Back to Journals » International Journal of General Medicine » Volume 11
Relapsing fever Borrelia in California: a pilot serological study
Authors Middelveen MJ , Shah JS , Fesler MC, Stricker RB
Received 6 June 2018
Accepted for publication 17 July 2018
Published 21 September 2018 Volume 2018:11 Pages 373—382
DOI https://doi.org/10.2147/IJGM.S176493
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Video abstract presented by Melissa C. Fesler.
Views: 2356
Marianne J Middelveen,1 Jyotsna S Shah,2 Melissa C Fesler,3 Raphael B Stricker3
1Atkins Veterinary Services, Calgary, AB, Canada; 2IGeneX Laboratories, Palo Alto, CA, USA; 3Union Square Medical Associates, San Francisco, CA, USA
Background: Borrelia spirochetes are tick-borne Gram-negative bacteria that cause disease in humans and animals. Although many studies have focused on Borrelia burgdorferi (Bb), the agent of Lyme disease, recent studies have examined the role of Relapsing Fever Borrelia (RFB) in human disease. In this pilot study, we have evaluated serological reactivity against Bb and RFB in patients residing in California.
Methods: Serological testing for reactivity to Bb and RFB antigens was performed in 543 patients with suspected tick-borne illness using a Western blot technique. Further evaluation of a subset of 321 patients residing in California was obtained. Serum samples were tested for IgM and IgG antibodies reactive with Bb and RFB, and samples were classified by county of residence according to Bb reactivity alone, RFB reactivity alone, and dual reactivity against Bb and RFB. Seroreactivity was ranked in counties with the highest absolute number and the highest prevalence of positive samples.
Results: Of the 543 total serum samples, 32% were positive for Bb, 22% were positive for RFB, and 7% were positive for both Bb and RFB. Of the 321 serum samples from patients residing in California, 33% were positive for Bb, 27% were positive for RFB, and 11% were positive for both Bb and RFB. In the California cohort, the highest rates of positive serological testing for Bb were found in Santa Clara, Alameda, and Contra Costa counties, while the highest rates of positive serological testing for RFB were found in Santa Clara, Alameda, Marin, and San Francisco counties. The highest rates of dual reactivity against Bb and RFB were found in Contra Costa, Alameda, and San Francisco counties. Among the 24 counties with patients who were tested, Bb seropositivity alone was found in four counties, RFB seropositivity alone was found in two counties, and seropositivity for both Bb and RFB was found in 14 counties.
Conclusion: Results of this pilot study suggest that seroreactivity against Bb and RFB is widespread in California, and dual exposure to Bb and RFB may complicate the diagnosis of tick-borne disease. Greater awareness of RFB and broader screening for this tick-borne infection is warranted.
Keywords: Lyme disease, Borrelia burgdorferi, relapsing fever Borrelia, Borrelia miyamotoi, tick-borne disease
Introduction
Borrelia spirochetes are best known for causing Lyme disease (LD), a tick-borne infection acquired from the bite of an Ixodes tick. The Borrelia spirochete complex encompasses approximately 52 species of Borrelia, of which 21 fall into the LD group (Borrelia burgdorferi, Bb) and 29 fall into the Relapsing Fever Borrelia (RFB) group that includes the agents of tick-borne and louse-borne relapsing fever.1–3 Two species remain unclassified.4
In the USA, several species of RFB have been reported to cause disease in humans, including B. miyamotoi, B. hermsii, B. lonestari, B. parkeri, and B. turicatae, with most cases occurring in the western USA.1,2,5–9 In the state of California, B. miyamotoi, B. hermsii, and B. parkeri have been shown to infect humans, and a fourth Borrelia species, B. coriaceae, infects ticks found in that state, although human infection has not yet been identified.10 Many of these RFB species are difficult to culture, and this limitation has hindered research into the diagnosis and treatment of infection with these spirochetes.1 Due to the emergence of genetically diverse RFB, infected individuals often present with a spectrum of symptoms, making diagnosis a challenge for clinicians unfamiliar with the disease.
