Back to Journals » Clinical Ophthalmology » Volume 10
Rebound macular edema following oral acetazolamide therapy for juvenile X-linked retinoschisis in an Italian family
Authors Galantuomo MS, Fossarello M , Cuccu A, Farci R, Preising MN, Lorenz B, Napoli PE
Received 8 June 2016
Accepted for publication 8 September 2016
Published 25 November 2016 Volume 2016:10 Pages 2377—2382
DOI https://doi.org/10.2147/OPTH.S114568
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Maria Silvana Galantuomo,1,* Maurizio Fossarello,1 Alberto Cuccu,1 Roberta Farci,1 Markus N Preising,2 Birgit Lorenz,2 Pietro Emanuele Napoli1,*
1Department of Surgical Sciences, Eye Clinic, University of Cagliari, Cagliari, Italy; 2Department of Ophthalmology, Faculty of Medicine, Justus-Liebig-University, Giessen, Germany
*These authors contributed equally to this work
Background: Juvenile X-linked retinoschisis (RS1, OMIM: 312700) is a hereditary vitreoretinal dystrophy characterized by bilateral foveal schisis and, in half of the patients, splitting through the nerve fiber layer in the peripheral retina. In the first decade of life, patients usually develop a decrease in visual acuity. Long-term visual outcomes can be poor due to the limited number of known successful treatments.
Purpose: The purposes of this study were to present, for the first time, a p.Arg197Cys missense mutation in the RS1 gene (OMIM: 300839) in a four-generation Italian family with RS1 and to examine the clinical response to the treatment with acetazolamide tablets alone or in combination with dorzolamide eye drops as assessed by spectral-domain optical coherence tomography (SD-OCT).
Methods: Eleven individuals, including two brothers with RS1 (patients 1 and 2), underwent a full medical history examination and a comprehensive ocular assessment that involved SD-OCT, fluorescein angiography, electroretinography and DNA analysis. Each RS1 patient received oral acetazolamide (375 mg daily) during the first three months. Thereafter, patient 1 continued only with dorzolamide eyedrops three times a day for a period of three months, while patient 2 spontaneously stopped both medications.
Results: Sequence analysis of the RS1 gene identified a hemizygous c.589C>T (p.Arg197Cys) missense mutation in exon 6, which has not been previously reported in an Italian family. A different response to the medical therapy was observed in the four eyes of the two affected brothers hemizygous for this abnormality. Of note, after acetazolamide interruption, a rebound effect on cystoid macular edema reduced the beneficial effects of the initial therapy for RS1 from p.Arg197Cys mutation. Indeed, a minimal rebound effect on cystoid macular edema, and an improvement in visual acuity, was observed in patient 1 during the six months of treatment. Conversely, in patient 2, an initial improvement in cystoid macular edema was not associated with visual acuity changes, followed by a marked rebound effect.
Conclusion: This study showed that the sequential use of acetazolamide tablets and dorzolamide eye drops should be considered and studied further as a possible treatment for macular edema and visual impairment in patients with RS1 from a hemizygous p.Arg197Cys mutation.
Keywords: juvenile X-linked retinoschisis, oral acetazolamide, topical dorzolamide, cystoid macular edema, macular schisis, foveal zone thickness
Introduction
Juvenile X-linked retinoschisis (RS1) is a hereditary vitreoretinal disorder that shows radiating cystoid changes in the macula with or without peripheral retinoschisis, early onset of visual loss, abnormal electroretinograms (ERGs) and, in some cases, vitreous hemorrhage and/or retinal detachment. RS1 affects predominantly male subjects. It is transmitted as an X-linked recessive trait and female carriers are usually asymptomatic. The penetrance of RS1 is almost complete but clinical expression is highly variable.1 As previously reported, the prevalence of RS1 is between 1:5,000 and 1:25,000.2,3 Visual impairment is usually mild until the fourth decade of life; thereafter, progressive visual deterioration often occurs.
The RS1 gene (OMIM: 300839) maps to Xp22.13, has six exons and encodes retinoschisin, a secreted 224 amino acid protein associated with the disease, abundantly expressed in both cone and rod photoreceptors and bipolar cells of the retina and the pineal gland.
