Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
Regulatory T Cells Conditioned Media Stimulates Migration in HaCaT Keratinocytes: Involvement of Wound Healing
Authors Kim D , Lo E , Kim D , Kang J
Received 6 March 2020
Accepted for publication 10 June 2020
Published 6 July 2020 Volume 2020:13 Pages 443—453
DOI https://doi.org/10.2147/CCID.S252778
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Dongsoo Kim, Eunji Lo, Dongju Kim, Junghwa Kang
Research & Development, IMMUNISBIO CO. Ltd., B2, International ST. Mary’s Hospital MTP Mall, Seo-gu, Incheon, Korea
Correspondence: Junghwa Kang
Research & Development, IMMUNISBIO CO. Ltd., B2, International ST. Mary’s Hospital MTP Mall, 31, Simgok-ro 100 Beon-gil, Seo-gu, Incheon, Korea
Tel +82-32-715-5868
Fax +82-32-715-5869
Email [email protected]
Purpose: Regulatory T (Treg) cells, a type of immune cell, play a very important role in the immune response as a subpopulation of T cells. In this study, we investigated the effects of Treg cells conditioned media (CM) on cell migration. Various cytokines and growth factors of Treg cells CM can effect on re-epithelialization stage during the wound healing.
Methods: Isolated CD4+CD25+ Treg cells from Peripheral Blood Mononuclear Cells (PBMCs) were cultured and CM obtained. HaCaT keratinocytes were treated with various concentration of Treg cells CM. Cell migration, proliferation and expression of proteins that are related to the Epithelial-Mesenchymal Transition (EMT) process, matrix metalloproteinase-1 (MMP-1) were analyzed.
Results: Above 90% CD4+CD25+ Treg cells were obtained from CD8+ depleted PBMCs and the CM have various cytokines and growth factors.One percent and 5% concentration of Treg cells CM increased HaCaT keratinocytes migration. The Treg cells CM stimulated EMT, which led to the down-regulation of E-cadherin in the HaCaT keratinocytes at the wound edge. The Treg cells CM increased MMP-1, which is involved in tissue remodeling.
Conclusion: Our results suggest that Treg cells CM which has various cytokines and growth factors promote wound healing by stimulating HaCaT keratinocytes migration.
Keywords: regulatory T cells, cells conditioned media; CM, keratinocytes, migration, Epithelial-Mesenchymal Transition; EMT, matrix mjetalloproteinase-1; MMP-1, wound healing
Introduction
The skin is the largest organ in the human body, and its main function is the protection against external damage and changes in temperature and environment.1,2 The skin consists of two distinct layers: epidermis and dermis.3 The epidermis is the outermost layer, and the inside dermis is supported by an extracellular matrix (ECM) to provide cushioning and tensile strength of the skin.1 The epidermis is composed mostly of keratinocytes, and cells multiply in the base layer and move to the outer exfoliate layer to be naturally eliminated.1,4
Wounds are defined as abnormalities in the skin caused by trauma or disease. The healing of the wound is very important because the persistence of such wounds can prevent the skin from performing its full functions.5 The wound healing process can be divided into hemostasis, inflammation, re-epithelialization, and tissue remodeling.6
The re-epithelialization stage is the process in which the epithelial cells move to the wound surface and cover the wound.7 In this wound healing stage, keratinocytes of epidermis are mainly involved. In order to create new epithelium at the wound site, keratinocytes undergo the process of migration, differentiation, and proliferation.8 In this process, keratinocytes undergo Epithelial-Mesenchymal Transition (EMT), a change from adherent phenotype to migratory phenotype.9 During the EMT process, it leads to the destabilizing of the adherent junction and down-regulation of E-cadherin.9,10 The EMT process in wound healing is essential and the response is controlled by matrix metalloproteinases (MMPs), adherent junction proteins such as E-cadherin, transcription factors such as Twist, Snail.11–14
The MMP family is proteinases that are closely related to ECM modeling.15–18 The ECM is degraded by various proteinases. Type I collagen, which is most abundant in skin, is resistant to most enzymes.19 Matrix metalloproteinase-1 (MMP-1) is known to be primarily involved in the turnover of collagen.19,20 The expressions of the MMPs are controlled in a very complex manner. In general, basal levels are maintained under normal condition, and selectively expressed and activated when tissue remodeling is required.17,18,21,22 In the wound healing process, proteinases are responsible for cell migration and tissue repair by removing or remodeling epithelial and interstitial ECM components.18
