Back to Journals » International Medical Case Reports Journal » Volume 11
Recurrent pterygium treatment using mitomycin C, double amniotic membrane transplantation, and a large conjunctival flap
Authors Monden Y , Hotokezaka F, Yamakawa R
Received 6 September 2017
Accepted for publication 14 December 2017
Published 7 March 2018 Volume 2018:11 Pages 47—52
DOI https://doi.org/10.2147/IMCRJ.S150969
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yu Monden, Fumi Hotokezaka, Ryoji Yamakawa
Department of Ophthalmology, Kurume University School of Medicine, Fukuoka, Japan
Purpose: The aim of this study was to evaluate the clinical outcomes of surgery for recurrent pterygia using mitomycin C (MMC), double amniotic membrane transplantation (AMT), and a large conjunctival flap.
Patients and methods: This retrospective case series included 31 eyes in 31 patients with recurrent pterygia. All patients underwent pterygium excision, application of MMC, double AMT, and placement of a large conjunctival flap. Outcome measures were visual acuity, astigmatism, and recurrence. Recurrence was defined as the presence of fibrovascular proliferative tissue crossing the limbus.
Results: The patients’ mean age was 68.2 years. The mean follow-up period was 3.6 years. The mean preoperative and postoperative best-corrected visual acuities (logMAR conversion) were 0.23 and 0.13, respectively. There was a significant difference between the mean preoperative (−3.85 D) and postoperative (−2.22 D) astigmatism. The recurrence rate was 3.2% (1/31 cases).
Conclusion: Surgical pterygium excision with application of MMC, double AMT, and placement of a large conjunctival flap was an effective treatment for recurrent pterygia.
Keywords: pterygium, amnion, MMC
Introduction
Patients with recurrent pterygia suffer from blurred vision because of the astigmatism. Various surgical procedures have been proposed for treating recurrent pterygia, including conjunctival autografting (CAU),1,2 conjunctival–limbal autografting (CLAU),2,3 amniotic membrane transplantation (AMT),4,5 and application of mitomycin C (MMC).6
The recurrence rates associated with these procedures vary among studies (1.0%–31.2%).1–5 Several techniques combining these procedures have been reported, including CAU, CLAU, or AMT combined with MMC3,7–9 or CLAU plus AMT.10 The recurrence rates with these combined procedures are lower (0%–20%) than they are with a single procedure.1–6 Other authors have combined more than two procedures, such as CLAU or limbal allograft combined with MMC and AMT.11–13 Although the recurrence rates with more than two procedures combined are lower (0%–11.9%) than they are with combined procedures, each procedure has its limitations. For example, pannus formation and the appearance of a pseudopterygium at the donor site have been noted after CLAU,7 and limbal allografting requires a donor cornea.
We report the clinical outcomes after treating recurrent pterygium with MMC, double AMT, and placement of a large conjunctival flap. Our combined procedure is optimal pterygium surgery that maximizes each procedure. The combined procedure has proved acceptable as a first surgical option for recurrent pterygium.
Patients and methods
The Kurume University institutional review board approved this study. The board waived the requirement for patient consent to review their medical records because identifiable private information was not included and confidentiality of patient data was ensured in this study. A written informed consent was obtained from the patient in the case report to have the case details and accompanying images published.
The study group comprised 31 eyes in 31 patients (9 men and 22 women) with recurrent pterygia. Eyes with severe symblepharon causing diplopia in primary gaze were excluded from this study. The pterygium surgery was performed at Kurume University Hospital from January 2003 to July 2016. All pterygia were large, crossing a point midway between the limbus and the pupillary margin, and 17 pterygia (55%) encroached on the pupillary axis. Mild symblepharon formation was noted in nine eyes (29%). Two eyes had double-headed pterygia.
