total scale, 0.74 for negative effect belief subscale and 0.80 for pain endurance belief subscale.14
Data were analyzed using the IBM® Statistical Package for Social Sciences (SPSS) Desktop version 24. Descriptive statistics was used, whereby categorical data were presented as frequency and percentage. Continuous data were tested for normality using the Shapiro–Wilk test.25 Normally distributed data such as age were presented as mean and standard deviation (SD), while non-normally distributed data such as duration of treatment with opioid analgesics, adherence, pain characteristics and pain and opioid analgesic beliefs were presented as median and interquartile range (IQR). Spearman’s rank-order correlation tests were run to determine the associations between sociodemographic factors, pain and opioid analgesic beliefs and pain characteristics with adherence.
Possible predictors of adherence to opioid analgesics investigated were age, gender, highest education level, type of ATC opioid analgesics, duration of treatment with opioid analgesics, pain intensity, pain interference, negative effects beliefs and pain endurance beliefs. Simple linear regression (univariate analysis) was conducted on these variables to identify significant independent variables (predictors) for multiple linear regression (multivariate analysis). Independent variables with p-values of <0.25 were included in the multivariate model. The standard or simultaneous method (“entry” method) was employed in the multivariate analysis to identify the best predictive model of opioid analgesic adherence. A 95% confidence interval was utilized and statistical significance was denoted by a p-value of <0.05.
Results
A total of 134 patients (95.7%) of 140 eligible patients agreed to participate in this study and completed all sections of the questionnaires. The mean age of the patients was 53.1 years (SD = 13.8). The majority of patients were females (61.9%), have completed secondary education (49.3%) and married (78.4%). The majority had breast cancer (24.6%), followed by colorectal cancer (18.7%). One-third of the patients were currently receiving chemotherapy (38.8%). Over two-thirds of the patients (76.9%) had metastatic disease to two or more sites (44.4%), most commonly to the bone and lung. Most of the patients were treated with ATC aqueous morphine (39.6%) for a median treatment period of 2 months (IQR = 4). Table 1 shows the patients’ sociodemographic data and clinical characteristics.
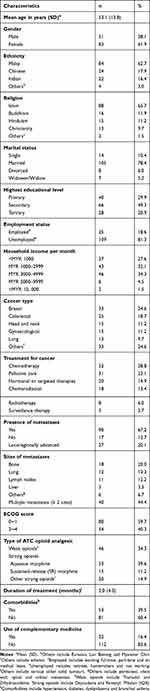 |
Table 1 Sociodemographic Data and Clinical Characteristics (n = 134) |
The patients’ adherence scores ranged from 52─100% with a median adherence score of 84% (IQR = 76─94). Although opioid analgesics were meant to be taken ATC, up to 28.4% of patients claimed that they frequently only took the opioid analgesics when not feeling well (ie, when in pain), while 14.9% frequently stopped taking the opioid analgesics when they felt healthy (ie, not in pain). Additionally, 17.2% of patients reported that they frequently reduced the dose of opioid analgesics when feeling well.
The patients’ pain characteristics were assessed using the BPI questionnaire. The median pain intensity composite score was 3.3 (IQR = 1.5─4.8) while the median pain interference composite score was 4.2 (IQR = 2.0─6.0). With regards to pain intensity, the median score for worst pain was 5 (IQR = 4─8), least pain 2 (IQR = 0─3), average pain 3 (IQR = 1─5) and present pain 2 (IQR = 0─4). Pain interference with enjoyment of life had the highest percentage of “high” pain interference (62.7%), followed by sleep (60.4%) and work (51.4%).
Pain and opioid analgesic beliefs were assessed using the POABS-CA questionnaire (Table 2). The median composite score for negative effect beliefs was 2.0 (IQR = 1.4─2.4) and 1.2 (IQR = 0.7─2.7) for pain endurance beliefs. Approximately one-third of patients agreed to each of the following ─ that opioids should only be used at the last stage of illness (38.1%), that the use of opioids indicated that the illness is terminal (35.8%), that opioids caused many adverse effects (40.3%), that an increased usage of opioids may lead to a life-long dependency (34.3%) and that adult cancer patients should bear with the pain as far as possible (30.6%).
 |
Table 2 Distribution of POABS-CA Scores (n = 134) |
There were no associations between adherence and age (rs = −0.07, p=0.439) or duration of treatment with opioids (rs = 0.04, p=0.665). On the other hand, there was a moderate, negative correlation between negative effect beliefs and adherence (rs = −0.53, p<0.001). There was also a moderate, negative correlation between pain endurance beliefs and adherence (rs = −0.49, p<0.001). For pain characteristics, there was no association between pain intensity and adherence (rs = −0.02, p=0.808). There was a weak association between pain interference and adherence, however, it was not statistically significant (rs =−0.11, p=0.201).