The gene contains a highly conserved region in exons 4–6 known as the discoidin domain, shared with a number of other proteins.4 The discoidin domain is implicated in cell–cell adhesion and phospholipid binding.5 Cell expression studies showed that disease-associated missense mutations result in aberrant protein synthesis and retention in the endoplasmic reticulum, producing non-functional products.
The ERGs of the most affected males demonstrate normal or near-normal a-waves, characteristic of photoreceptor function, but often substantially reduced b-waves, originating from inner retinal cell activity.6 For several decades, the ERG has been the major diagnostic technique for RS1.
Relatively recently, optical coherence tomography (OCT) has changed the diagnostic approach for various ocular diseases in both anterior and posterior segments,7–12 including RS1. Indeed, at present spectral domain OCT (SD-OCT) is the most useful diagnostic tool for this disease. Even in a non-cooperative child, a single OCT B-scan of the foveal area is sufficient to detect RS1 signs and to make a differential diagnosis with other diseases causing visual loss in young boys.
Long-term visual outcomes can be poor due to the limited number of known successful treatments. A beneficial effect from the use of a topical or, alternatively, systemic carbonic anhydrase inhibitor (CAI) has been reported in patients with cystoid macular edema in retinal dystrophies including RS1. One study described the successful treatment of a patient with a p.Arg197Cys, and other mutations, in RS1.13 No follow-up was reported in this study after the discontinuation of the therapy with acetazolamide. Further studies on CAI treatment showed a rebound effect in RS1 in a small subset of patients only.14 A different response to treatment with oral CAI might be possible for various genotypes in RS1 (including the side effects), as previously reported.15–18
The purposes of this study were, therefore, to report for the first time a p.Arg197Cys missense mutation in the RS1 gene in an Italian family with RS1 and to explore the clinical response, as assessed by SD-OCT, in two affected brothers hemizygous for this mutation, to the treatment with oral acetazolamide alone or in combination with dorzolamide eye drops.
Materials and methods
Subjects and study procedure
This study was conducted at the Eye Clinic of the Department of Surgical Sciences, University of Cagliari, Italy. The study adhered to the tenets of the Declaration of Helsinki and was approved by the Office of Research Ethics, University of Cagliari. All subjects signed informed consents prior to their participation in the study.
Individuals from a four-generation family with RS1 were recruited at Budduso, a village in Olbia Province, Italy. The family consisted of 20 members with four affected subjects (Figure 1). Eleven individuals, including two brothers with RS1, 18 and 20 years old (patients 1 and 2, respectively), underwent a full medical history examination and a comprehensive ocular assessment, which involved best-corrected visual acuity, slit lamp examinations, fundus examination in mydriasis, SD-OCT scans, fluorescein angiography, electrophysiology and genetic analysis.
Patients were diagnosed as having RS1 based on the clinical findings, which included a decrease in visual acuity (VA), stellate-shaped cavities in the macular region on the retinal exam, and a decrease in the b-wave amplitude on ERG examination. The diagnosis was confirmed by molecular genetic analysis of RS1 gene, which revealed a p.Arg197Cys missense mutation (Figure 1).
Genetic investigation
Peripheral blood samples with EDTA anticoagulant were collected from each participant of the study. Genomic DNA was extracted using GeneCatcher™ gDNA Automated Blood Kit. All six exons and the flanking intronic regions of the RS1 gene were amplified by polymerase chain reaction with oligonucleotide primers published.4 The PCR products were analyzed on 2% agarose gel stained with ethidium bromide and purified with a DNA extraction kit (QIA-quick Gel Extraction Kit; Qiagen, Hilden, Germany). PCR products from all exons were directly sequenced with the Bigdyes termination method.