Immune cells play a very important role in wound healing.6,23 Immune cells contribute to the removal of foreign antigens when wounds are formed and inflammatory reactions occur.6,24 In addition, immune cells secrete a variety of growth factors and cytokines during the wound healing, which affects tissue remodeling and cell migration.24–27 The immune system is regulated by a very complex crosstalk, which plays an important role in maintaining homeostasis.28 Regulatory T (Treg) cells, a type of immune cell, play a very important role in the immune response as a subpopulation of T cells. Treg cells suppress activation of the immune system and prevent pathological self-reactivity such as autoimmune disease.29 In addition to the direct response by the receptor, such as CTLA-4, the ability of the Treg cells is also achieved through the secretion of cytokines.30–33 Cytokines are essential for the interaction and communication of cells with each other and are involved in cell migration, proliferation, and inflammatory responses.34–38 The IL-8, for example, has been reported to significantly increase the migration of keratinocytes.39
Wound healing is a complex process. Impaired wound healing can affect cosmetic problems such as scar formation, as well as disease problems such as chronic inflammatory reactions.40,41 In this study, we obtained conditioned media (CM) through the culturing of Treg cells, a type of immune cell, and confirmed the effects on the cell migration of HaCaT keratinocytes by treating the Treg cells CM. During the treating process, EMT and MMP-1 expression were confirmed to elucidate the mechanism of regulating wound healing.
Materials and Methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), Penicillin-streptomycin solution and Fetal bovine serum (FBS) were purchased from Gibco (Life technologies Korea, Seoul, Korea). 3-(4,5-dimethylhiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT), Bicinchoninic acid (BCA) solution, Fluorescein isothiocyanate (FITC)-conjugated secondary antibody, Sodium dodecyl sulfate (SDS), Dimethyl sulfoxide (DMSO) were obtained from Sigma-Aldrich (St, Louis, MO, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was obtained from Santa-Curz Biotechnology (Santa Cruz, CA, USA). E-cadherin and MMP-1 antibodies were obtained through Cell Signaling Technology (Danvers, MA, USA). Enhanced chemiluminescence (ECL) reagent was obtained from GE Healthcare BIO-Sciences (Piscataway, NJ, USA). Anti-human CD4 FITC antibodies, Anti-human CD25 antibodies, Staining buffers were purchased from eBioscience (eBioscience, INC, San Diego, CA). Treg sol was provided by IMMUNISBIO. Co. Ltd. (Incheon, Korea) CD8+ T cell isolation kit was purchased from MACS (Miltenyi Biotec, Auburn, CA, USA). KBM502 medium was purchased from KOHJIN Bio (Salcado city, Saitama, Japan). Human cytokine antibody array 5 was purchased from RayBiotech (Raybiotech, Norcross, GA, USA).
Culture of HaCaT Keratinocytes
HaCaT cells (ATCC12192), spontaneously immortalized human keratinocyte cell line, were incubated at 37°C and 5% CO2 in 10% FBS DMEM (v/v), 100 U/L Penicillin, 100 μg/mL Streptomycin were added. And then, the cells were incubated with 1% FBS DMEM for 24 hours before the cells were treated.
Preparation of Treg Cells Isolated from PBMCs and Conditioned Media
Treg cells were isolated from PBMCs. The depletion of CD8+ T cells was performed using CD8 Microbeads and the MS column with a good manufacturing practice procedure. The suspended cells in MACs buffer and CD8 microbeads were mixed in a 4:1 ratio to remove CD8+ cells. CD8+ depleted unstaining cells were obtained by MS column. The obtaining cells were incubated with Treg sol that was specially made for Treg differentiation, L-glutamine, and plasma in KBM502 medium at 37°C, 5% CO2, for 2 days with 2.0×107 cells/flask. The Treg sol was provided by IMMUNISBIO. Co. Ltd. After that, the subculture was performed every two or three days and also added the Treg sol at same time. After 14 days of incubation, we centrifuged cell suspension of cells at 500 × g and supernatant was obtained as Treg cells CM.
Flow Cytometry
For staining of Treg cells, cells were harvested and washed. And centrifuged at 350 × g for 5 min and resuspended in ice cold FACS buffer. Flow cytometry to confirm surface expression of CD4, CD8, CD25 molecules was performed after a 30 mins incubation at 4°C in the presence of saturating concentration of antibodies. After washing, Treg cells were fixed by intracellular fixation buffer for 30 mins at room temperature. Then cells were reacted with Permeabilizatioin buffer and Foxp3 antibody for 30 mins at room temperature. The washed and stained cells were analyzed using FACS analysis by BD AccuriTM C6 Plus.