Starting in 2016, our practice has performed serological testing for RFB on patients with suspected tick-borne disease. Our findings indicate that the genotypic makeup of spirochetal infection in the USA may be more complex than acknowledged at present.
Materials and methods
Patients and data collection
Between October 2016 and May 2018, patients were recruited from a medical practice located in San Francisco, CA, specializing in the diagnosis and treatment of tick-borne diseases. Patients of either sex qualified for the study if they were at least 18 years old and reported musculoskeletal, neuropsychiatric and/or cardiac symptoms consistent with LD.11 Since the primary goal was to assess exposure and not necessarily active infection with select Borrelia genospecies, a known tick bite or erythema migrans rash was not required for participation in the study. Written informed consent for data collection was obtained from each patient, and the anonymous retrospective data collection protocol was approved by the Western Institutional Review Board (WIRB), Puyallup, WA. Blood was drawn at independent laboratories including BioReference® (Elmwood Park, NJ, USA), Laboratory Corporation of America® (Burlington, NC, USA), and AnyLabTestNow® (Alpharetta, GA, USA), and serum samples were sent via overnight mail for tick-borne disease testing. Anonymous patient samples from California were coded according to the patient’s county of residence.
Laboratory assessment
Testing for Bb and RFB was performed through IGeneX Clinical Laboratory in Palo Alto, CA. IGeneX is a high-complexity testing laboratory that has Clinical Laboratory Improvement Amendments (CLIA) certification. Serological testing for Bb and RFB was performed using Western blot techniques that detect IgM and IgG antibodies.12,13 The Bb Western blot detects antibodies reactive with two strains of B. burgdorferi (B31 and 297), as previously described.12 The RFB Western blot detects antibodies reactive with two species of relapsing fever spirochetes, B. hermsii and a fast-growing strain related to B. turcica, that are representative of RFB species known to infect humans.13 Since many RFB species are difficult to culture, the two RFB variants were chosen because they could be grown in the laboratory to serve as reliable test substrates. The reports for RFB Western blot testing in this study did not distinguish between the species.
A positive IgM or IgG test for Bb was based on seroreactivity with six significant protein bands on the Western blot, as previously described.12 The significant bands have molecular weights of 23–25, 31, 34, 39, 41, and 83–93 kDa. The Western blot was interpreted as positive if at least two of the six bands were detected, but with the following exceptions: the test was interpreted as indeterminate if only bands 31 and 41 kDa or only bands 31 and 83–93 kDa were detected. The test was interpreted as negative if only bands 41 and 83–93 kDa were detected, or if less than two bands were detected.
A positive IgM or IgG test for RFB was based on seroreactivity with protein bands from either B. hermsii or the B. turcica-related strain on the Western blot. An aliquot equivalent to 36 µg of sonicated spirochete cell lysate (300 µg/mL) was fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 11.3% acrylamide gel and transferred to Protran BA nitrocellulose membrane (Schleicher & Schuell, Keene, NH) in Tris-glycine-methanol transfer buffer pH 8.3 [25 mM Tris, 192 mM glycine, 20% (vol/vol) methanol]. Detection of serum antibodies against the fractionated RFB protein bands was performed as described for Bb.12 RFB IgM and IgG Western blots were considered positive if test sera reacted with two of the following four antigens from B. hermsii: 21–23, 37 (GlpQ), 41, and 70–75 kDa. RFB IgM and IgG Western blots were considered positive if test sera reacted with two of the following four antigens from the B. turcica-related strain: 21–23, 41, 45 (GlpQ), and 70–75 kDa. The test was interpreted as negative if less than two protein bands were detected. Interpretation was based on internal validation studies using confirmed positive and negative serum samples.