Clinical assessment
Both patients with RS1 from p.Arg197Cys mutation (patients 1 and 2) were orally treated with 375 mg acetazolamide tablets once daily simultaneously and the treatment was maintained for three months (Diamox; Teofarma s.r.l., Via F.lli Cervi, 8 27010 Valle Salimbene, PV, Italy). At the end of the 3rd month, patient 1 continued therapy only with 2% dorzolamide collyrium (Trusopt; Santen Switzerland SA, Geneva, Switzerland) three times daily in both eyes, while the older brother (patient 2) refused any further treatment.
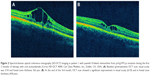
The response to the treatment was monitored by measuring VA and by performing SD-OCT (Cirrus HD-OCT 4000; Carl Zeiss Meditec, Inc., Dublin, CA, USA) using the macular cube 512×128 and HD 5 line raster scanning protocol, at baseline, at the end of the 3rd and 6th months. Changes in the foveal zone thickness (FZT), as detected in the central subfield of the macular thickness map (ie, in the central 1,000 μm of the foveal region in the ETDRS grid, from ILM to RPE), were calculated from the start to the end of the medical treatment. As previously reported, more than 19.6% reduction in FZT was considered to be substantial, as well as a change in logMAR score of 0.12 (based on previously reported normal variability between visits in non-treated eyes).19
Results
Sequence analysis of the RS1 gene identified a hemizygous c.589C>T (p.Arg197Cys) missense mutation in exon 6. The patients’ mother and the unaffected sister were heterozygous for the mutation c.589C>T. The nucleotide change in the patients predicted an amino acid change from arginine (CGC) to cysteine (TGC). This mutation was previously reported in a British patient but not in patients with Italian ethnicity.13,20
Fluorescein angiography showed that macular schisis cavities were not associated with late leakage. ERG examination showed that both patients had a significantly reduced rod response and decreased cone responses for the amplitudes of a-waves and b-waves.
A different response to the medical therapy was observed in the four eyes of the two brothers on examination by SD-OCT. Relative changes were calculated with respect to baseline values (Tables 1 and 2).
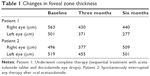
Patient 1 underwent complete therapy. At the start of treatment, the FZT was 563 μm in the right eye (RE) and 501 μm in the left eye (LE), and VA was 3/10 (logMAR 0.522) in both eyes (OU) on the Snellen chart. After the first three months of acetazolamide treatment (Figure 2), the FZT was 430 μm in the RE (−23.62%) and 371 μm in the LE (−25.94%), and VA had improved to 6/10 in the RE (logMAR 0.221) and 5/10 in the LE (logMAR 0.301). At the end of the 6th month, after treatment with 2% dorzolamide three times a day for three months, the FZT was 440 μm in the RE (−21.84%) and 277 μm in the LE (−44.71%), and VA remained 6/10 in the RE (logMAR 0.221) and 5/10 in the LE (logMAR 0.301) (Table 1).
In patient 2, the FZT was 496 μm in the RE and 519 μm in the LE, and VA was 4/10 (logMAR 0.397) in the RE and 5/10 (logMAR 0.301) in the LE before treatment. After acetazolamide treatment, the FZT was 377 μm in RE (−23.99%) and 455 μm in the LE (−12.33%), but VA was unchanged in OU. At the end of the 6th month, the FZT was 509 μm in the RE (+2.62%) and 501 μm in the LE (−3.46%), and VA was unchanged in OU (Table 2).