Simple linear regression was conducted to identify possible predictors of adherence to opioid analgesics. Subsequently, variables with a p-value <0.25 (ie, negative effect belief, pain endurance belief, pain interference, percentage pain relief provided by opioid analgesics and type of ATC opioid analgesic (aqueous morphine, SR morphine and strong opioids)) were included in the multiple linear regression to establish a predictive model for adherence to opioid analgesics. The results of simple linear regression (univariate analysis) and multiple linear regression (multivariate analysis) are shown in Table 3. In the regression model, it was found that negative effect beliefs (p<0.001), pain endurance beliefs (p<0.001), type of ATC opioid analgesic (aqueous morphine) (p=0.003) and pain interference (p=0.018) were significant predictors for adherence to opioid analgesics. The R2 value was 0.47; therefore, the preliminary final model fits reasonably well and 47% of the variation in adherence scores could be explained by the four predictors in the regression model. Moreover, the model was a significant predictor of adherence, F (7, 126) = 15.75, p<0.001.
 |
Table 3 Univariate and Multivariate Analyses on Factors Influencing Adherence to Opioid Analgesics |
The standardized beta coefficient (β) was used to evaluate the effect of each individual variable on adherence to opioid analgesics when the variance explained by all other variables in the model was controlled. Negative effect belief (β = −0.39, p<0.001) and aqueous morphine use (β = −0.34, p<0.001) were the strongest statistically significant, unique contributors to the model.
Discussion
The most prevalent type of cancer in this study was breast cancer, owing to the slightly larger number of female patients. In Malaysia, there is a higher incidence of cancer in females (54.8%) compared to males (45.2%), and the most common cancer in Malaysian females is breast cancer. Therefore, the prevalence rates of cancer types in this study are similar to the national incidence, according to the Malaysian National Cancer Registry report.26
Approximately two-thirds of patients in this study had metastases. This high incidence could be attributed to the inclusion criteria for this study, which meant that only patients who were prescribed with opioid analgesics for cancer pain were eligible to participate. Opioid analgesics are the drugs of choice for moderate to severe cancer pain.27 In this study, half of the patients were prescribed with either aqueous morphine or SR morphine. The tendency towards prescribing morphine matches the national incidence, whereby the most commonly used strong opioid in Malaysia is morphine,28 likely due to its cost-effectiveness and extensive experience among physicians in using morphine for cancer pain.
The considerably high median level of adherence to opioid analgesics in this study corresponds with results from a similar study by Nguyen et al, which also utilized a Likert scale-type questionnaire (ie, Modified Morisky Medication Adherence Scale) to assess adherence rates of patients prescribed with opioid analgesics for cancer pain. In that study, 82% of patients had high levels of adherence.29 The high level of adherence found in the present study could be due to adequate knowledge on the role of ATC opioids for cancer pain. Nonetheless, it could also be due to reporting bias usually seen with self-reported questionnaires, where patients tend to give socially desirable answers.