Cytokine Array
The CM obtained from the Treg cells was tested according to RayBio® C-Series Human Cytokine Antibody Array C5 kit manual. Briefly, each cytokine array membrane was blocked for 30 mins at room temperature with a blocking buffer and then reacted overnight at 4°C with Biotinylated antibody cocktail. After washing, the reaction was overnight at 4°C using HRP-streptavidin solution. After washing the membrane, the reaction of mixture buffer was done for 2 mins at room temperature and detected using Davinch-chemidoc (Davinch-K, Seoul, Korea).
Cell Proliferation Assay
HaCaT cells were cultured at 10% FBS DMEM for 24 hours with 8.0×103 cells/well in a 96-well culture plate, followed by 1% FBS DMEM for 24 hours. Thereafter, HaCaT keratinocytes were treated with Treg cells CM of various concentrations in 1% FBS DMEM for 24 hours, and then the viability of the cells was determined by MTT assay. In brief, 10 μL of 5 mg/mL MTT solution in Phosphate-buffered saline was treated in each well and incubated at 37°C for 4 hours. The generated formazan was dissolved in 200 μL of DMSO and the absorbance was detected at 560 nm.
Scratch Assay of Cell Migration
To measure the migration of HaCaT keratinocytes treated with Treg cells CM, we used Scratch assay. The HaCaT keratinocytes were cultured 95%~100% confluence and treated with Treg cells CM for 24 hours before scratching the cells monolayer. Using a 200 μL plastic pipette tip, we created a straight scratch and washed it with PBS to remove the cells debris. Then they were incubated with fresh medium without Treg cells CM. After 24 hours, images were taken at the same location using an Olympus CKX41 microscope and an IMT cam 3 digital camera (Olympus Corp, Tokyo, Japan). The migration rate of the cells was measured using ImageJ software to determine the area reduction rate of the scratched area.
Western Blotting
Whole cells were lysised using a Radioimmunoprecipitation assay (RIPA) lysis buffer (150 mM NaCl, 1% Triton X-100, 0.5% Sodium deoxycholate, 0.1% SDS, 50 mM Tris-HCl, pH 8.0) containing a Protease/phosphatase inhibitor cocktail. After obtained proteins, concentration was measured using a BCA reagent. In brief, media or lysate proteins were separated into 10% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to Polyvinylidene fluoride (PVDF) membrane. Proteins transferred to membrane were treated with 5% Skim milk in Tris-buffered saline containing 0.1% Tween 20 for non-specific blocking. Blot was reacted with an appropriate concentration of primary antibody at 4°C for 12 hours. Then, Horseradish peroxidase-conjugated secondary antibody was reacted for 2 hours at room temperature and detected using ECL reagent. Bands were densimetrically quantified using ImageJ software.
Immunofluorescence Staining of E-Cadherin
HaCaT keratinocytes cells were cultured to be 95% to 100% confluence on glass coverslips at 6 well plates. After 24 hours of treatment with various concentrations of Treg cells CM, the cells monolayer was linearly scratched using a 200 μL plastic pipette tip. HaCaT keratinocytes were washed with PBS and incubated with fresh medium for 24 hours. Thereafter, incubation was performed with 1% Bovine serum albumin in PBS with 0.1% Tween 20 for 30 mins. The reaction was carried out at 4°C for 12 hours using an E-cadherin antibody, and then, FITC-conjugated secondary antibody for 2 hours at room temperature. FITC fluorescence images were obtained using a LEICA DMi8 fluorescence microscope (Leica microsystems, Wetzlar, Germany).
Statistical Analysis
Statistical analyses were performed using the Student’s t-test. A P-value (< 0.05) was considered significant for all tests. The results were expressed as the means and standard deviations. All experiments were performed in triplicate at least.