Results
A summary of Bb and RFB testing for all patients is shown in Table 1. Serum samples from a total of 543 patients were collected from October 2016 through May 2018 and tested for IgM and IgG antibodies against Bb and RFB. Of these patients, 511 resided in the USA, 22 were residents of Mexico, six resided in Canada, two were residents of Ireland, one resided in Denmark and one was a resident of New Zealand. Serological testing for Bb yielded 171/543 (32%) positive results, 94/543 (17%) indeterminate results and 278/543 (51%) negative results. Among the samples tested, 54 (10%) had an isolated IgM response, 98 (18%) had an isolated IgG response, and 19 (3%) had a combined IgM/IgG response. Serological testing for RFB yielded 121/543 (22%) positive results and 422/543 (78%) negative results. Among the samples tested, 61 (11%) had an isolated IgM response, 53 (10%) had an isolated IgG response, and 7 (1%) had a combined IgM/IgG response. Positive testing for both Bb and RFB was found in 39/543 (7%) of the serum samples (Table 1).
  | Table 1 Total Lyme and RFB Western Blot Summary, October 2016 through May 2018 Notes: aCombined Lyme/RFB in 39 positive cases (7% of total). Abbreviation: RFB, Relapsing Fever Borrelia. |
A summary of Bb and RFB testing for patients residing in California is shown in Table 2. Patients residing in California comprised 321/543 (59%) of the total tested. Serological testing for Bb yielded 106/321 (33%) positive results, 67/321 (21%) indeterminate, and 148/321 (46%) negative results. Among the samples tested, 37 (12%) had an isolated IgM response, 63 (19%) had an isolated IgG response, and 6 (2%) had a combined IgM/IgG response. Serological testing for RFB yielded 87/321 (27%) positive results and 234/321 (73%) negative results. Among the samples tested, 52 (17%) had an isolated IgM response, 29 (9%) had an isolated IgG response, and 6 (2%) had a combined IgM/IgG response. Positive testing for both Bb and RFB was found in 36/321 (11%) of the serum samples (Table 2).
  | Table 2 California Lyme and RFB Western Blot Summary, October 2016 through May 2018 Notes: aCombined Lyme/RFB in 36 positive cases (11% of total). Abbreviation: RFB, Relapsing Fever Borrelia. |
The results of Bb Western blot reactivity, sorted by California counties into positive IgM, IgG, and combined IgM/IgG responses and total positive, indeterminate, and negative responses are summarized in Table 3. Positive test results for Bb seroreactivity were obtained for patients residing in 18 California counties. The counties with the highest numbers of Bb responses were: Santa Clara, 19 positive results; San Francisco, 16 positive results; Alameda, 15 positive results; and Contra Costa, 14 positive results.
  | Table 3 Lyme Western Blot by California County, October 2016 through May 2018 |
The results of RFB Western blot reactivity, sorted by California counties into positive IgM, IgG and combined IgM/IgG responses, and total positive and negative responses are summarized in Table 4. Positive test results for RFB seroreactivity were obtained for patients residing in 16 California counties. The counties with the highest numbers of RFB responses were: Santa Clara, 21 positive results; Alameda, 16 positive results; Marin, 12 positive results; and San Francisco, 12 positive results.
  | Table 4 RFB Western Blot by California County, October 2016 through May 2018 Abbreviation: RFB, Relapsing Fever Borrelia. |
The results of individual Bb, RFB, and dual Bb and RFB seroreactivity sorted by California county are summarized in Table 5. Positive test results for dual Bb and RFB seroreactivity were obtained for patients residing in 11 California counties. Counties with the highest numbers of patients with dual positive Bb and RFB results were: Contra Costa, eight positive results; Alameda, seven positive results; San Francisco, seven positive results; and Santa Clara, six positive results.