In this study, negative effect beliefs were moderately, negatively correlated with adherence, and similarly for pain endurance beliefs. These findings corresponded with results from other studies, which also found that the more negative beliefs the patient had regarding opioids30 and cancer pain,17 the poorer their adherence to ATC analgesics. This is likely because patients who have more concerns regarding cancer pain and analgesic use would more likely to be hesitant to use analgesics, thereby resulting in lower adherence levels. A randomized controlled study showed that cancer patients who were in a pain education program had a significantly improved analgesic adherence rate after eight weeks compared to those in standard care. These findings attributed to the reduction in the patients’ “barriers” (concerns) about pain management and analgesics, which led them to be more accepting of analgesics.31
In relation to cancer pain characteristics, this study found no association between pain intensity and opioid adherence. There was a weak association between pain interference and adherence; however, it was not statistically significant. A study by Liang et al among cancer patients reported no significant relationships between opioid adherence and any of the measures of pain experience.30 Another study conducted among cancer patients only found a weak, significant positive correlation between pain interference and adherence but no correlation between pain intensity and analgesic adherence.18 A similarity between the study by Liang et al and the current study was the assessment of adherence to opioid analgesics only, but not to other adjuvant analgesics (co-analgesics), which the patients might be taking for other causes of cancer pain.30 For instance, anticonvulsants for neuropathic pain or bisphosphonates for bone pain in patients with bone metastasis.19 Therefore, there were missing gaps in this study pertaining to the role of co-analgesics, which may have been necessary to fully understand the relationship between analgesic adherence and cancer pain characteristics. Patients were diagnosed with different types of cancer and prescribed with different types of opioids for cancer pain, which was unstandardized. These may have influenced the pain intensity and its association with opioid adherence. Further research is warranted to investigate the impact of opioid adherence on pain control in order to ascertain the need for interventions to improve adherence for better pain management outcome.
Half of the patients in this study reported high pain interference with work. This possibly explains the high unemployment found in this study. These findings were similar to a study conducted on cancer patients across ten countries in Asia, which found that 77.6% of cancer patients were unemployed. Of these, 41.8% stopped working due to cancer pain. Moreover, of the patients who were employed, 69.7% cited that cancer pain affected their work performance.32 The current study also found that approximately two-thirds of patients reported high pain interference with sleep, which was lower than the aforementioned study whereby cancer pain affected sleep patterns in 85.9% of patients.32 The high pain interference with sleep was possibly because the most prevalent type of ATC opioid in this study is aqueous morphine, dosed every four-hourly in view of its short half-life.33 According to the Malaysian Clinical Practice Guideline for the Management of Cancer Pain, a double dose of aqueous morphine at bedtime is recommended for convenience and to prevent the patient from being woken up by pain at night.20 Patients with high scores for pain interference with sleep were possibly unaware of this and not counselled to double the dose at bedtime. Frequently, healthcare providers do not mention that a double dose is recommended at bedtime, partly because they may be wary of medication errors when doubling doses for opioids.34 It is also common for cancer patients undergoing chemotherapy to experience sleep problems.35 Furthermore, long-term use of opioids for chronic pain may worsen overall sleep quality.36
Multiple linear regression on significant predictors of adherence (ie, negative effect beliefs, pain endurance beliefs, pain interference composite score, aqueous morphine, SR morphine and strong opioids) was applied to produce a predictive model of opioid analgesic adherence. The final model, which fits the data well judging from its R2 value, contained four significant predictors of adherence, which were negative effect beliefs, pain endurance beliefs, pain interference composite score and use of aqueous morphine. Controlling for the effects of the other independent variables, negative effect beliefs made the strongest contribution to the model based on its β value, and uniquely explained approximately 12.0% of the variance in analgesic adherence. This is followed by the use of aqueous morphine, which explained slightly less than 5.0% of the variance.
Since only 47% of the variance in adherence was explained in this study, further research is needed to determine other factors which could influence analgesic adherence in cancer patients. For future studies, efforts should be made to explore other pain barrier domains such as “fatalism” (ie, fatalistic beliefs about cancer pain and its management), “be good” (ie, “good” patients do not complain of pain) and “distract” (ie, reports of pain distract the physician from treating the underlying disease) using the validated Barriers Questionnaire II tool.37 Other barriers to effective cancer pain management such as healthcare system-related barriers, healthcare provider barriers,38 societal attitudes towards pain management and other patient-related related barriers27 should ideally be assessed together to build a more holistic picture on the overall barriers to cancer pain management.
There were certain limitations to the current study. It was conducted at hospitals located in an urban area. Thus, the findings of this study cannot be extrapolated to a larger population. Sampling bias might have occurred due to the convenience sampling method used in this study. It also relied on self-administered questionnaires by the patients, which might have resulted in reporting bias. Furthermore, this study did not investigate the appropriateness of the type of opioids (weak vs strong opioids or aqueous morphine vs SR morphine) prescribed to patients for cancer pain management. The variations in the types of opioids used may not be ideal for exploring the relationship between patients’ adherence and beliefs. Nonetheless, this study provides an overview on the adherence to opioid analgesics among patients with cancer pain in our local setting.