Results
Characteristics of Regulatory T Cells and Conditioned Media
Culture of cells isolated from CD8+ depleted PBMCs treated with Treg sol in the KBM502 medium allowed us to get a population of Treg cells. Treg cells were expressed CD4+, CD25+, Foxp3+ (Figure 1). The X chromosome-encoded forkhead transcription factor, Foxp3, was recently identified as a key player in Treg cells.42 The Foxp3 is the master regulator of regulatory pathways in the development and function of regulatory T cells.42,43 As shown in Figure 1, CD4+CD25+ Treg cells accounted for 94.6% of CD8+ depleted PBMCs, and 87.98% of isolated CD4+CD25+ Treg cells expressed Foxp3. This Foxp3 population is responsible for the actual regulation of immunity. However, in the cells not treated with Treg sol, the proportion of CD4+CD25+ cells were hardly differentiated to 1.6%, and the expression rate of Foxp3 was rarely expressed as 1.04%.
Regulatory T cells not only show a direct self-tolerance effect, but also secrete various substances such as cytokines, growth factors to affect other immune cells.44–46 Cytokines are a category of signaling molecules that regulate immunity, inflammation and hematopoiesis.47,48 The cytokine array was performed to identify cytokines contained in Treg cells CM. Our results show that the expression levels of IL-6, IL-8, IL-10, IL-13, VEGF, IP-10, MCP-1 and RANTES are significantly increased (Figure 2). IL-6, IL-8, IL-10, IL-13, IP-10, MCP-1 and RANTES are reported that increase cell migration, especially the migration of keratinocytes.39,49-51 Through Cytokine array, we demonstrated that IL-6 was increased by about 70 times, and IL-8, IL-10, IP-10, MCP-1 and RANTES were increased by about 40 times, over the basal.
Treg Cells CM Effects on the Cell Proliferation
We performed The MTT assay to determine the viability effect of Treg cells CM on HaCaT keratinocytes. The groups treated with Treg cells CM showed a slight decrease in cells proliferation compared to untreated control in dose-dependent manner. In 0.5% and 1% Treg cells CM treatment groups, there was no significant difference compared to the Control. The cells treated with Treg cells CM for 24 hours showed a slight reduction in cells proliferation, 91.7±8.5% at 5% Treg CM (p < 0.01), 91.3±5.7% at 10% Treg cells CM (p < 0.001) and 74.3±6.8% at 20% Treg cells CM (p < 0.001) (Figure 3).
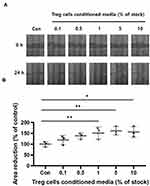
Treg Cells CM Stimulates the Cell Migration
We also performed a Scratch cell migration assay to determine the migration rate of HaCaT keratinocytes. The HaCaT keratinocytes were cultured 95%~100% confluence and treated with Treg cells CM for 24 hours before scratching the cells' monolayer. Cell migration were confirmed by measuring the reduction of scratch area (Figure 4A). HaCaT keratinocytes treated with Treg cells CM increased reduction of scratch area compared to untreated control groups. Figure 4B showed that pretreatment of Treg cells CM increased cell migration (Figure 4B). Taken together, these results suggest that treatment within 5% of Treg cells CM significantly increases cell migration without affecting the proliferation of HaCaT cells.
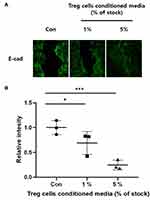
Treg Cells CM Stimulates EMT of HaCaT Keratinocyte
During the EMT, epithelial cells undergo cytoskeleton rearrangement, weaken cell-to-cell junctions, apical-basal polarity, and ECM binding, and gain motility, one of the mesenchymal features.9,11,52,53 In general, studies related to EMT confirm the expression of epithelial markers such as E-cadherin.9–11 We confirmed the expression of the marker protein of EMT to see the effect of Treg cells CM on HaCaT keratinocytes. Figure 5 is an Immunofluorescence image of E-cadherin, an epithelial cells marker. HaCaT keratinocytes were treated with Treg cells CM for 24 hours and then cells monolayer was scratched. After culture for 24 hours, immunostaining was performed. There was a significant reduction in dose-dependent manner of E-cadherin in HaCaT keratinocytes pretreatment with Treg cells CM. These results show that treatment of Treg cells CM can stimulate migration of HaCaT keratinocytes by promoting EMT at the wound site.
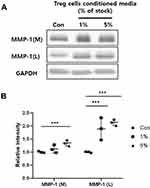
Treg Cells CM Reduces Expression of Matrix Metalloproteinase-1
MMPs play an important role in the wound healing by enabling cell migration and tissue remodeling through modifying the wound matrix.15–18 The MMP-1 cleaves Type I collagen to produce cleaved fragments and makes them low adhesive binding ligand. Thus, the ability of this MMP-1 loosens the binding with the ECM and causes cellular movement.19,20 The Western blot was performed to investigate the MMP-1 expression from HaCaT keratinocytes treated with Treg cells CM. Figure 6 shows that treatment of Treg cells CM for 24 hours significantly increases both expression and secretion of MMP-1 in dose-dependent. These results show that treatment of Treg cells CM activates ECM degradation and remodeling at the wound site.