Amniotic membrane preparation
Placenta was obtained from healthy mothers undergoing cesarean section with their written informed consent. Amniotic membrane was peeled off from the chorion and washed several times with saline containing gentamicin (Gentacin; MSD K.K., Tokyo, Japan). The amniotic membrane was cut into pieces measuring about 4 by 4 cm and each piece was rinsed in 0.5 M dimethylsulfoxide (DMSO; Nacalai Tesuque, Inc., Kyoto, Japan) dissolved in PBS, and then in 1.0 and 1.5 M DMSO in PBS. The amniotic membrane was placed in a plastic container containing 1.5 M DMSO in PBS and preserved at −80°C. The protocol for amniotic membrane preparation was changed for the amniotic membrane used in surgery after November 2015 (seven eyes). In brief, the amniotic membrane was washed thoroughly with PBS containing gentamicin sulfate solution (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and cut into pieces measuring about 5 by 5 cm. The amniotic membrane was placed in a plastic container containing a mixture of 250 mL DMEM (Wako Pure Chemical Industries, Ltd.), 250 mL glycerol, and 50 µL gentamicin sulfate solution and preserved at −80°C.
Surgical procedure
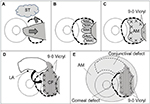
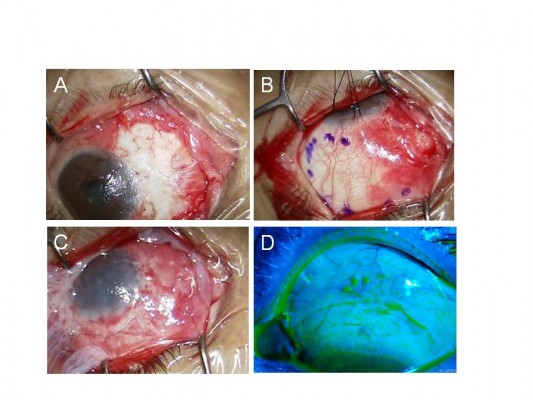
One surgeon (YM) performed all the surgical procedures. Written informed consent was obtained from each patient before the surgical procedure. All patients received topical anesthesia using 4% lidocaine eye drops (Xylocaine; AstraZeneca K.K., Osaka, Japan). Sub-Tenon’s anesthesia with 2% lidocaine was used when the patient experienced pain. Blunt dissection was used to remove the pterygium head from the cornea, the epithelium of the pterygium body from the subconjunctival fibrous tissue, and then the subconjunctival fibrous tissue from the sclera. Removal of the subconjunctival fibrous tissue was extensive (Figure 1A). Pieces of sponge soaked in 0.04% MMC were then applied to the adjacent subconjunctival fibrous tissue for 3 minutes (Figure 1B). After removing the sponge material, the areas were thoroughly irrigated with 200 mL of balanced salt solution. Patients were asked to look in the opposite direction to the pterygium excision site to fully expose the bare sclera. Amniotic membrane was placed over the bare sclera with the epithelial side up. The amniotic membrane was larger in size than the bare sclera. The amniotic membrane overlying the surrounding conjunctival edge was tucked subconjunctivally, and the amniotic membrane overlying the cornea was trimmed. The amniotic membrane was sutured to the sclera with interrupted 9-0 Vicryl sutures (Figure 1C). Patients were again asked to look in the opposite direction to the pterygium excision site to calculate the size of the defect covered by amniotic membrane and to determine the size of conjunctival flap. A large conjunctival epithelium (pedicle) flap from the superior area, excluding the limbal area (1–2 mm), was rotated, positioned, and secured on the amniotic membrane with 9-0 Vicryl sutures (Figure 1D). The conjunctival flap was large enough to cover the entire amniotic membrane. Another amniotic membrane was placed on the ocular surface with 10-0 nylon sutures with the epithelial side down. This second application of amniotic membrane was to cover the conjunctival defect, corneal defect, and 9-0 Vicryl sutures placed on the conjunctival flap (Figure 1E). A subconjunctival injection of dexamethasone (Rinderon, Shionogi & Co., Ltd., Osaka, Japan) and gentamicin (Gentacin, MSD K.K.) was given at the inferior temporal fornix, and a therapeutic contact lens was placed over the amniotic membrane.
