Back to Journals » Research and Reports in Neonatology » Volume 4
Recent advances in the management of neonatal jaundice
Authors Watchko J
Received 9 September 2014
Accepted for publication 30 September 2014
Published 17 November 2014 Volume 2014:4 Pages 183—193
DOI https://doi.org/10.2147/RRN.S52373
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Robert Schelonka
Jon F Watchko
Division of Newborn Medicine, Department of Pediatrics, University of Pittsburgh School of Medicine, Magee-Womens Research Institute, Pittsburgh, PA, USA
Abstract: Advances in the clinical assessment strategies used to identify neonates at risk for the development of severe hyperbilirubinemia and bilirubin neurotoxicity, as well as the treatment measures to control hyperbilirubinemia in newborns, continue to be made. They include, among others, universal predischarge birth hospitalization bilirubin screening, the confirmation that hemolysis is an important risk factor for bilirubin neurotoxicity, the use of a numeric scoring system to help stage the severity of acute bilirubin encephalopathy, the potential advantages of turquoise-light phototherapy, and the potential role of heme-oxygenase inhibitors in preventing the need for exchange transfusions, all of which are reviewed here.
Keywords: phototherapy, exchange transfusion, bilirubin, free bilirubin, bilirubin encephalopathy, kernicterus
Introduction
Neonatal hyperbilirubinemia is the most common clinical condition in the newborn requiring evaluation and management and remains a frequent reason for hospital readmission during the first week of postnatal life.1,2 The high prevalence of neonatal hyperbilirubinemia reflects developmental red blood cell, hepatic, and gastrointestinal immaturities that result in an imbalance favoring bilirubin production over hepatic–enteric bilirubin clearance.3,4 For most neonates, hyperbilirubinemia is a benign postnatal transitional phenomenon of no overt clinical effect. A subset of infants, however, will develop more significant hyperbilirubinemia. The estimated occurrence of hyperbilirubinemia based on peak total serum bilirubin (TSB) severity has been reported as: more than 17 mg/dL (291 μmol/L), defined as significant, at ~1 in 10; more than 20 mg/dL (342 μmol/L), defined as severe, at ~1:70; more than 25 mg/dL (428 μmol/L), defined as extreme, at ~1:700; and more than 30 mg/dL (513 μmol/L), defined as hazardous, at ~1:10,000 live births.5,6
Marked hyperbilirubinemia can lead to acute bilirubin encephalopathy (ABE) and evolve into chronic bilirubin encephalopathy (CBE), a devastating, permanently disabling neurologic disorder7–10 synonymous with kernicterus.11 The central nervous system sequelae of kernicterus reflect both a predilection of bilirubin toxicity for neurons (relative to glial cells) and the regional topography of bilirubin-induced neuronal damage characterized by prominent involvement of the globus pallidus and subthalamic nuclei, the eighth cranial nerve and cochlear nuclei, the dorsal midbrain periaqueductal grey, and the dentate nuclei of the cerebellum.7,10,12,13 CBE is classically characterized by dystonia, athetosis, auditory neuropathy spectrum disorder, paresis of vertical gaze, and dental enamel dysplasia.7,10 The prevention of kernicterus remains a serious clinical concern for neonatal caregivers worldwide.9,14,15
Despite the implementation of evaluation and treatment guidelines for neonatal hyperbilirubinemia by the American Academy of Pediatrics (AAP)11,16 and others to “reduce the incidence of severe hyperbilirubinemia and bilirubin encephalopathy,” cases of kernicterus are still occurring in the United States, Canada, Europe, and elsewhere.17 In developed countries, population-based estimates for kernicterus in term infants range from 1 in 30,000 to 1 in 200,000 live births.17
The current review focuses on recent advances in our approach to risk assessment for the development of severe hyperbilirubinemia and bilirubin neurotoxicity, as well as treatment measures to control hyperbilirubinemia in newborns. The crux of hyperbilirubinemia and bilirubin-induced neurotoxicity risk evaluation remains the measurement of total serum bilirubin interpreted in an hour-specific fashion,17,18 and the mainstays of intervention are phototherapy and exchange transfusion.19,20 These are detailed, along with other evaluation strategies, clinical tools, and adjunctive therapies, including several currently under study that hold promise for potential future application to the management of neonatal jaundice.
Universal predischarge birth hospitalization bilirubin screening
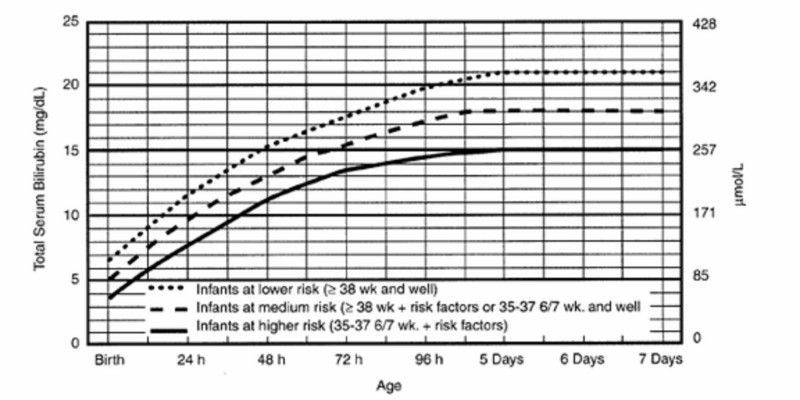
Experience with implementation of the 2004 AAP clinical practice guideline on the management of hyperbilirubinemia in the newborn at 35 or more weeks’ gestation coupled with several subsequent clinical studies led to a 2009 update with clarifications.16 The update was notable for recommending universal predischarge birth hospitalization bilirubin screening using TSB or transcutaneous (TcB) measurements to help assess the risk for subsequent severe hyperbilirubinemia.16 The predischarge bilirubin measurement combined with the gestational age of the infant make up a particularly robust predictor of subsequent severe hyperbilirubinemia16,21–23 that is consistent with the importance of immaturity as a risk factor for jaundice (Figure 1).16,22 Studies consistently demonstrate that implementation of routine predischarge bilirubin screening is associated with significantly reduced numbers of infants with extreme or hazardous hyperbilirubinemia,24–26 most recently showing a decrease from 11.5 cases of hazardous bilirubin (TSB ≥30 mg/dL) per 100,000 live births before universal bilirubin screening to 4.3 per 100,000 after implementation of universal bilirubin screening in Kaiser Permanente Northern California facilities.26 TSB and TcB measurement interpreted in an hour-specific fashion is an integral part of currently applied clinical guidelines in the management of the jaundiced neonate.11,16