Discussion
RS1 is caused by mutations of the RS1 gene that encodes for retinoschisin. Retinoschisin promotes cell adhesion and its dysfunction leads to the formation of cystoid cavities in the inner nuclear and outer plexiform layers of the retina.15 Clinical effects of CAI are presumed to be attributed to their facilitation effect on the fluid transport across the retinal pigment epithelium, decreasing the volume of the subretinal space and increasing retinal adhesiveness.21 Apushkin and Fishman19 in their study demonstrated a reduction in the foveal thickness in seven of the eight patients affected by RS1, who were treated with 2% dorzolamide eye drops, and a concomitant improvement in the VA in five patients.19 In a further small case study, it was found that the response to dorzolamide eye drops was independent of the type of the underlying mutation.15
To date, 196 different mutations in the RS1 gene are known to cause RS1 (Leiden Open Variation Database, LOVD, version 2.0, Build 35; http://grenada.lumc.nl/LOVD2/eye/home.php?select_db=RS1). More than 80% are nucleotide substitutions, with missense mutations being the predominant type, mostly found in exons 4–6 encoding the discoidin domain. However, the functional impact of most missense variants is not well known. In general, a lack of relationship between the response to dorzolamide and the type of mutation in RS1 gene has been observed by different authors.13,15 In particular, structure-based analysis of p.Arg197Cys mutation predicts a mild change in retinoschisin protein, which should not affect the protein fold.22
A case report on the use of oral acetazolamide in an 8-year-old child diagnosed with RS1 demonstrated a near complete resolution of macular edema, with an increased VA even when the macular edema recurred on cessation of treatment.14
Long-term treatment of oral acetazolamide may be contraindicated in some patients because of potential systemic side effects, such as electrolyte imbalance, weakness, renal dysfunction, depression, metabolic acidosis, and mental lethargy. Because of these reasons, a topical medication would be preferable for therapy over a prolonged period of time. Clearly, topical dorzolamide may also lead to side effects, which are prevalently local, such as burning sensation of eyes, eye pruritus/discomfort, and local ocular hypersensitivity reaction.
Our study showed that oral treatment of acetazolamide for 3 months substantially reduced macular thickness in both patients and increased VA in one (patient 1), without any systemic adverse drug effects. The data of our study also showed that after the cessation of acetazolamide treatment, a rebound effect on cystoid macular edema may reduce the beneficial effects of initial therapy for RS1 from p.Arg197Cys mutation. This aspect highlights the difficulties in choosing the correct treatment strategy after the cessation of oral acetazolamide.
Of note, in patient 1 (who underwent subsequent treatment with dorzolamide eyedrops) the appearance of macular thickening was limited by sustained therapy with topical CAI, avoiding the return to the pretreatment levels (baseline values). In fact, the sequential use of topical 2% dorzolamide resulted only in a slight increase in FZT without any changes in VA. Overall, during the 6 months of therapy, sequential administration of CAI, orally and topically, induced a significant improvement in both cystoid macular edema and VA, without any systemic adverse drug effects.
Conversely, in patient 2, the initial improvement in cystoid macular edema was not associated with VA changes (during therapy with oral acetazolamide), followed by a marked rebound effect after cessation of the therapy. Whether the absence of an effect on VA predicts the rebound effect is speculative since nonresponders in VA to CAI treatment have been reported previously.16,17,23
Conclusion
In conclusion, the results of this study suggest that cessation of oral acetazolamide may result in a rebound effect on cystoid macular edema in patients with RS1 from a specific gene mutation in the RS1 (c.589C>T, p.Arg197Cys).
Also, the combined use of oral and topical CAI should be considered and studied further for a possible treatment of maculopathy and visual impairment in affected subjects with this abnormality. In this sense, CAI withdrawal should be gradual, and the dilution of the medication with eyedrops may be desirable. Consequently, until long-term efficacy and safety of a proper treatment protocol for XLRS maculopathy is established, we recommend caution in suddenly interrupting the therapeutic regimen with CAI.
Disclosure
The authors report no conflicts of interest in this work.