The amniotic membrane on the ocular surface was removed 1 week after the surgery. The therapeutic contact lens was left on the cornea for about 1 month. Postoperatively, the patients were treated with topical dexamethasone 0.1% and antibiotics for more than a year, after which their use was tapered slowly until they were medication free.
Outcomes
Outcome measures were visual acuity, corneal astigmatism, and recurrence. Decimal visual acuities were converted to logMAR units for the analyses. The degree of corneal astigmatism was measured using an automated keratometer (KR8100PA; Topcon, Tokyo, Japan). Recurrence was defined as the presence of fibrovascular proliferative tissue crossing the limbus.
Results
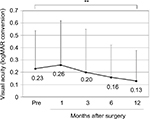
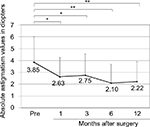
The mean (±SD) age of the patients was 68.2±11.3 years (range, 39–89 years). The mean number of prior pterygium excisions was 1.5±0.9 (range, 1–4). The mean postoperative follow-up duration was 3.6±2.9 years (range, 0.5–10.6 years). There was a significant difference between preoperative (0.23±0.31) and postoperative (0.13±0.25 after 12 months) best-corrected visual acuity (logMAR conversion; p<0.01, Wilcoxon signed-rank test; Figure 2). Preoperative astigmatism was measured in 23 eyes. Seven eyes had astigmatism that was so high that it could not be measured by the automated keratometer. Astigmatism measurement was not conducted in one eye. There was a significant difference between preoperative (−3.85±2.16 D) and postoperative (−2.63±1.62 D after 1 month, −2.75±1.78 D after 3 months, −2.10±1.58 D after 6 months, −2.22±1.70 D after 12 months) astigmatism (p<0.05, p<0.05, p<0.01, and p<0.01, respectively, Wilcoxon signed-rank test; Figure 3).
Recurrence was documented in one eye after 4 months, with a recurrence rate of 3.2% (1/31 eyes). The recurrent case was a 61-year-old woman who had undergone pterygium excision two times in the past. The recurrent pterygium developed 1 mm from the limbus, but did not progress for 10 years. There were no recurrences in eyes with double-headed pterygia. Symblepharon was alleviated in all patients. Corneal epithelialization was observed when the amniotic membrane on the ocular surface was removed 1 week after the surgery.
There were no complications associated with the donor conjunctival site. The conjunctiva in all patients had epithelialized within 11.8±9.7 days. A recurrent epithelial defect of the conjunctival flap was seen in one eye 6 months after surgery. Amniotic membrane was placed on the defect and the conjunctiva epithelialized.
Representative case report
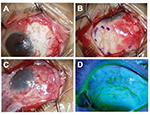
A 57-year-old woman underwent excision of a recurrent pterygium combined with application of MMC, double AMT, and a large conjunctival flap (Figure 4). Her best-corrected visual acuity (logMAR conversion) was 0.3 before surgery and 0.1 afterward. Astigmatism was −2.0 D before the surgery and −0.5 D after the surgery. So far, there has been no recurrence for 1.9 years (Figure 5).
  | Figure 5 Preoperative and postoperative appearances in the representative case. Notes: (A) Before the surgery. (B) At 1.9 years after the surgery. |
Discussion
A pterygium can induce a significant amount of astigmatism in proportion to its size.14 All pterygia in this study were large, with the mean preoperative astigmatism being −3.85 D in 23 eyes. Seven eyes had astigmatism that was so high that it could not be measured by the automated keratometer. Astigmatism measurement was not conducted in one eye.