  | Figure 1 Gestational age impacts risk for severe hyperbilirubinemia. |
Some brief comments on TcB are warranted. Strictly speaking, TcB is a measure of the yellow color of blanched skin and subcutaneous tissue, not the serum, and only an indirect proxy of TSB.16 As such, TcB is a screening tool to help determine whether a TSB should be measured.16 TcB accuracy is best at lower TSB levels, and in general, the TcB tends to underestimate TSB, particularly at higher TSB concentrations.16,27 Thus, it is important to adopt approaches to avoid missing a high TSB when using TcB to screen for hyperbilirubinemia; for example, obtaining a TSB whenever the TcB reading is higher than 13 mg/dL.16
Neurotoxicity risk factors
The major determinants of bilirubin neurotoxicity currently in clinical use are the TSB, the gestational age of the neonate, and the presence of neurotoxicity risk factors. The latter are clinical conditions that might increase the risk for brain damage in an infant with severe hyperbilirubinemia and, as outlined in the 2004 AAP clinical practice guideline, include isoimmune hemolytic disease, glucose-6-phosphate dehydrogenase (G6PD) deficiency, asphyxia, sepsis, acidosis, and albumin levels lower than 3.0 g/dL.11 Each condition is used along with the hour-specific TSB and gestational age in the calculus to determine phototherapy (Figure 2) and exchange transfusion treatment thresholds for newborns.11,16 As highlighted in the 2009 update and the 2004 AAP guideline, these interventions are recommended at lower TSB when any of the neurotoxicity risk factors are present.11,16
  | Figure 2 American academy of pediatrics phototherapy guidelines. |
TSB and neurotoxicity risk
TSB is the measure of albumin-bound bilirubin, whereas the small circulating fraction not bound to albumin or other serum proteins is indexed by the unbound or “free” (Bf) bilirubin level.9,28,29 The latter is in dynamic equilibrium with the extravascular tissues, including the central nervous system, and provides a measure of the relative amount of bilirubin that will exit the vascular space at a given level of TSB.9,28,29 TSB alone is of limited value in predicting neurologic impairment and kernicterus in hyperbilirubinemic newborns,9,28–32 a conclusion reaffirmed in recent clinical studies.33,34 Nevertheless, it is clear that marked elevations in TSB are associated with kernicterus risk,6,31,33,35,36 and TSB remains for all intents and purposes the only biochemical measurement of bilirubin widely available to clinicians.
There continues to be a keen interest in Bf, the bilirubin-albumin (B/A) ratio as a proxy of Bf, and the Bf/TSB ratio as potential more reliable indices of neurotoxicity risk than the TSB.9,28,29 At this time, however, no commercial instrument is available to measure Bf, although a newly developed fluorescence sensor for quantifying Bf in plasma has been detailed37 and applied in research reports.38,39 This novel methodology is less complex than the previously studied horseradish peroxidase method, requires only a small sample volume, and therefore holds promise for clinical application in the future.37–39 In the interim, the most frequently used proxy for Bf is the ratio of TSB (mg/dL) to serum albumin (g/dL), an index that correlates with measured circulating Bf.40 Use of the B/A ratio has been endorsed by the AAP in determining the need for exchange transfusion, albeit in conjunction with, but not in lieu of, the TSB.11
Evidence from the recent prospective randomized multicenter Bilirubin Albumin Ratio Trial (BART) in The Netherlands, however, showed that the neurodevelopmental outcome of preterm infants treated according to the B/A ratio or TSB (whichever was exceeded first) was not superior to those treated according to the TSB threshold alone.41 Although this study may have been underpowered (only 30% of infants in the experimental group were treated on the basis of the B/A ratio, as opposed to TSB), it is also possible that B/A ratios selected for study lacked sensitivity and that a lower B/A ratio than those used for intervention in the BART might better index neurotoxicity risk. Indeed, preliminary data from Japan suggest that lower B/A ratios may be more sensitive to unbound bilirubin levels in the putative neurotoxic range, and to bilirubin-induced abnormalities on brainstem auditory evoked responses, particularly in preterm neonates.42 For example, in infants of less than 30 weeks’ gestational age, a B/A molar ratio of 0.40 (equivalent to B/A of 3.4 mg/g) was predictive of a Bf of more than 1 μg/dL and abnormal brainstem auditory evoked responses, with 100% sensitivity and 85% specificity.42 In contrast to these, Iskander et al were unable to show improved sensitivity in predicting ABE, CBE, and hearing loss risk using B/A as compared with TSB in a large cohort of infants with TSB levels higher than 25 mg/dL in Cairo.35 In fact, only a B/A molar ratio of 1:1 or greater (B/A ratio of ≥8.6 mg/g) was revelatory of ABE risk.35 The nature of the differences across these studies merit clarification to determine the potential utility of B/A as a proxy of Bf and a predictor of neurotoxicity risk.