Conclusion
Overall, cancer patients experiencing pain had good level of adherence to opioid analgesics. Negative effect beliefs were found to be the strongest predictor of opioid adherence. Therefore, efforts to develop a pain education programme targeting cancer patients with high negative beliefs about pain and opioid analgesics would be a priority. It is envisaged that changing pain beliefs would ultimately result in optimizing their adherence to opioid analgesics.
Acknowledgments
We would like to thank the Director General of Health Malaysia for his permission to publish this article. We would also like to express our heartfelt gratitude to patients who participated in this study.
Disclosure
The authors have no financial or other conflict of interest to disclose. This study was not funded by any organisation.
References
1. Ministry of Health Malaysia. Health facts 2016. Available from: http://www.moh.gov.my/moh/images/gallery/publications/KKM%20HEALTH%20FACTS%202016.pdf.
2. Ibrahim NA, Oludara MA. Socio-demographic factors and reasons associated with delay in breast cancer presentation: a study in Nigerian women. Breast. 2012;21(3):416–418. doi:10.1016/j.breast.2012.02.006
3. Ghazali SM, Othman Z, Cheong KC, et al. Non-Practice of breast self examination and marital status are associated with delayed presentation with breast cancer. Asian Pac J Cancer Prev. 2013;14(2):1141–1145. doi:10.7314/APJCP.2013.14.2.1141
4. Cheng ML, Ling DY, Nanu P, et al. Factors influencing late stage of breast cancer at presentation in a district hospital—Segamat Hospital, Johor. Med J Malaysia. 2015;70(3):148–152.
5. Mohd Mujar NM, Dahlui M, Emran NA, et al. Complementary and alternative medicine (CAM) use and delays in presentation and diagnosis of breast cancer patients in public hospitals in Malaysia. PLoS One. 2017;12(4):e0176394. doi:10.1371/journal.pone.0176394
6. Raja Lexshimi RG, Oranye NO, Ho SE, et al. Complementary and alternative medicine use among cancer patients. Malaysian J Public Health Med. 2013;13(1):11–19.
7. Zulkipli AF, Islam T, Mohd Taib NA, et al. Use of complementary and alternative medicine among newly diagnosed breast cancer patients in Malaysia: an early report from the MyBCC study. Integr Cancer Ther. 2018;17(2):312–321. doi:10.1177/1534735417745248
8. Van Den Beuken-van Everdingen MHJ, Hochstenbach LMJ, Joosten EAJ, et al. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51:1070–1090. doi:10.1016/j.jpainsymman.2015.12.340
9. Mansor M, Cardosa MS, Vijayan R Prevalence of pain as a symptom in advanced cancer patients.
10. Hui D, Bruera E. A personalized approach to assessing and managing pain in patients with cancer. J Clin Oncol. 2014;32:1640–1646. doi:10.1200/JCO.2013.52.2508
11. World Health Organization. WHO guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents; 2018. Available from: https://apps.who.int/iris/bitstream/handle/10665/279700/9789241550390-eng.pdf?ua=1.
12. World Health Organisation. Adherence to long-term therapies: evidence for action; 2003. Available from: https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1.
13. Miaskowski C, Dodd MJ, West C, et al. Lack of adherence with the analgesic regimen: a significant barrier to effective cancer pain management. J Clin Oncol. 2001;19:4275–4279. doi:10.1200/JCO.2001.19.23.4275
14. Lai YH, Keefe FJ, Sun WZ, et al. Relationship between pain-specific beliefs and adherence to analgesic regimens in Taiwanese cancer patients: a preliminary study. J Pain Symptom Manage. 2002;24:415–423. doi:10.1016/S0885-3924(02)00509-2
15. Janz NK, Becker MH. The health belief model: a decade later. Health Educ Behav. 1984;11:1–47.
16. Ward SE, Goldberg N, Miller-McCauley V, et al. Patient-related barriers to management of cancer pain. Pain. 1993;52:319–324. doi:10.1016/0304-3959(93)90165-L
17. Lucenteforte E, Vagnoli L, Pugi A, et al. A systematic review of the risk factors for clinical response to opioids for all-age patients with cancer-related pain and presentation of the paediatric STOP pain study. BMC Cancer. 2018;18(1):568. doi:10.1186/s12885-018-4478-3
18. Valeberg BT, Miaskowski C, Hanestad BR, et al. Prevalence rates for and predictors of self-reported adherence of oncology outpatients with analgesic medications. Clin J Pain. 2008;24:627–636. doi:10.1097/AJP.0b013e31816fe020
19. Ripamonti CI, Bandieri E, Roila F. Management of cancer pain: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22:vi69–vi77. doi:10.1093/annonc/mdr390
20. Ministry of Health Malaysia. Clinical practice guidelines: management of cancer pain 1st ed; 2010. Available from: file:///C:/Users/Asus/Downloads/CPG%20Management%20of%20Cancer%20Pain%20(2).pdf.