References
Sieving PA, Ziccardi L. Juvenile retinoschisis. In: Traboulsi E, editor. Genetic Diseases of the Eye. New York: Oxford University Press; 1998:347–355. | ||
Kim JE, Ruttum MS, Koeberl MJ, Hassemer EL, Sidjanin DJ. Genetic and clinical evaluation of juvenile retinoschisis. J AAPOS. 2009;13(2):215–217. | ||
Sikkink SK, Biswas S, Parry NRA, Stanga PE, Trump D. X-linked retinoschisis: an update. J Med Genet. 2007;44(4):225–232. | ||
Sauer CG, Gehrig A, Warneke-Wittstock R, et al. Positional cloning of the gene associated with X-linked juvenile retinoschisis. Nat Genet. 1997;17(2):164–170. | ||
Vogel W. Discoidin domain receptors: structural relations and functional implications. FASEB J. 1999;(13 Suppl):S77–S82. | ||
Sieving PA, Bingham EL, Kemp J, Richards J, Hiriyanna K. Juvenile X-linked retinoschisis from XLRS1 Arg213Trp mutation with preservation of the electroretinogram scotopic b-wave. Am J Ophthalmol. 1999;128(2):179–184. | ||
Napoli PE, Coronella F, Satta GM, Galantuomo MS, Fossarello M. Evaluation of the adhesive properties of the cornea by means of optical coherence tomography in patients with meibomian gland dysfunction and lacrimal tear deficiency. PLoS One. 2014;9(12):e115762. | ||
Napoli PE, Coronella F, Satta GM, Fossarello M. A novel technique of contrast-enhanced optical coherence tomography imaging in evaluation of clearance of lipids in human tears. PLoS One. 2014;9(11):e109843. | ||
Napoli PE, Nioi M, d’Aloja E, Fossarello M. Post-mortem corneal thickness measurements with a portable optical coherence tomography system: a reliability study. Sci Rep. 2016;6:30428. | ||
Cruz-Herranz A, Balk LJ, Oberwahrenbrock T, et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2016;86(24):2303–2309. | ||
Drexler W, Sattmann H, Hermann B, et al. Enhanced visualization of macular pathology with the use of ultrahigh-resolution optical coherence tomography. Arch Ophthalmol. 2003;121(5):695–706. | ||
Staurenghi G, Sadda S, Chakravarthy U, Spaide RF; International Nomenclature for Optical Coherence Tomography (IN•OCT) Panel. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN•OCT consensus. Ophthalmology. 2014;121(8):1572–1578. | ||
Khandhadia S, Trump D, Menon G, Lotery AJ. X-linked retinoschisis maculopathy treated with topical dorzolamide, and relationship to genotype. Eye (Lond). 2011;25(7):922–928. | ||
Ghajarnia M, Gorin MB. Acetazolamide in the treatment of X-linked retinoschisis maculopathy. Arch Ophthalmol. 2007;125(4):571–573. | ||
Walia S, Fishman GA, Molday RS, et al. Relation of response to treatment with dorzolamide in X-linked retinoschisis to the mechanism of functional loss in retinoschisin. Am J Ophthalmol. 2009;147(1):111.e1–115.e1. | ||
Collison FT, Genead MA, Fishman GA, Stone EM. Resolution of mid-peripheral schisis in x-linked retinoschisis with the use of dorzolamide. Ophthalmic Genet. 2014;35(2):125–127. | ||
Gurbaxani A, Wei M, Succar T, McCluskey PJ, Jamieson RV, Grigg JR. Acetazolamide in retinoschisis: a prospective study. Ophthalmology. 2014;121(3):802–803.e3. | ||
Cordovez JA, Traboulsi EI, Capasso JE, et al. Retinal dystrophy with intraretinal cystoid spaces associated with mutations in the crumbs homologue (CRB1) gene. Ophthalmic Genet. 2015;36(3):257–264. | ||
Apushkin MA, Fishman GA. Use of dorzolamide for patients with X-linked retinoschisis. Retina. 2006;26(7):741–745. | ||
Simonelli F, Cennamo G, Ziviello C, et al. Clinical features of X linked juvenile retinoschisis associated with new mutations in the XLRS1 gene in Italian families. Brit J Ophthalmol. 2003;87(9):1130–1134. | ||
Wolfensberger TJ. The role of carbonic anhydrase inhibitors in the management of macular edema. Doc Ophthalmol. 1999;97(3–4):387–397. | ||
Sergeev YV, Vitale S, Sieving PA, et al. Molecular modeling indicates distinct classes of missense variants with mild and severe XLRS phenotypes. Hum Mol Genet. 2013;22(23):4756–4767. | ||
Genead MA, Fishman GA, Walia S. Efficacy of sustained topical dorzolamide therapy for cystic macular lesions in patients with X-linked retinoschisis. Arch Ophthalmol. 2010;128(2):190–197. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.