Should Bf measurements become clinically available, it will be important to remember that Bf and TSB are not competing independent determinants of bilirubin toxicity but, rather, critically interrelated and interdependent factors in estimating risk, a point highlighted by Ahlfors et al in several publications.28,29,40,43 TSB is needed to gauge the size of the bilirubin load, and Bf its distribution.29 Ahlfors et al therefore suggest that the Bf-to-TSB ratio may be the best risk predictor of all (Figure 3).29,43 It will be of interest to study this possibility in a larger cohort once Bf measurements are available in the clinical arena.
  | Figure 3 Bf/TBC ratio improves prediction of bilirubin-induced brainstem response abnormalities. |
An alternative or complementary approach to Bf and the Bf/TSB ratio in predicting bilirubin-induced neurotoxicity is the bilirubin binding capacity (BBC), using hematofluorometry.44 The latter index provides a measure of the neonate’s capacity to handle a bilirubin load.44 BBC encompasses both the albumin level and the nuances of albumin–bilirubin binding, which can be affected by several clinical conditions, including gestational age.44–46 Interest in the BBC has been rekindled by the development of a point-of-care hematofluorometric device now being trialed in the clinical arena.46
Collectively, these observations show that much work remains to be done in effectively applying bilirubin measurements (TSB, Bf, Bf/TSB, BBC) to identify infants at neurotoxicity risk. In theory, we should be able to do better than TSB alone, but in clinical reality, that is not yet the case. Finally, although Bf has biologic effects in the brain, the Bf level alone is not likely to dictate the risk for bilirubin encephalopathy. Bilirubin-induced neurotoxicity depends on a complex interaction between the level and duration of CNS Bf exposure and the innate cellular characteristics of the developing CNS that may predispose or protect against bilirubin-induced neuronal injury.9,47
Hemolysis and neurotoxicity risk
The clinical impression that hemolysis potentiates bilirubin neurotoxicity in neonates is long-standing and dates back to the early work on Rh isoimmunization and the first reports correlating increasing TSB and the risk for bilirubin encephalopathy.48 Hemolytic conditions remain prevalent contributors to both hyperbilirubinemia and bilirubin neurotoxicity risk.4,49 They encompass immune-mediated hemolytic disorders (Rhesus, non-Rhesus, and ABO hemolytic disease), red cell enzyme defects (G6PD, pyruvate kinase deficiencies), and red cell membrane abnormalities (hereditary spherocytosis, elliptocytosis, and others), as well as some unstable hemoglobins.3,4,49 Although the mechanism or mechanisms underlying the neurotoxicity-intensifying effect is unclear, recent data provide strong corroboration that hemolysis augments bilirubin neurotoxicity in neonates, with Rh hemolytic disease and G6PD deficiency playing particularly prominent etiologic roles.26,33,35 More specifically, Gamaleldin et al showed that the TSB threshold for identifying 90% of babies with bilirubin encephalopathy was 25.4 mg/dL (434 μmol/L) in infants with neurotoxicity risk factors (primarily hemolytic disorders) in contrast to 31.5 mg/dL (539 μmol/L) in those without.33 The presence of Rh hemolytic disease alone greatly increased the risk for bilirubin encephalopathy (odds ratio, 48.6; 95% confidence interval, 14–168).33
Of concern, it is increasingly apparent that the diagnosis of hemolysis in neonates remains problematic and, as a result, underrecognized. Several reports demonstrate that the etiology of extreme or hazardous hyperbilirubinemia is often unclear and not identified,26,36,50 when almost assuredly a hemolytic process is an important contributor to their genesis in many, if not most, cases.49,51,52 Indeed, Christensen et al recently reported that when an exhaustive search, including “next-generation” sequencing of a panel of gene variants involved in neonatal hyperbilirubinemia, was performed, a specific diagnosis was made in all infants with extreme hyperbilirubinemia (TSB >25 mg/dL) and was explained by an underlying hemolytic condition in each instance.52
These data suggest that clinical indices to detect accelerated red blood cell turnover may be useful adjuncts in identifying neonates with hemolysis. The approach that has received the most attention in this regard is the measurement of end-tidal carbon monoxide (ETCO) corrected for ambient CO or ETCOc.53–56 The catabolism of heme derived from red cell hemoglobin produces equimolar amounts of CO and bilirubin, providing the physiologic basis for ETCOc to index hemolysis.53,55,56 Previous studies have demonstrated that elevated levels of ETCOc correlate with blood carboxyhemoglobin concentrations.53,55,56 Recent studies have detailed measurements of ETCOc levels in neonates and are nicely summarized in a succinct review by Tidmarsh and colleagues.55 Collectively, they suggest an ETCOc cutoff of more than 2.5 parts per million to predict the presence of clinically significant hemolysis.55 Although no commercial ETCOc device is currently available, one is in development and undergoing preliminary testing.55 Such an instrument holds potential for point-of-care application in helping identify neonates with hemolysis once normative data are first established. One can envision the measurement of ETCOc as an adjunct in helping to identify infants at risk for subsequent severe hyperbilirubinemia and in further stratifying treatment criteria.55,56
Identifying neonates with intermediate to advanced ABE
ABE defines an encephalopathic state induced by hazardous hyperbilirubinemia during the first days of postnatal life and is characterized by a constellation of abnormal clinical signs typically progressive in their severity. In term (≥370/7 weeks’ gestation) and late preterm (340/7–366/7 weeks’ gestation) infants, the initial phase of ABE is characterized by stupor (lethargy), hypotonia, and poor sucking.7,10 These nonspecific signs are seen in numerous clinical contexts, but in a hyperbilirubinemic infant, they should raise the possibility of early ABE. Clinical signs of intermediate to advanced stages of ABE are increasingly more specific to bilirubin-induced neurotoxicity and herald a marked increased risk for permanent injury.7,10 These include hypertonia, often manifested by retrocollis, and opisthotonus, fever, and high-pitched cry.7,10 Inability to feed and apnea may ensue.36.57 There is growing evidence that bilirubin-induced neurotoxicity resulting in brainstem injury may manifest clinically as symptomatic apneic events and presage intermediate to advanced stages of ABE.57 Infants younger than 34 weeks’ gestation less frequently show these classic abnormal neuromotor signs.57–59 Recurrent apnea and desaturations may be the only clinical manifestations of ABE in preterm infants during the neonatal period, if any appear at all.57–59 In contrast, CBE defines the permanent clinical sequelae of bilirubin toxicity that become evident in the first year of life and is synonymous with kernicterus.7,10,11
Recently, a numeric scoring system for quantifying the degree of ABE has been outlined; it is detailed in Table 1.36,60 This scoring system of bilirubin-induced neurologic dysfunction may prove to be a useful clinical tool in identifying infants with intermediate to advanced ABE, conditions that pose significant risk for CBE and are an indication for the urgent application of bilirubin reduction strategies. Indeed, preliminary observations suggest this numeric approach may be quite reliable in characterizing the severity of ABE33,35 and may prove helpful in managing infants with hazardous hyperbilirubinemia. Notably, as detailed in the section on exchange transfusion, the AAP recommends immediate exchange transfusion in any infant who is jaundiced and manifests signs of intermediate to advanced stages of ABE (hypertonia, arching, retrocollis, opisthotonos, fever, high-pitched cry), even if the TSB is falling.11