Discussion
The wound healing process is very complex and finely controlled by the involvement of various factors.11–14 During the wound healing process, keratinocytes migrate to the wounding site and participate in re-epithelialization.7,8 EMT is essential for keratinocytes to have motility.9 EMT causes cells to have motility, decreases the expression of epithelial marker proteins and increases the expression of mesenchymal marker proteins.54 MMPs degrade ECM and perform tissue remodeling of the wound sites during healing.15–18,21,22
When the wound forms and an inflammatory reaction occurs, the immune cell contributes to the wound healing by secreting various Cytokines and growth factors.24,26,27 Treg cells play an important role in immune response, not only in the direct interaction by CTLA-4 but also in the cell migration, wound healing, and being involved in inflammatory reactions by secreting various cytokines.29–38 As shown in the cytokine array (Figure 2) which confirmed the cytokines contained in CM obtained by culturing Treg cells, it is shown that Treg cells CM contains a large amount of IL-6, IL-8, IL-10, etc. and these cytokines increase cell migration of keratinocytes.39,49,50 Therefore, it is thought that Treg cells CM can implicate the wound healing. However, there is little research on the effects of cell migration using Treg cells CM. Because cell migration is essential for the wound healing process, we identified cell migration, EMT marker protein, and MMP expression in Treg cells CM. As shown in our results, Treg cells CM increased cell migration without affecting cells proliferation (Figures 3 and 4). At 5% treatment of the Treg cells CM, there was a slight decrease without significant difference in viability of the cells, but the wound closure showed the maximum rate at that concentration. These results show that treatment within 5% of Treg cells CM can increase the migration of keratinocytes and have an effect on the wound healing without significantly affecting cells viability.
Since EMT is essential in cell migration, we confirmed the expression of E-cadherin, an epithelial marker protein, by Immunofluorescence. The E-cadherin is an adherent junction protein that functions to maintain cell-to-cell junction and epithelial phenotype.55 As shown in our experimental results, the intensity of E-cadherin in HaCaT cells treated with Treg cells CM decreased dose-dependently. This shows that keratinocytes from which EMT has been induced gain motility, and enable cell migration.
In the tissue remodeling stage, MMPs reduce the adhesion of keratinocytes and ECM, allowing the migration of keratinocytes and restructuring tissue.15,16 MMP-1 degrades collagen, which accounts for the majority of skin ECM, and plays an important role in cell signaling by being involved in the secretion of various growth factors and cytokines.16,19 Our results show that the treatment of Treg cells CM affects the expression and release of MMP-1. The treatment of Treg cells CM in keratinocytes increased the release of MMP-1 (Figure 6). The results show that the Treg cells CM can increase both expression and release of MMP-1 to affect cell migration and ECM degradation. However, our study does not include the mechanism by which Treg cells CM affects HaCaT keratinocytes. So we need to find out more about which signaling pathway, by which Treg cells CM influences keratinocytes.
Conclusion
Our results demonstrated that Treg cells CM have various cytokines and growth factors. And our results showed that Treg cells CM stimulates migration of HaCaT keratinocytes by EMT and up-regulation of MMP-1. These mean that Treg cells CM which have various cytokines and growth factors mixture stimulates cell migration and so can promote wound healing.
Acknowledgments
This work was supported by the IMMUNISBIO. Co. Ltd.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kolarsick PAJ, Kolarsick MA, Goodwin C. Anatomy and physiology of the skin. J Dermatol Nurses Assoc. 2011;3(4):203–213. doi:10.1097/JDN.0b013e3182274a98
2. Kanitakis J. Anatomy, histology and immunohistochemistry of normal human skin. Eur J Dermatol. 2001;12(4):390–399.
3. Rognoni E, Watt FM. Skin cell heterogeneity in development, wound healing, and cancer. Trends Cell Biol. 2018;28(9):709–722. doi:10.1016/j.tcb.2018.05.002
4. Chu DH. Overview of biology, development, and structure of skin. Fitzpatrick’s Dermatology in General Medicine. 2012;8(3).