  | Table 5 Lyme, RFB, and Lyme/RFB Western Blot Summary by California County, October 2016 through May 2018 Abbreviation: RFB, Relapsing Fever Borrelia. |
The prevalence of Bb, RFB, and dual Bb and RFB seroreactivity in California counties is shown in Table 6. The population estimates for these counties were derived from financial records as of January 1, 2018 (http://www.dof.ca.gov/Forecasting/Demographics/Estimates/E-1/). For the 24 counties tested, the highest overall prevalence of Borrelia seroreactivity was found in Marin, Humboldt, and Santa Cruz counties. The highest prevalence of Bb seroreactivity was found in Mendocino, Napa, and Santa Cruz counties, while the highest prevalence of RFB seroreactivity was found in Marin, Humboldt, and Santa Clara counties. The reason for the discrepancy between Bb and RFB county prevalence is unclear at present.
  | Table 6 Prevalence of Positive Lyme, RFB, and Lyme/RFB Western Blots by California County, October 2016 through May 2018 Notes: County population estimates were derived from financial records (http://www.dof.ca.gov/Forecasting/Demographics/Estimates/E-1/). Numbers in bold represent counties with the highest prevalence of Lyme and/or RFB. Abbreviation: RFB, Relapsing Fever Borrelia. |
Figure 1 shows a Venn diagram of the 321 California patients who were tested for Bb and RFB in the study. Of the 157 patients who were seropositive for Bb and/or RFB, 70 (45%) were seropositive for Bb alone, 51 (32%) were seropositive for RFB alone, and 36 (23%) were seropositive for both Bb and RFB. There were 164 patients who were seronegative for both Bb and RFB.
  | Figure 1 Venn diagram of 321 California patients tested for Lyme, RFB, and dual Lyme + RFB seropositivity, October 2016 through May 2018. Abbreviation: RFB, Relapsing Fever Borrelia. |
Figure 2 shows the distribution of California counties with patients who were positive for Bb alone, RFB alone, and both Bb and RFB. Patients from 24 counties were tested. Bb testing alone was positive in patients from four counties, RFB testing alone was positive in patients from two counties, Bb and RFB testing was positive in patients from 14 counties, and no positive testing was found in patients from four counties.
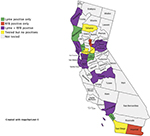  | Figure 2 Map showing distribution of Lyme, RFB and Lyme + RFB testing in California counties, October 2016 through May 2018. Notes: Patients from 24 counties were tested. Lyme testing alone was positive in four counties, RFB testing alone was positive in two counties, Lyme + RFB testing was positive in 14 counties, and no positive testing was found in four counties. Map created using https://mapchart.net/.45 Abbreviation: RFB, Relapsing Fever Borrelia. |
Discussion
Studies of humans and reservoir hosts in California suggest that RFB may be a growing problem in the state. Krause et al conducted a retrospective study of sera collected between 1988 and 1989 from residents of southern Mendocino County at high risk for LD. Their study showed that RFB was present in 2%–7% of those individuals decades before infection with RFB was even recognized. Infection with B. miyamotoi and B. hermsii could not be distinguished with certainty, but findings suggested that both RFB species were present in that county.10 Two studies of rodents in Southern California using serology and molecular detection methods in 2009 and 2014 found that up to 7.7% were infected with RFB.7,14 B. miyamotoi was also found in Ixodes pacificus ticks from 24 of 48 California counties that were surveyed over a 13 year period, and the prevalence of this RFB strain in adult ticks was similar to the prevalence of Bb.15
Our serological study of California patients who were tested for tick-borne disease suggests that Bb and RFB may have a similar prevalence in these patients (33%vs 27%), and evidence for dual seropositivity with Bb and RFB was found in 11% of patients (Table 2 and Figure 1). Seropositivity for Bb was noted in patients from 18 of 24 counties surveyed, while seropositivity for RFB was found in 16 of 24 counties surveyed. Patients who were seropositive for Bb and RFB were found in 14 counties, while dual seropositivity for both Bb and RFB was noted in patients from 11 counties (Tables 3–5). Although our numbers are small so far, the results suggest that RFB could be widespread in California based on the limited data available at present (Table 6 and Figure 2).