  | Table 1 Clinical bilirubin-induced neurological dysfunction (BIND) score of onset, severity, and progression of acute bilirubin encephalopathy, as elicited by history and physical examination |
Phototherapy
Phototherapy is the mainstay of hyperbilirubinemia treatment in neonates across the gestational age spectrum. In widespread application since the 1970s, phototherapy has resulted in a marked reduction in the need to perform exchange transfusions to prevent hazardous hyperbilirubinemia and bilirubin encephalopathy.4,19,61 The effectiveness of phototherapy is determined by the irradiance, the surface area of exposure, and the light spectrum used.4,19,61
Irradiance is the radiant power, and the irradiance in a specific wavelength band is termed the spectral irradiance and is expressed as micro-Watts per centimeter squared per nanometer.4,19,61 There is a direct dose–response relationship between the efficacy of phototherapy and the irradiance used, and the level of irradiance is related to the distance between the light and the infant.4,19,61 In contrast to the early data from Tan,62 Vandborg et al recently demonstrated a linear relationship between irradiance and the decrease in the TSB with no evidence of a saturation point.63
The surface area of the infant exposed to phototherapy and the spectrum of light delivered are also key elements in determining the efficacy of phototherapy.4,19,61 The spectrum of light delivered by the phototherapy unit is determined by the type of light source and any filters used. Unconjugated bilirubin absorbs light most strongly in the blue region of the spectrum, near 460 nm, and the penetration of tissue by light increases with increasing wavelength.4,19,61 Only wavelengths that penetrate tissue and are absorbed by bilirubin have a phototherapeutic effect.4,19,61 Recently, Lamola et al detailed the important effect hematocrit has on the efficacy of phototherapy for neonatal jaundice.64,65 More specifically, they calculated that the wavelength at which the highest fraction of light is absorbed by bilirubin across a range of hematocrits peaked at 476 nm, not 460 nm.64 On the basis of this previously unrecognized hematocrit effect, they speculate that narrow-band light-emitting diode phototherapy at 476 nm would be 60% more effective than blue fluorescent lamps,64 a conclusion consistent with observations that blue-green (turquoise) light with emission peak at 490 nm and bandwidth of 65 nm is more effective than blue light.66,67 Others, however, have observed no difference between turquoise and blue light phototherapy effectiveness.68 Further study is necessary to clarify the potential advantage of turquoise phototherapy.
The benefit of timely phototherapy application in infants who show marked, potentially hazardous hyperbilirubinemia is clear and highlighted by the work of Mreihil et al, who report that configurational photoisomerization of bilirubin occurs almost instantaneously and is detectable in appreciable amounts in the blood of newborns within 15 minutes of initiating intensive phototherapy.69 These photoisomers can account for up to 20%–30% of the total unconjugated bilirubin and are less lipophilic than native bilirubin, and therefore are less likely to cross the blood–brain barrier.69 So the immediate detoxification of some bilirubin, even before it is excreted, is a possible additional benefit of phototherapy.4,19,61,69 Clearance of the structural photoisomer lumirubin is felt to be primarily responsible for the ultimate bilirubin-lowering effect of phototherapy, although the actual contributions of individual isomers remain uncertain.4,19,61 Photooxidation of bilirubin is a slow process and a minor contributor to the elimination of bilirubin during phototherapy.4,19,61
The last few years have witnessed the publication of several phototherapy treatment guidelines for premature infants of less than 35 weeks’ gestational age that complement those previously published for 35 weeks’ gestation and older newborns.11,58 As there are limited data for evidence-based recommendations in preterm neonates, these guidelines are, of necessity, consensus-based and provided by different experts, none of whom would make any claim for the greater validity of one approach over another.58,70
One subgroup of preterm neonates treated with phototherapy has recently been carefully studied, and this work merits specific comment. The Neonatal Research Network performed a prospective randomized controlled trial in extremely low birth weight (<1,000 g) infants of aggressive phototherapy (used prophylactically and started at 23±9 hours after birth) versus conservative phototherapy (started when the TSB level was ≥8 mg/dL [137 μmol/L] for infants 500–750 g, or ≥10 mg/dL [171 μmol/L] for infants 751–1,000 g).71 There was no difference in the primary outcome of death or neurodevelopmental impairment at 18–20 months of corrected age, but among survivors, when compared with conservative phototherapy, aggressive phototherapy produced a significant decrease in neurodevelopmental impairment, hearing loss, profound impairment, and athetosis.71
An unexpected finding in this study was a 5% increase in mortality in infants with birth weights 501–750 g who received aggressive phototherapy (relative risk, 1.05; 95% confidence interval, 0.90–1.22]).71–73 The difference was not statistically significant, but a post hoc, Bayesian analysis estimated an 89% probability that aggressive phototherapy increased the rate of deaths in this subgroup.71–73 In an earlier Eunice Kennedy Shriver National Institute of Child Health and Human Development study, infants with birth weights of 1,000 g or less who received phototherapy had a 19% increase in mortality compared with control infants (no phototherapy; P=0.14).58,74 The reasons for these findings are not clear, but these tiny, immature infants have gelatinous, thin skin, through which light will penetrate readily, reaching more deeply into the subcutaneous tissue.58 There is some evidence that phototherapy can produce oxidative injury to cell membranes, and such injury could have an adverse effect on these immature infants.58 In the Neonatal Research Network study, the average irradiance level was reported as 22–23 μw/cm2/nm, and the “target irradiance level” was 15–40 μw/cm2/nm.71
Because of the reported increase in mortality in infants with birth weights 501–750 g, it has been recommended that phototherapy be initiated at lower irradiance levels in extremely low birth weight neonates and that these levels are only increased, or the surface area of the infant exposed to phototherapy is increased, if the TSB continues to rise.58 One caveat to this approach is that the irradiance in the earlier Eunice Kennedy Shriver National Institute of Child Health and Human Development study from the 1970s was presumably low.73–75 As a result, intermittent or cycled, as opposed to continuous, phototherapy is being given consideration as a potential alternative treatment strategy in the extremely low birth weight neonate.73,75
Exchange transfusion
Exchange transfusion remains an important, if infrequently required, clinical intervention to prevent or reduce the risk for kernicterus.4,19,20 The prevention of Rh (D) hemolytic disease with Rh (D) immunoglobulin and the more effective use of intensive phototherapy have led to a dramatic decline in the number of exchange transfusions performed.4,19 Although there is little new to report on exchange transfusion per se, it is important to highlight a few clinical issues that remain relevant to its effective use.