5. Shaw TJ, Martin P. Wound repair at a glance. J Cell Sci. 2009;122(Pt 18):3209–3213. doi:10.1242/jcs.031187
6. Larouche J, Sheoran S, Maruyama K, Martino MM. Immune regulation of skin wound healing: mechanisms and novel therapeutic targets. Adv Wound Care. 2018;7(7):209–231. doi:10.1089/wound.2017.0761
7. Chen JD, Lapiere JC, Sauder DN, Peavey C, Woodley DT. Interleukin-1α stimulates keratinocyte migration through an epidermal growth factor/transforming growth factor-α-independent pathway. J Invest Dermatol. 1995;104(5):729–733. doi:10.1111/1523-1747.ep12606970
8. Safferling K, Sutterlin T, Westphal K, et al. Wound healing revised: a novel reepithelialization mechanism revealed by in vitro and in silico models. J Cell Biol. 2013;203(4):691–709. doi:10.1083/jcb.201212020
9. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi:10.1016/j.cell.2009.11.007
10. Kuwahara M, Hatoko M, Tada H, Tanaka A. E-cadherin expression in wound healing of mouse skin. J Cutan Pathol. 2001;28(4):191–199. doi:10.1034/j.1600-0560.2001.028004191.x
11. Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166(1):21–45. doi:10.1016/j.cell.2016.06.028
12. Cano A, Pérez-Moreno MA, Rodrigo I, et al. The transcription factor Snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi:10.1038/35000025
13. Yan C, Grimm WA, Garner WL, et al. Epithelial to mesenchymal transition in human skin wound healing is induced by tumor necrosis factor-α through bone morphogenic protein-2. Am J Pathol. 2010;176(5):2247–2258. doi:10.2353/ajpath.2010.090048
14. Terao M, Ishikawa A, Nakahara S, et al. Enhanced epithelial-mesenchymal transition-like phenotype in N-acetylglucosaminyltransferase V transgenic mouse skin promotes wound healing. J Biol Chem. 2011;286(32):28303–28311. doi:10.1074/jbc.M111.220376
15. Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91(4):439–442. doi:10.1016/S0092-8674(00)80429-8
16. Ravanti L, Kahari VM. Matrix metalloproteinases in wound repair (Review). Int J Mol Med. 2000;6(4):391–798.
17. Mäkelä M, Larjava H, Pirilä E, et al. Matrix metalloproteinase 2 (gelatinase a) is related to migration of keratinocytes. Exp Cell Res. 1999;251(1):67–78. doi:10.1006/excr.1999.4564
18. Caley MP, Martins VL, O’Toole EA. Metalloproteinases and wound healing. Adv Wound Care. 2015;4(4):225–234. doi:10.1089/wound.2014.0581
19. Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC. The activity of collagenase-1 is required for keratinocyte migration on a type i collagen matrix. J Cell Biol. 1997;137(6):1445–1457. doi:10.1083/jcb.137.6.1445
20. Saarialho-Kere UK, Kovacs SO, Pentland AP, Olerud JE, Welgus HG, Parks WC. Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J Clin Invest. 1993;92(6):2858–2866. doi:10.1172/JCI116906
21. Matrisian LM. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990;6(4):121–125. doi:10.1016/0168-9525(90)90126-Q
22. Woessner JF. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5(8):2145–2154. doi:10.1096/fasebj.5.8.1850705
23. Ellis S, Lin EJ, Tartar D. Immunology of wound healing. Curr Dermatol Rep. 2018;7(4):350–358. doi:10.1007/s13671-018-0234-9
24. Canedo-Dorantes L, Canedo-Ayala M. Skin acute wound healing: a comprehensive review. Int J Inflam. 2019;2019:3706315. doi:10.1155/2019/3706315
25. Tamagawa-Mineoka R. Important roles of platelets as immune cells in the skin. J Dermatol Sci. 2015;77(2):93–101. doi:10.1016/j.jdermsci.2014.10.003
26. Nami N, Feci L, Napoliello L, et al. Crosstalk between platelets and PBMC: new evidence in wound healing. Platelets. 2016;27(2):143–148. doi:10.3109/09537104.2015.1048216
27. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11(8):519–531. doi:10.1038/nri3024
28. Rankin LC, Artis D. Beyond host defense: emerging functions of the immune system in regulating complex tissue physiology. Cell. 2018;173(3):554–567. doi:10.1016/j.cell.2018.03.013