A possible concern is serological cross-reactivity between Bb and RFB antigens on Western blots. Cross-reactivity is unlikely for the following reasons: First, when we tested Bb-reactive rabbit serum on the RFB Western blot and RFB-reactive rabbit serum on the Bb Western blot, we found that only the 41 kDa antigen was weakly cross-reactive. The other five significant Bb proteins and the other three significant RFB proteins did not cross-react (data not shown). Second, we found that 89% of patients in California had seroreactivity to either Bb or RFB antigens but not both, and the lack of widespread dual reactivity indicates that cross-reactivity in our distinct Western blot format was unlikely.
B. miyamotoi, a relapsing fever spirochete that was first identified in Japan over 20 years ago, was thought to have been recently introduced into the Western hemisphere.9,15 B. miyamotoi infection has a prevalence of 1%–3% in the northeastern USA, but until recently had never been thought to be on the West Coast. With advances in molecular testing, however, identification of specific Borrelia spp. now challenges pre-existing dogma surrounding the geographic distribution and occurrence of RFB.16 A recent study from Russia found that about 23% of Borrelia infections were associated with positive molecular testing for B. miyamotoi.17 Between 1987 and 2000, 450 human cases of RFB, predominantly B. hermsii, were reported in the USA, most of which occurred in the western states.9 In Northwest Morocco, B. hispanica was reported to have caused over 20% of unexplained fever cases, and in Senegal B. crocidurae infection had a reported incidence of 14 per 100 person-years.9
Interestingly, B. miyamotoi and B. lonestari are transmitted by hard ticks, leading to the proposed distinction of hard tick-borne relapsing fever (HTBRF) or B. miyamotoi disease (BMD).1,10,18,19 In the USA, B. miyamotoi strains have been detected in the hard ticks Ixodes scapularis and Ixodes pacificus,6,9,20,21 while B. lonestari DNA has been detected in the hard tick Amblyomma americanum.5B. miyamotoi also varies genetically depending on geographic region, leading to the concept of the B. miyamotoi sensu lato complex.6,19,22–26
The genetic diversity of Borrelia spp. has consequences for the performance of LD diagnostic tests, and this is reflected in the dismal sensitivity of commercial serological testing.27,28 To date, most commercially available kits for serological testing are based on detection of just one Bb strain, B31.12,28–31 Testing for Borrelia spirochetes ideally should include the entire spectrum of organisms that encompass both Bb and RFB. RFB infection is only sporadically detected because commercially available serologic testing is not readily available.32 The development of testing that reflects the complexity and diversity of Borrelia species worldwide is essential for improving the accuracy of LD diagnosis and treatment (Table 1).
RFB should be considered a major public health concern as infected individuals can develop cyclical fevers with flu-like symptoms and possible central nervous system involvement, especially in immunocompromised patients.19 Dissemination of the RFB spirochete within the bloodstream occurs at a rate 100–1000 times that of the Lyme spirochete and results in a mortality rate of approximately 4%–10% if left untreated, particularly in species endemic to Asia and Africa.33,34 While blood smears obtained at times when the patient is febrile may be diagnostic, blood microscopy can be complicated by the presence of pseudospirochetes, which are filaments derived from erythrocytes that can be confused with living spirochetes such as Leptospira and Borrelia.35,36 Thus, diagnosis through microscopy should ideally be confirmed by other more specific testing such as serological assays.
As with Bb, infection with RFB requires prompt antibiotic treatment to ensure a positive clinical response, and antibiotic therapy can trigger a Jarisch–Herxheimer reaction.37,38 Symptoms of Borrelia and other tick-borne infections are not specific, and patients can have mixed infections with significant overlap of symptoms.39,40 Testing for both Bb and RFB allows a greater diversity of Borrelia genotypes to be detected and will enhance our understanding of geographical distribution and infection rates through surveillance and monitoring.