To what hematocrit should the donor blood be reconstituted for exchange transfusion?
Given that bilirubin is bound to albumin in the vascular compartment, the efficacy of a double-volume exchange transfusion is a function of the plasma volume, and most important, the mass of albumin exchanged.76,77 It follows that donor blood of high plasma volume, that is, low hematocrit (~40%), to enhance the amount of bilirubin-free albumin introduced into the infant’s circulation during exchange is preferred.76–78 Surprisingly, this fundamental aspect of performing an exchange transfusion is not consistently highlighted in current reviews or textbook recommendations.
Exchange transfusion in intermediate to advanced stages of ABE
The 2004 AAP statement on hyperbilirubinemia specifies that “immediate exchange transfusion is recommended in any infant who is jaundiced and manifests signs of intermediate to advanced stages of acute bilirubin encephalopathy (hypertonia, arching, retrocollis, opisthotonos, fever, high-pitched cry) even if the TSB is falling.”11 This recommendation is consistent with reports79,80 that at least some infants so treated may escape unscathed without chronic bilirubin encephalopathy (kernicterus) and with earlier observations that a decline in TSB coincident with the clinical onset of intermediate to advanced signs of ABE can occur and is often a prognostic sign for poor outcome, as opposed to a sign of clinical improvement.81
Should emergency release uncross-matched blood be used in exchange transfusion for advanced ABE?
Given the urgency of an exchange transfusion in an infant with intermediate to advanced stages of ABE, it has been suggested that emergency release uncross-matched blood be used.82 This recommendation merits specific comment. First, emergency release blood is in the form of packed red blood cells with a hematocrit that approximates 70%–75%, and therefore could not be used immediately in an exchange transfusion because of polycythemia and hyperviscosity. The unit would need to be reconstituted in type AB fresh frozen plasma to an appropriate hematocrit (target, 35%–40%) to be both safe and effective. The time required to thaw the fresh frozen plasma and reconstitute the packed red blood cells would approximate 1.5–2.0 hours, which is more than sufficient time for the blood bank to identify and cross-match a compatible unit that could then be reconstituted with the thawed AB fresh frozen plasma. Emergency units are typically cytomegalovirus safe, leukoreduced, and stored in citrate-phosphate-dextrose less than 7 days old instead of adenosine, dextrose, saline, mannitol (Adsol; Baxter, Deerfield, IL, USA), and so would not require washing. Although blood for double-volume exchange would need to be irradiated, this could be accomplished in about 5 minutes with an irradiator on site. However, it is quite important to use mother’s serum or cord blood to perform a cross-match when red cell alloantibodies are the cause, or possible cause, of hazardous hyperbilirubinemia, as this ensures compatibility of the chosen unit. When the cause of hazardous hyperbilirubinemia and advanced bilirubin encephalopathy is known to be unrelated to red cell antibodies, then there is no significant advantage to using cross-matched blood, but there would be little to no time savings in using an uncross-matched unit, given these processing requirements. It is notable in this regard that in Hansen’s report on the reversibility of acute intermediate phase bilirubin encephalopathy,80 the interval between hospital admission and initiation of double-volume exchange transfusion was between 3.5 and 9 hours, with a mean of 6.5 hours (TWR Hansen, Rikshospitalet, Oslo, Norway, personal communication, January, 2013).
Pharmacologic treatment
Intravenous immunoglobulin
Early studies4,19 and a systematic review83 suggested that the administration of intravenous immunoglobulin (IVIG) to infants with Rh hemolytic disease would significantly reduce the need for exchange transfusion. Two recent randomized controlled trials, however, showed no benefit from the administration of IVIG to newborns with Rh hemolytic disease,84,85 and a Cochrane meta-analysis86 concluded that the efficacy of IVIG was not conclusive in Rh or ABO hemolytic disease of the newborn. The mechanism of action of IVIG is unknown, but it is possible that it might alter the course of immune-mediated hemolytic disease by blocking Fc receptors, thereby inhibiting hemolysis.4,19 The 2004 AAP guideline reserves the use of IVIG to direct Coombs-positive infants whose TSB continues to rise despite intensive phototherapy or whose TSB is within 2–3 mg/dL of the exchange level.11
Heme oxygenase inhibitors
The rate-limiting step in bilirubin production is the conversion of heme to biliverdin by heme oxygenase.4,19,87 Certain synthetic metalloporphyrins are powerful competitive inhibitors of heme oxygenase and suppress the formation of bilirubin.4,19,87 One such compound that has been studied extensively is tin mesoporphyrin (SnMP).4,19,87 A series of controlled clinical trials have demonstrated that SnMP is a potent inhibitor of heme oxygenase and is highly effective in reducing TSB levels and the requirements for phototherapy in term and preterm neonates.4,19 The only reported adverse effect has been a transient erythema that disappeared without sequelae in infants who received white light phototherapy after SnMP administration.4,19 This drug is still awaiting approval by the US Food and Drug Administration, although it can be obtained for compassionate use (InfaCare Pharmaceutical Corp, Trevose, PA, USA).4 If approved, SnMP could find immediate application in preventing the need for exchange transfusion in infants who are not responding to phototherapy.4,19 Other metalloporphyrin formulations are currently under study that hold the additional promise of being orally bioavailable and nonphotosensitizing.87,88 A concise current review of ongoing research efforts to optimize metalloporphyrin effectiveness and safety profile is provided by Schulz et al.87
Acknowledgment
The author thanks Darrell J Triulzi, medical director, Institute of Transfusion Medicine, University of Pittsburgh Medical Center, PA, USA, for his many insightful suggestions and comments.