The recurrence rate of recurrent pterygia varies, depending on the surgical procedure and duration of follow-up.13 Previous studies with a minimum follow-up of 6 months reported recurrence rates following a single procedure at 10.0% and 31.2% after CAU,1,2 1.0% and 14.6% after CLAU,2,3 9.5% and 12.5% after AMT,4,5 and 12.5% after MMC.6 The recurrence rates after combined procedures were 12.5% and 13.3% after CAU/MMC,3,7,8 zero and 2.1% after CLAU/MMC,7,9 8%–20% after AMT/MMC,5,8–10 and zero after CLAU/AMT.10 The recurrence rates after combining more than two procedures were zero after CLAU/MMC/AMT11 and zero and 11.9% after limbal allograft/MMC/AMT.12,13 The recurrence rate was 3.2% after our procedure.
Limbal stem cells may play an important role in the pathogenesis of pterygia as recurrence rates after procedures involving limbal stem cells were found to be low (0%–14.6%).2,3,7,9–11 Procedures with low recurrence rates have limitations, however. For example, localized pannus formation and the appearance of a pseudopterygium were reported to develop at the donor site after CLAU.7 In addition, limbal stem cell deficiency can occur at the donor site of CLAU, and a limbal allograft requires a donor cornea. CLAU or limbal allografting should be performed as a last resort for multi-recurrent pterygia. Our surgical procedure – pterygium excision combined with application of MMC, double AMT, and a large conjunctival flap – had no complications associated with the donor conjunctival site, and the procedure does not require a donor cornea.
Amniotic membrane has antifibrotic and anti-inflammatory effects.15,16 We used two pieces of amniotic membrane. After excising the recurrent pterygium, one piece of amniotic membrane is placed on the bare sclera and under the large conjunctival flap. Because the conjunctival flap in our procedure involves only epithelium, the amniotic membrane may also substitute for Tenon’s capsule under the flap. When we place the first amniotic membrane, we ask patients to look in the opposite direction to the pterygium excision site, necessitating a large amniotic membrane. The other piece of amniotic membrane is placed on the ocular surface. This second application facilitates epithelialization at the donor conjunctival site and the area of the corneal defect after pterygium excision. The amniotic membrane also may reduce the pain caused by the conjunctival sutures. In most previous reports,5,8–10 one piece of amniotic membrane was placed on the bare sclera, and in another report,4 two layers of amniotic membrane were placed on the bare sclera and the muscle. We employed double AMT for recurrent pterygium to maximize the effects of amniotic membrane. It is a novelty in that we employ the amniotic membrane graft to have antifibrotic and anti-inflammatory effects, a large conjunctival flap to completely cover the defect, and the amniotic membrane patch to facilitate epithelialization.
To completely cover the large amniotic membrane placed on the defect area, a large conjunctival flap was required in this study, and it was not necessary to wait for epithelialization. The large conjunctival flap involves only epithelium, with Tenon’s capsule and limbal stem cells at the donor site in place. The donor site epithelializes promptly without scar formation because the amniotic membrane and the upper eyelid cover Tenon’s and stem cells.
MMC, an antibiotic–antineoplastic agent, selectively inhibits the synthesis of DNA and cellular RNA, preventing cellular division and duplication. Intraoperative application of MMC (0.02%–0.025%) for 1–5 minutes has been used successfully to prevent recurrence.3,5–13 Intraoperatively applied MMC is associated with a severe complication, necrotizing scleritis. The scleritis has been treated successfully with AMT and systemic steroids.17 Our surgical procedure also used MMC. An epithelial defect was seen in one eye after the surgery, but necrotizing scleritis did not appear during the follow-up. The combined use of AMT and a topical steroid might have prevented the development of necrotizing scleritis. The safety of intraoperative use of MMC is still open to debate, however, and warrants further study.3
We report the clinical outcomes of recurrent pterygium surgery using MMC, double AMT, and a large conjunctival flap. This combined procedure was effective in the treatment of recurrent pterygium.
Disclosure
The authors report no conflicts of interest in this work.