Our study found that Bb-seropositive patients tended to have more frequent IgG reactivity, while RFB-seropositive patients tended to have more frequent IgM reactivity (Table 2). Although this difference may reflect the timing of testing, persistent IgM reactivity has been reported in patients with LD and suggests that Bb is viable throughout the course of the illness.41–43 The patients in this study were evaluated for Bb and RFB exposure because they had symptoms consistent with tick-borne infection, such as musculoskeletal, neuropsychiatric and/or cardiac problems. Persistent Bb infection despite treatment with antibiotics coupled with prolonged IgM reactivity has been found in humans.42,44 Our study suggests that prolonged IgM reactivity may be a feature of RFB infection as well and suggests that this infection may be persistent. Recognition of prolonged IgM reactivity to RFB should shape recommended testing protocols for accurate diagnosis.
Our study has a number of limitations. RFB seropositivity does not necessarily indicate active infection, and conversely some RFB-infected patients may be seronegative. The presence of positive serology indicates exposure to the spirochete, however, and testing for active infection using other methods should be considered based on the serological results. The number of subjects in our study is small and this limitation is particularly relevant when applying figures by county. The county of residence does not necessarily indicate that the patient was infected in that county, but the demographics provide a basic idea of where RFB-exposed patients can be found. In addition, the Western blot used in this study does not indicate the exact RFB species detected in each case, and reactivity with other RFB species might have been missed. Thus, our pilot study may have underestimated the prevalence of RFB exposure in our patients. More sophisticated immunoblot testing will determine the exact RFB species and will help to refine our understanding of RFB prevalence in California.
In summary, exposure to Bb and RFB appears to be a growing concern in California. RFB infection may explain “Lyme-like” symptoms in patients who are seronegative for Bb, and dual infection with Bb and RFB may confound the diagnosis of tick-borne disease. Greater awareness of RFB and broader screening for this tick-borne infection is warranted.
Acknowledgments
The authors thank Dave Franklin and Brian McElroy for technical assistance. Supported by a grant from the Lindorf Family Foundation, Newark, OH.
Author contributions
All authors contributed toward data analysis, drafting, and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
JSS is President and Laboratory Director of IGeneX Clinical Laboratory, Palo Alto, CA. RBS is the owner of Union Square Medical Associates, a medical practice that treats tick-borne diseases in San Francisco, CA. The other authors report no conflicts of interest in this work.
References
Cutler SJ. Relapsing Fever Borreliae: A Global Review. Clin Lab Med. 2015;35(4):847–865. | ||
CDC [webpage on the Internet]. Tick-borne relapsing fever (TBRF). Distribution; 2015. Available from: https://www.cdc.gov/relapsing-fever/distribution/index.html. Accessed May 27, 2018. | ||
CDC [webpage on the Internet]. Louse-borne relapsing fever (LBRF); 2015. Available from: https://www.cdc.gov/relapsing-fever/resources/louse.html. Accessed May 27, 2018. | ||
Cutler SJ, Ruzic-Sabljic E, Potkonjak A. Emerging borreliae – Expanding beyond Lyme borreliosis. Mol Cell Probes. 2017;31:22–27. | ||