Disclosure
The author reports no conflicts of interest in this work.
References
Maisels MJ, Kring E. Length of stay, jaundice, and hospital readmission. Pediatrics. 1998;101(6):995–998. | |
Escobar GJ, Greene JD, Hulac P, et al. Rehospitalisation after birth hospitalisation: patterns among infants of all gestations. Arch Dis Child. 2005;90(2):125–131. | |
Watchko JF. Indirect hyperbilirubinemia in the neonate. In: Maisels MJ, Watchko JF, editors. Neonatal Jaundice. Amsterdam, The Netherlands: Harwood Academic Publishers; 2000:51–66. | |
Maisels MJ. Jaundice. In: MacDonald MG, Seshia MMK, Mullett MD, editors. Neonatology: Pathophysiology and Management of the Newborn. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005:768–846. | |
Newman TB, Escobar GJ, Gonzales VM, Armstrong MA, Gardner MN, Folck BF. Frequency of neonatal bilirubin testing and hyperbilirubinemia in a large health maintenance organization. Pediatrics. 1999; 104(5 Pt 2):1198–1203. | |
Bhutani VK, Johnson LH, Jeffrey Maisels M, et al. Kernicterus: epidemiological strategies for its prevention through systems-based approaches. J Perinatol. 2004;24(10):650–662. | |
Shapiro SM. Kernicterus. In: Stevenson DK, Maisels MJ, Watchko JF, editors. Care of the Jaundiced Neonate. New York: McGraw-Hill; 2012:229–242. | |
Watchko JF. Kernicterus and the molecular mechanisms of bilirubin-induced CNS injury in newborns. Neuromolecular Med. 2006; 8(4):513–529. | |
Watchko JF, Tiribelli C. Bilirubin-induced neurologic damage – mechanisms and management approaches. N Engl J Med. 2013;369(21):2021–2030. | |
Volpe JJ. Bilirubin and brain injury. In: Volpe JJ, editor. Neurology of the Newborn. 5th ed. Philadelphia: Saunders/Elsevier; 2008:619–651. | |
American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114(1):297–316. | |
Ahdab-Barmada M, Moossy J. The neuropathology of kernicterus in the premature neonate: diagnostic problems. J Neuropathol Exp Neurol. 1984;43(1):45–56. | |
Ahdab Barmada M. The neuropathology of kernicterus: definitions and debate. In: Maisels MJ, Watchko JF, editors. Neonatal Jaundice. Amsterdam: Harwood Academic Publishers; 2000:75–88. | |
Bhutani VK, Zipursky A, Blencowe H, et al. Neonatal hyperbilirubinemia and Rhesus disease of the newborn: incidence and impairment estimates for 2010 at regional and global levels. Pediatr Res. 2013;74(Suppl 1):86–100. | |
Olusanya BO, Ogunlesi TA, Slusher TM. Why is kernicterus still a major cause of death and disability in low-income and middle-income countries? Arch Dis Child. 2014. Epub ahead of print. Available from: http://dx.doi.org/10.1136/archdischild-2013-305506. | |
Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF. Hyperbilirubinemia in the newborn infant ≥35 weeks’ gestation: an update with clarifications. Pediatrics. 2009;124(4):1193–1198. | |
Maisels MJ, Newman TB. Prevention, screening, and postnatal management of neonatal hyperbilirubinemia. In: Stevenson DK, Maisels MJ, Watchko JF, editors. Care of the Jaundiced Neonate. New York, NY: McGraw Hill; 2012:175–194. | |
Bhutani VK, Johnson L, Sivieri EM. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant hyperbilirubinemia in healthy term and near-term newborns. Pediatrics. 1999; 103(1):6–14. | |
Maisels MJ, Stevenson DK, Watchko JF, McDonagh AF. Phototherapy and other treatments. In: Stevenson DK, Maisels MJ, Watchko JF, editors. Care of the Jaundiced Neonate. New York, NY: McGraw Hill; 2012:195–227. | |
Watchko JF. Exchange transfusion in the management of neonatal hyperbilirubinemia. In: Maisels MJ, Watchko JF, editors. Neonatal Jaundice. Amsterdam, The Netherlands: Harwood Academic Publishers; 2000:169–176. | |
Keren R, Luan X, Friedman S, Saddlemire S, Cnaan A, Bhutani VK. A comparison of alternative risk-assessment strategies for predicting significant neonatal hyperbilirubinemia in term and near-term infants. Pediatrics. 2008;121(1):e170–e179. | |
Newman TB, Liljestrand P, Escobar GJ. Combining clinical risk factors with serum bilirubin levels to predict hyperbilirubinemia in newborns. Arch Pediatr Adolesc Med. 2005;159(2):113–119. | |
Maisels MJ, Deridder JM, Kring EA, Balasubramaniam M. Routine transcutaneous bilirubin measurements combined with clinical risk factors improve the prediction of subsequent hyperbilirubinemia. J Perinatol. 2009;29(9):612–617. | |
Eggert LD, Wiedmeier SE, Wilson J, Christensen RD. The effect of instituting a prehospital-discharge newborn bilirubin screening program in an 18-hospital health system. Pediatrics. 2006;117(5):e855–e862. | |
Mah MP, Clark SL, Akhigbe E, et al. Reduction of severe hyperbilirubinemia after institution of predischarge bilirubin screening. Pediatrics. 2010;125(5):e1143–e1148. | |
Kuzniewicz MW, Wickremasinghe AC, Wu YW, et al. Incidence, etiology, and outcomes of hazardous hyperbilirubinemia in newborns. Pediatrics. 2014;134(3):504–509. | |
Maisels MJ. Transcutaneous bilirubinometry. Neoreviews. 2006;7(5):e217–e225. | |
Ahlfors CE, Wennberg RP, Ostrow JD, Tiribelli C. Unbound (free) bilirubin: improving the paradigm for evaluating neonatal jaundice. Clin Chem. 2009;55(7):1288–1299. | |
Ahlfors CE. Predicting bilirubin neurotoxicity in jaundiced newborns. Curr Opin Pediatr. 2010;22(2):129–133. | |