29. Sojka DK, Huang YH, Fowell DJ. Mechanisms of regulatory T-cell suppression - a diverse arsenal for a moving target. Immunology. 2008;124(1):13–22. doi:10.1111/j.1365-2567.2008.02813.x
30. Jain N, Nguyen H, Chambers C, Kang J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc Natl Acad Sci U S A. 2010;107(4):1524–1528. doi:10.1073/pnas.0910341107
31. Bettini M, Vignali DA. Regulatory T cells and inhibitory cytokines in autoimmunity. Curr Opin Immunol. 2009;21(6):612–618. doi:10.1016/j.coi.2009.09.011
32. Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–532. doi:10.1038/nri2343
33. Vignali D. How many mechanisms do regulatory T cells need? Eur J Immunol. 2008;38(4):908–911. doi:10.1002/eji.200738114
34. Odenthal J, Takes R, Friedl P. Plasticity of tumor cell invasion: governance by growth factors and cytokines. Carcinogenesis. 2016;37(12):1117–1128. doi:10.1093/carcin/bgw098
35. Sokol CL, Luster AD. The chemokine system in innate immunity. Cold Spring Harb Perspect Biol. 2015;7(5):a016303. doi:10.1101/cshperspect.a016303
36. Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naïve, central memory and effector memory CD4+ T cells. Pathol Biol. 2003;51(2):64–66. doi:10.1016/S0369-8114(03)00098-1
37. Dang T, Liou G-Y. Macrophage cytokines enhance cell proliferation of normal prostate epithelial cells through activation of ERK and Akt. Sci Rep. 2018;8(1):7718. doi:10.1038/s41598-018-26143-8
38. Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(2):27–37. doi:10.1097/AIA.0b013e318034194e
39. Jiang WG, Sanders AJ, Ruge F, Harding KG. Influence of interleukin-8 (IL-8) and IL-8 receptors on the migration of human keratinocytes, the role of PLC-γ and potential clinical implications. Exp Ther Med. 2012;3(2):231–236. doi:10.3892/etm.2011.402
40. Landen NX, Li D, Stahle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73(20):3861–3885.
41. Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127(3):514–525. doi:10.1038/sj.jid.5700701
42. Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi:10.1038/ni904
43. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi:10.1016/j.cell.2008.05.009
44. Chen Y, Kuchroo VK, Inobe J-I, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265(5176):1237–1240. doi:10.1126/science.7520605
45. Bridoux F, Badou A, Saoudi A, et al. Transforming growth factor β (TGF-β)-dependent inhibition of T helper cell 2 (Th2)-induced autoimmunity by self–major histocompatibility complex (MHC) class II–specific, regulatory CD4+ T cell lines. J Exp Med. 1997;185(10):1769–1775. doi:10.1084/jem.185.10.1769
46. Han H-S, Jun H-S, Utsugi T, Yoon J-W. Molecular role of TGF-β, secreted from a new type of CD4+suppressor T cell, NY4.2, in the prevention of autoimmune IDDM in NOD mice. J Autoimmun. 1997;10(3):299–307. doi:10.1006/jaut.1997.0137
47. Chen Z, Bozec A, Ramming A, Schett G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol. 2019;15(1):9–17. doi:10.1038/s41584-018-0109-2
48. Zhang CC, Lodish HF. Cytokines regulating hematopoietic stem cell function. Curr Opin Hematol. 2008;15(4):307–311. doi:10.1097/MOH.0b013e3283007db5
49. Gallucci RM, Sloan DK, Heck JM, Murray AR, O’Dell SJ. Interleukin 6 indirectly induces keratinocyte migration. J Invest Dermatol. 2004;122(3):764–772. doi:10.1111/j.0022-202X.2004.22323.x
50. Michel G, Kemeny L, Peter RU, et al. Interleukin-8 receptor-mediated chemotaxis of normal human epidermal cells. FEBS Lett. 1992;305(3):241–243.
51. Szabo I, Wetzel MA, Rogers TJ. Cell-density-regulated chemotactic responsiveness of keratinocytes in vitro. J Invest Dermatol. 2001;117(5):1083–1090.
52. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi:10.1038/nrm3758
53. Lim J, Thiery JP. Epithelial-mesenchymal transitions: insights from development. Development. 2012;139(19):3471–3486. doi:10.1242/dev.071209
54. Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119(6):1429–1437. doi:10.1172/JCI36183
55. Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988;102:639–655.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.