References
Ti SE, Chee SP, Dear KB, Tan DT. Analysis of variation in success rates in conjunctival autografting for primary and recurrent pterygium. Br J Ophthalmol. 2000;84(4):385–389. | ||
Al Fayez MF. Limbal-conjunctival vs conjunctival autograft transplant for recurrent pterygia: a prospective randomized controlled trial. JAMA Ophthalmol. 2013;131(1):11–16. | ||
Mutlu FM, Sobaci G, Tatar T, Yildirim E. A comparative study of recurrent pterygium surgery: limbal conjunctival autograft transplantation versus mitomycin C with conjunctival flap. Ophthalmology. 1999;106(4):817–821. | ||
Solomon A, Pires RT, Tseng SC. Amniotic membrane transplantation after extensive removal of primary and recurrent pterygia. Ophthalmology. 2001;108(3):449–460. | ||
Ma DH, See LC, Hwang YS, Wang SF. Comparison of amniotic membrane graft alone or combined with intraoperative mitomycin C to prevent recurrence after excision of recurrent pterygia. Cornea. 2005;24(2):141–150. | ||
Mastropasqua L, Carpineto P, Ciancaglini M, Enrico Gallenga P. Long term results of intraoperative mitomycin C in the treatment of recurrent pterygium. Br J Ophthalmol. 1996;80(4):288–291. | ||
Kheirkhah A, Hashemi H, Adelpour M, Nikdel M, Rajabi MB, Behrouz MJ. Randomized trial of pterygium surgery with mitomycin C application using conjunctival autograft versus conjunctival-limbal autograft. Ophthalmology. 2012;119(2):227–232. | ||
Katırcıoglu YA, Altiparmak U, Engur Goktas S, Cakir B, Singar E, Ornek F. Comparison of two techniques for the treatment of recurrent pterygium: amniotic membrane vs conjunctival autograft combined with mitomycin C. Semin Ophthalmol. 2015;30(5–6):321–327. | ||
Chen R, Huang G, Liu S, Ma W, Yin X, Zhou S. Limbal conjunctival versus amniotic membrane in the intraoperative application of mitomycin C for recurrent pterygium: a randomized controlled trial. Graefes Arch Clin Exp Ophthalmol. 2017;255(2):375–385. | ||
Fallah MR, Golabdar MR, Amozadeh J, Zare MA, Moghimi S, Fakhraee G. Transplantation of conjunctival limbal autograft and amniotic membrane vs mitomycin C and amniotic membrane in treatment of recurrent pterygium. Eye (Lond). 2008;22(3):420–424. | ||
Yao YF, Qiu WY, Zhang YM, Tseng SC. Mitomycin C, amniotic membrane transplantation and limbal conjunctival autograft for treating multirecurrent pterygia with symblepharon and motility restriction. Graefes Arch Clin Exp Ophthalmol. 2006;244(2):232–236. | ||
Miyai T, Hara R, Nejima R, Miyata K, Yonemura T, Amano S. Limbal allograft, amniotic membrane transplantation, and intraoperative mitomycin C for recurrent pterygium. Ophthalmology. 2005; 112(7):1263–1267. | ||
Ono T, Mori Y, Nejima R, Tokunaga T, Miyata K, Amano S. Long-term follow-up of transplantation of preserved limbal allograft and amniotic membrane for recurrent pterygium. Graefes Arch Clin Exp Ophthalmol. 2016;254(12):2425–2430. | ||
Mohammad-Salih PA, Sharif AF. Analysis of pterygium size and induced corneal astigmatism. Cornea. 2008;27(4):434–438. | ||
Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999;179(3):325–335. | ||
Solomon A, Rosenblatt M, Monroy D, Ji Z, Pflugfelder SC, Tseng SC. Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br J Ophthalmol. 2001;85(4):444–449. | ||
Karalezli A, Kucukerdonmez C, Borazan M, Akova YA. Successful treatment of necrotizing scleritis after conjunctival autografting for pterygium with amniotic membrane transplantation. Orbit. 2010;29(2):88–90. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.