Ip S, Chung M, Kulig J, et al; American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. An evidence-based review of important issues concerning neonatal hyperbilirubinemia. Pediatrics. 2004;114(1):e130–e153. | |
Newman TB, Liljestrand P, Jeremy RJ, et al; Jaundice and Infant Feeding Study Team. Outcomes among newborns with total serum bilirubin levels of 25 mg per deciliter or more. N Engl J Med. 2006;354(18):1889–1900. | |
Watchko JF. Neonatal hyperbilirubinemia – what are the risks? N Engl J Med. 2006;354(18):1947–1949. | |
Gamaleldin R, Iskander I, Seoud I, et al. Risk factors for neurotoxicity in newborns with severe neonatal hyperbilirubinemia. Pediatrics. 2011;128(4):e925–e931. | |
Oh W, Stevenson DK, Tyson JE, et al; NICHD Neonatal Research Network Bethesda MD. Influence of clinical status on the association between plasma total and unbound bilirubin and death or adverse neurodevelopmental outcomes in extremely low birth weight infants. Acta Paediatr. 2010;99(5):673–678. | |
Iskander I, El Houchi S, El Shenawy A, et al. Validation of bilirubin/albumin ratio as a predictor of bilirubin-induced neurologic dysfunction (BIND). Presented at: Pediatric Academic Societies Annual Meeting; May 7, 2013. Washington, DC. | |
Johnson L, Bhutani VK, Karp K, Sivieri EM, Shapiro SM. Clinical report from the pilot USA Kernicterus Registry (1992 to 2004). J Perinatol. 2009;29(Suppl 1):S25–S45. | |
Huber AH, Zhu B, Kwan T, Kampf JP, Hegyi T, Kleinfeld AM. Fluorescence sensor for the quantification of unbound bilirubin concentrations. Clin Chem. 2012;58(5):869–876. | |
Hegyi T, Kathiravan S, Stahl GE, Huber AH, Kleinfeld A. Unbound free fatty acids from preterm infants treated with intralipid decouples unbound from total bilirubin potentially making phototherapy ineffective. Neonatology. 2013;104(3):184–187. | |
Huber AH, Hegyi T, Kleinfeld AM. Free fatty acids (FFA) produced from intralipid and sulfisoxazole displace similar amounts of bilirubin from albumin. Presented at: Pediatric Academic Societies Annual Meeting; May 6, 2014; Vancouver, British Columbia. | |
Ahlfors CE. Criteria for exchange transfusion in jaundiced newborns. Pediatrics. 1994;93(3):488–494. | |
Hulzebos CV, Dijk PH, van Imhoff DE, et al; BARTrial Study Group. The bilirubin albumin ratio in the management of hyperbilirubinemia in preterm infants to improve neurodevelopmental outcome: a randomized controlled trial – BARTrial. PLoS ONE. 2014;9(6):e99466. | |
Nakamura H, Koda T, Yokota T, et al. Bilirubin/albumin molar ratios at the critical level of serum unbound bilirubin in hyperbilirubinemic neonates. Presented at: Pediatric Academic Societies Annual Meeting; May 7, 2014; Vancouver, British Columbia. | |
Ahlfors CE, Amin SB, Parker AE. Unbound bilirubin predicts abnormal automated auditory brainstem response in a diverse newborn population. J Perinatol. 2009;29(4):305–309. | |
Amin SB, Lamola AA. Newborn jaundice technologies: unbound bilirubin and bilirubin binding capacity in neonates. Semin Perinatol. 2011;35(3):134–140. | |
Bender GJ, Cashore WJ, Oh W. Ontogeny of bilirubin-binding capacity and the effect of clinical status in premature infants born at less than 1300 grams. Pediatrics. 2007;120(5):1067–1073. | |
Lamola AA, Du L, Bhutani VK. Albumin binding capacity for bilirubin increases with gestational age and validates recent expert recommendations for interventions. Presented at: Pediatric Academic Societies Annual Meeting; May 6, 2014; Vancouver, British Columbia. | |
Lasky RE, Church MW, Orlando MS, et al. The effects of aggressive vs conservative phototherapy on the brainstem auditory evoked responses of extremely-low-birth-weight infants. Pediatr Res. 2012;71(1):77–84. | |
Hsai DY, Allen FH, Gellis SS, Diamond LK. Erythroblastosis fetalis – studies of serum bilirubin in relation to kernicterus. N Engl J Med. 1952;247:668–671. | |
Kaplan M, Hammerman C. Hemolytic disorders and their management. In: Stevenson DK, Maisels MJ, Watchko JF, editors. Care of the Jaundiced Neonate. New York: McGraw-Hill; 2012:145–173. | |
Christensen RD, Lambert DK, Henry E, et al. Unexplained extreme hyperbilirubinemia among neonates in a multihospital healthcare system. Blood Cells Mol Dis. 2013;50(2):105–109. | |
Maisels MJ, Kring E. The contribution of hemolysis to early jaundice in normal newborns. Pediatrics. 2006;118(1):276–279. | |
Christensen RD, Nussenzveig RH, Yaish HM, Henry E, Eggert LD, Agarwal AM. Causes of hemolysis in neonates with extreme hyperbilirubinemia. J Perinatol. 2014;34(8):616–619. | |
Stevenson DK, Vreman HJ, Oh W, et al. Bilirubin production in healthy term infants as measured by carbon monoxide in breath. Clin Chem. 1994;40(10):1934–1939. | |
Herschel M, Karrison T, Wen M, Caldarelli L, Baron B. Evaluation of the direct antiglobulin (Coombs’) test for identifying newborns at risk for hemolysis as determined by end-tidal carbon monoxide concentration (ETCOc); and comparison of the Coombs’ test with ETCOc for detecting significant jaundice. J Perinatol. 2002;22(5):341–347. | |
Tidmarsh GF, Wong RJ, Stevenson DK. End-tidal carbon monoxide and hemolysis. J Perinatol. 2014;34(8):577–581. | |
Stevenson DK, Vreman HJ, Wong RJ. Bilirubin production and its measurement. In: Stevenson DK, Maisels MJ, Watchko JF, editors. Care of the Jaundiced Neonate. New York: McGraw-Hill; 2012:29–39. | |