Burkot TR, Mullen GR, Anderson R, Schneider BS, Happ CM, Zeidner NS. Borrelia lonestari DNA in adult Amblyomma americanum ticks, Alabama. Emerg Infect Dis. 2001;7(3):471–473. | ||
Bunikis J, Tsao J, Garpmo U, Berglund J, Fish D, Barbour AG. Typing of Borrelia relapsing fever group strains. Emerg Infect Dis. 2004;10(9):1661–1664. | ||
Schwan TG, Raffel SJ, Schrumpf ME, et al. Tick-borne relapsing fever and Borrelia hermsii, Los Angeles County, California, USA. Emerg Infect Dis. 2009;15(7):1026–1031. | ||
Vuyyuru R, Liu H, Manser T, Alugupalli KR. Characteristics of Borrelia hermsii infection in human hematopoietic stem cell-engrafted mice mirror those of human relapsing fever. Proc Natl Acad Sci U S A. 2011;108(51):20707–20712. | ||
Wagemakers A, Staarink PJ, Sprong H, Hovius JW. Borrelia miyamotoi: a widespread tick-borne relapsing fever spirochete. Trends Parasitol. 2015;31(6):260–269. | ||
Krause PJ, Carroll M, Fedorova N, et al. Human Borrelia miyamotoi infection in California: Serodiagnosis is complicated by multiple endemic Borrelia species. PLoS One. 2018;13(2):e0191725. | ||
Cameron DJ, Johnson LB, Maloney EL. Evidence assessments and guideline recommendations in Lyme disease: the clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev Anti Infect Ther. 2014;12(9):1103–1135. | ||
Shah JS, Cruz D I, Narciso W, Lo W, Harris NS. Improved sensitivity of Lyme disease Western blots prepared with a mixture of Borrelia burgdorferi strains 297 and B31. Chronic Dis Int. 2014;1(2):7. | ||
Güner ES, Hashimoto N, Kadosaka T, Imai Y, Masuzawa T. A novel, fast-growing Borrelia sp. isolated from the hard tick Hyalomma aegyptium in Turkey. Microbiology. 2003;149(Pt 9):2539–2544. | ||
Nieto NC, Teglas MB. Relapsing fever group Borrelia in Southern California rodents. J Med Entomol. 2014;51(5):1029–1034. | ||
Padgett K, Bonilla D, Kjemtrup A, et al. Large scale spatial risk and comparative prevalence of Borrelia miyamotoi and Borrelia burgdorferi sensu lato in Ixodes pacificus. PLoS One. 2014;9(10):e110853. | ||
Gugliotta JL, Goethert HK, Berardi VP, Telford SR. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N Engl J Med. 2013;368(3):240–245. | ||
Karan L, Makenov M, Kolyasnikova N, Stukolova O, Toporkova M, Olenkova O. Dynamics of Spirochetemia and early PCR detection of Borrelia miyamotoi. Emerg Infect Dis. 2018;24(5):860–867. | ||
Krause PJ, Barbour AG. Borrelia miyamotoi: The Newest infection brought to us by deer ticks. Ann Intern Med. 2015;163(2):141–142. | ||
Telford SR, Goethert HK, Molloy PJ, et al. Borrelia miyamotoi disease: neither lyme disease nor relapsing fever. Clin Lab Med. 2015;35(4):867–882. | ||
Nguyen NTT, Röttgerding F, Devraj G, Lin YP, Koenigs A, Kraiczy P. The complement binding and inhibitory protein CbiA of Borrelia miyamotoi degrades extracellular matrix components by interacting with plasmin(ogen). Front Cell Infect Microbiol. 2018;8:23. | ||
Cook VJ, Fedorova N, Macdonald WP, Lane RS, Barbour AG. Unique strain of Borrelia miyamotoi in Ixodes pacificus ticks, California, USA. Emerg Infect Dis. 2016;22(12):2205–2207. | ||
Wroblewski D, Gebhardt L, Prusinski MA, Meehan LJ, Halse TA, Musser KA. Detection of Borrelia miyamotoi and other tick-borne pathogens in human clinical specimens and Ixodes scapularis ticks in New York State, 2012-2015. Ticks Tick-borne Dis. 2017;8(3):407–411. | ||
Mun J, Eisen RJ, Eisen L, Lane RS. Detection of a Borrelia miyamotoi sensu lato relapsing-fever group spirochete from Ixodes pacificus in California. J Med Entomol. 2006;43(1):120–123. | ||