21. Hassan NB, Hasanah CI, Foong K, et al. Identification of psychosocial factors of noncompliance in hypertensive patients. J Hum Hypertens. 2006;20:23–29. doi:10.1038/sj.jhh.1001930
22. Zahrina AK, Norsa’adah B, Hassan NB, et al. Adherence to capecitabine treatment and contributing factors among cancer patients in Malaysia. Asian Pac J Cancer Prev. 2014;15:9225–9232. doi:10.7314/APJCP.2014.15.21.9225
23. Cleeland C The brief pain inventory user guide. The Brief Pain Inventory; 2009. Available from: https://www.mdanderson.org/documents/Departments-and-Divisions/Symptom-Research/BPI_UserGuide.pdf.
24. Aisyaturridha A, Naing L, Nizar AJ. Validation of the Malay brief pain inventory questionnaire to measure cancer pain. J Pain Symptom Manage. 2006;31:13–21. doi:10.1016/j.jpainsymman.2005.06.011
25. Ahad NA, Yin TS, Othman AR, et al. Sensitivity of normality tests to non-normal data. Sains Malays. 2011;40:637–641.
26. Malaysian National Cancer Registry Report (MNCR) 2012–2016. Available from: https://drive.google.com/file/d/1BuPWrb05N2Jez6sEP8VM5r6JtJtlPN5W/view.
27. Scarborough BM, Smith CB. Optimal pain management for patients with cancer in the modern era. CA Cancer J Clin. 2018;68(3):182–196. doi:10.3322/caac.21453
28. Ministry of Health Malaysia. Malaysian statistics on medicine 2015–2016. Pharmaceutical Services Programme; 2020: 97. Available from: https://www.pharmacy.gov.my/v2/sites/default/files/document-upload/malaysian-statistics-medicines-2015–2016.pdf.
29. Nguyen LMT, Rhondali W, De la Cruz M, et al. Frequency and predictors of patient deviation from prescribed opioids and barriers to opioid pain management in patients with advanced cancer. J Pain Symptom Manage. 2013;45:506–516. doi:10.1016/j.jpainsymman.2012.02.023
30. Liang S-Y, Chen K-P, Tsay S-L, et al. Relationship between belief about analgesics, analgesic adherence and pain experience in Taiwanese cancer outpatients. Asian Pac J Cancer Prev. 2013;14:713–716. doi:10.7314/APJCP.2013.14.2.713
31. Oldenmenger WH, Sillevis Smitt PAE, de Raaf PJ, et al. Adherence to analgesics in oncology outpatients: focus on taking analgesics on time. Pain Pract. 2017;17:616–624. doi:10.1111/papr.12490
32. Kim Y-C, Ahn JS, Calimag MMP, et al. Current practices in cancer pain management in Asia: a survey of patients and physicians across 10 countries. Cancer Med. 2015;4:1196–1204. doi:10.1002/cam4.471
33. Corbett AH, Dana WJ, Fuller MA, et al. Drug Information Handbook (Lexicomp).
34. Gallagher R. Killing the symptom without killing the patient. Can Fam Physician. 2010;56:544–546.
35. Palesh O, Peppone L, Innominato PF, et al. Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nat Sci Sleep. 2012;4:151–162. doi:10.2147/NSS.S18895
36. Bohra MH, Kaushik C, Temple D, et al. Weighing the balance: how analgesics used in chronic pain influence sleep? Br J Pain. 2014;8(3):
37. Gunnarsdottir S, Donovan HS, Serlin RC, et al. Patient-related barriers to pain management: the Barriers Questionnaire II (BQ-II). Pain. 2002;99:385–396. doi:10.1016/S0304-3959(02)00243-9
38. Kwon JH. Overcoming barriers in cancer pain management. J Clin Oncol. 2014;32:1727–1733. doi:10.1200/JCO.2013.52.4827
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