Amin SB, Bhutani VK, Watchko JF. Apnea in acute bilirubin encephalopathy. Semin Perinatol. Epub September 16, 2014. | |
Maisels MJ, Watchko JF, Bhutani VK, Stevenson DK. An approach to the management of hyperbilirubinemia in the preterm infant less than 35 weeks of gestation. J Perinatol. 2012;32(9):660–664. | |
Amin SB. Clinical assessment of bilirubin-induced neurotoxicity in premature infants. Semin Perinatol. 2004;28(5):340–347. | |
Johnson L, Brown AK, Bhutani V. BIND – a clinical score for bilirubin-induced neurologic dysfunction in newborns. Pediatrics. 1999;104:746–747. | |
Maisels MJ, McDonagh AF. Phototherapy for neonatal jaundice. N Engl J Med. 2008;358(9):920–928. | |
Tan KL. The pattern of bilirubin response to phototherapy for neonatal hyperbilirubinaemia. Pediatr Res. 1982;16(8):670–674. | |
Vandborg PK, Hansen BM, Greisen G, Ebbesen F. Dose-response relationship of phototherapy for hyperbilirubinemia. Pediatrics. 2012;130(2):e352–e357. | |
Lamola AA, Bhutani VK, Wong RJ, Stevenson DK, McDonagh AF. The effect of hematocrit on the efficacy of phototherapy for neonatal jaundice. Pediatr Res. 2013;74(1):54–60. | |
Lamola AA, Russo M. Fluorescence excitation spectrum of bilirubin in blood: a model for the action spectrum for phototherapy of neonatal jaundice. Photochem Photobiol. 2014;90(2):294–296. | |
Ebbesen F, Madsen P, Støvring S, Hundborg H, Agati G. Therapeutic effect of turquoise versus blue light with equal irradiance in preterm infants with jaundice. Acta Paediatr. 2007;96(6):837–841. | |
Ebbesen F, Agati G, Pratesi R. Phototherapy with turquoise versus blue light. Arch Dis Child Fetal Neonatal Ed. 2003;88(5):F430–F431. | |
Seidman DS, Moise J, Ergaz Z, et al. A prospective randomized controlled study of phototherapy using blue and blue-green light-emitting devices, and conventional halogen-quartz phototherapy. J Perinatol. 2003;23(2):123–127. | |
Mreihil K, McDonagh AF, Nakstad B, Hansen TW. Early isomerization of bilirubin in phototherapy of neonatal jaundice. Pediatr Res. 2010;67(6):656–659. | |
Maisels MJ, Watchko JF. Treatment of jaundice in low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 2003;88(6):F459–F463. | |
Morris BH, Oh W, Tyson JE, et al; NICHD Neonatal Research Network. Aggressive vs conservative phototherapy for infants with extremely low birth weight. N Engl J Med. 2008;359(18):1885–1896. | |
Tyson JE, Pedroza C, Langer J, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Does aggressive phototherapy increase mortality while decreasing profound impairment among the smallest and sickest newborns? J Perinatol. 2012;32(9):677–684. | |
Arnold C, Pedroza C, Tyson JE. Phototherapy in ELBW newborns: does it work? Is it safe? The evidence from randomized clinical trials. Semin Perinatol. 2014;38(7):452–464. | |
Lipsitz PJ, Gartner LM, Bryla DA. Neonatal and infant mortality in relation to phototherapy. Pediatrics. 1985;75(2 Pt 2):422–426. | |
Hansen TWR. Let there be light-but should there be less? J Perinatol. 2012;32(9):649–651. | |
Valaes T. Bilirubin distribution and dynamics of bilirubin removal by exchange transfusion. Acta Paediatr. 1963;52(6):604–605. | |
Sproul A, Smith L. Bilirubin equilibration during exchange transfusion in hemolytic disease of the newborn. J Pediatr. 1964;65(1):12–26. | |
Watchko JF. 50 years ago in the Journal of Pediatrics: Bilirubin equilibration during exchange transfusion in hemolytic disease of the newborn. J Pediatr. 2014;165(1):64. | |
Harris MC, Bernbaum JC, Polin JR, Zimmerman R, Polin RA. Developmental follow-up of breastfed term and near-term infants with marked hyperbilirubinemia. Pediatrics. 2001;107(5):1075–1080. | |
Hansen TWR, Nietsch L, Norman E, et al. Reversibility of acute intermediate phase bilirubin encephalopathy. Acta Paediatr. 2009;98(10):1689–1694. | |
Ackerman BD, Dyer GY, Taylor PM. Decline in serum bilirubin concentration coincident with clinical onset of kernicterus. Pediatrics. 1971;48(4):647–650. | |
Sims ME. Legal briefs: Kernicterus still preventable. Neo Reviews. 2011;12(12):e727–e730. | |
Gottstein R, Cooke RWI. Systematic review of intravenous immunoglobulin in haemolytic disease of the newborn. Arch Dis Child Fetal Neonatal Ed. 2003;88(1):F6–F10. | |
Smits-Wintjens VEHJ, Walther FJ, Rath MEA, et al. Intravenous immunoglobulin in neonates with rhesus hemolytic disease: a randomized controlled trial. Pediatrics. 2011;127(4):680–686. | |
Santos MC, Sá C, Gomes SC Jr, Camacho LA, Moreira ME. The efficacy of the use of intravenous human immunoglobulin in Brazilian newborns with rhesus hemolytic disease: a randomized double-blind trial. Transfusion. 2013;53(4):777–782. | |
Alcock GS, Liley H. Immunoglobin infusion for isoimmune hemolytic jaundice in neonates. Cochrane Database Syst Rev. 2002;(3):CD003313. | |
Schulz S, Wong RJ, Vreman HJ, Stevenson DK. Metalloporphyrins – an update. Front Pharmacol. 2012;3:68. | |
Wong RJ, Espadas C, Inayathullah M, et al. Polymeric particulate delivery of zinc protoporphyrin for the chemoprevention of neonatal jaundice. Presented at: Pediatric Academic Societies Annual Meeting; May 7, 2014; Vancouver, British Columbia. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