Barbour AG. Phylogeny of a relapsing fever Borrelia species transmitted by the hard tick Ixodes scapularis. Infect Genet Evol. 2014;27:551–558. | ||
Takano A, Toyomane K, Konnai S, et al. Tick surveillance for relapsing fever spirochete Borrelia miyamotoi in Hokkaido, Japan. PLoS One. 2014;9(8):e104532. | ||
Mukhacheva TA, Salikhova II, Kovalev SY. Multilocus spacer analysis revealed highly homogeneous genetic background of Asian type of Borrelia miyamotoi. Infect Genet Evol. 2015;31:257–262. | ||
Stricker RB, Johnson L. Lyme disease diagnosis and treatment: lessons from the AIDS epidemic. Minerva Med. 2010;101(6):419–425. | ||
Stricker RB, Johnson L. Lyme disease: the next decade. Infect Drug Resist. 2011;4:1–9. | ||
Wormser GP, Liveris D, Hanincová K, et al. Effect of Borrelia burgdorferi genotype on the sensitivity of C6 and 2-tier testing in North American patients with culture-confirmed Lyme disease. Clin Infect Dis. 2008;47(7):910–914. | ||
Ogden NH, Margos G, Aanensen DM, et al. Investigation of genotypes of Borrelia burgdorferi in Ixodes scapularis ticks collected during surveillance in Canada. Appl Environ Microbiol. 2011;77(10):3244–3254. | ||
Sperling J, Middelveen M, Klein D, Sperling F. Evolving perspectives on lyme borreliosis in Canada. Open Neurol J. 2012;6:94–103. | ||
Fiecek B, Chmielewski T, Tylewska-Wierzbanowska S. Borrelia miyamotoi – new etiologic agent of neuroborreliosis. Prezgl Epidemiol. 2017;71(4):531–538. | ||
CDC [webpage on the Internet]. Tick-borne relapsing fever (TBRF). Information for clinicians; 2016. Available from: https://www.cdc.gov/relapsing-fever/clinicians/index.html. Accessed May 27, 2018. | ||
Barbour AG. Relapsing Fever and Other Borrelia Diseases. In: Tropical Infectious Diseases: Principles, Pathogens and Practice. 3rd ed. Guerrant RL, Walker DH, Weller PF, editors. Edinburgh: W.B. Saunders; 2011:295–302. | ||
Smith TF, Wold AD, Fairbanks VF, Washington JA, Wilkowske CJ. Pseudospirochetes, a cause of erroneous diagnoses of leptospirosis. Am J Clin Pathol. 1979;72(3):459–463. | ||
Greene RT, Walker RL, Greene CE. Pseudospirochetes in animal blood being cultured for Borrelia burgdorferi. J Vet Diagn Invest. 1991;3(4):350–352. | ||
Koton Y, Bisharat N. Tick-borne relapsing fever with severe jarisch-herxheimer reaction. Isr Med Assoc J. 2018;20(1):62–63. | ||
Wieser A, Löscher T, Schunk M, et al. Relapsing fever: an almost forgotten disease in focus again. Dtsch Med Wochenschr. 2016;141(14):1009–1013. | ||
Stone BL, Brissette CA. Host Immune Evasion by Lyme and Relapsing Fever Borreliae: Findings to lead future studies for Borrelia miyamotoi. Front Immunol. 2017;8:12. | ||
Eickhoff C, Blaylock J. Tickborne diseases other than Lyme in the United States. Cleve Clin J Med. 2017;84(7):555–567. | ||
Craft JE, Fischer DK, Shimamoto GT, Steere AC. Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J Clin Invest. 1986;78(4):934–939. | ||
Lomholt H, Lebech AM, Hansen K, Brandrup F, Halkier-Sørensen L. Long-term serological follow-up of patients treated for chronic cutaneous borreliosis or culture-positive erythema migrans. Acta Derm Venereol. 2000;80(5):362–366. | ||
Kalish RA, Leong JM, Steere AC. Early and late antibody responses to full-length and truncated constructs of outer surface protein A of Borrelia burgdorferi in Lyme disease. Infect Immun. 1995;63(6):2228–2235. | ||
Middelveen MJ, Sapi E, Burke J, et al. Persistent Borrelia infection in patients with ongoing symptoms of Lyme disease.. Healthcare. 2018;6(2):33. | ||
mapchart.net. Available from: https://mapchart.net/. Accessed September 10, 2018. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
