Back to Journals » Chronic Wound Care Management and Research » Volume 6
Rare chemical burns: first response, early hospitalization and first treatment: retrospective analysis from a single center
Authors Akelma H , Karahan ZA, Tarıkçı Kılıç E
Received 23 April 2019
Accepted for publication 26 June 2019
Published 11 July 2019 Volume 2019:6 Pages 71—81
DOI https://doi.org/10.2147/CWCMR.S213100
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Marco Romanelli
Hakan Akelma,1 Zeki Ayhan Karahan,2 Ebru Tarıkçı Kılıç3
1Department of Anesthesiology and Reanimation, Diyarbakır Gazi Yaşargil Training and Research Hospital, Diyarbakır, Turkey; 2Department of General Surgery, Diyarbakır Gazi Yaşargil Training and Research Hospital, Diyarbakır, Turkey; 3Department of Anesthesiology and Perioperative Care, Ümraniye Training and Research Hospital, İstanbul, Turkey
Background: Chemical burns are common enough not to be underestimated and may cause significant physical, psychological and economic burdens in poor and rural areas. The most important point in the evaluation and management of such injuries is to identify the chemical substance, to be informed about the damage to be done and to make the first provision.
Objective: The objective of this study was to evaluate the outcomes of chemical burns and clinical variables of chemical burns.
Methods: A retrospective analysis was performed on all patients admitted between 2014 and 2018, at our burn unit of our hospital due to chemical burns. The patients were evaluated with respect to demographic aspects, type of chemicals, burn percentage, burn level, burn site, length of hospital stay, first application type, hospitalization day, presence of additional pathology and treatment.
Results: Twelve patients were male and six were female. The patients were hospitalized after the burns, 6 of them on the 1st day, 6 on the 2nd day, 4 on the 5th day, 1 on the 10th day and 1 on the 14th day of the burn (3,44±3,53 days). The mean hospital stay was 5.5 days. Chemical burn types consisted of cement (n=3), superglue (n=2), air bag gas (n=2), liquid oxygen (n=2), sink opener (n=3), nail polish remover (n=2), expigment cream (n=1), hot tar (n=1), IL-33 solution (n=1), white vinegar (n=1) and fridge gas (n=1).
Discussion and Conclusion: Chemical burn injuries are an important part of total burn injuries. They can be largely preventable and if managed properly can have a good outcome.
Keywords: rare chemical burns, first response, early hospitalization, first treatment
Introduction
Burn is a trauma causing physiological changes in the tissue creating impairments of form, organ loss and death. Burn care and treatment is extremely difficult and includes complex procedures.1
Chemical burns result from exposure to various chemical substances commonly found in the home, workplace or external surroundings, because of carelessness or neglect. Chemical substances are knowingly used by people in many areas, primarily in domestic and work environments. Approximately 6 million variations of chemical substances are used by people. Chemical burns constitute 3% of all burns, and 30% of chemical burns result in death.1
The most common causes of chemical burns are acids such as sulfuric, hydrofluoric, hydrochloric and acetic acid, bases such as sodium and potassium hydroxide and calcium hydroxide, oxidants used in the home such as chlorides and peroxides, and various other substances such as hair dyes and airbag injuries. Acidic agents cause coagulation necrosis leading to cytotoxicity. Alkaline substances are more toxic than acidic agents due to the irreversible changes in protein and lipid tissue damage.2,3
The prognosis of a chemical burn depends on the type of chemical and the degree of injury. Most small lesions heal well but larger wounds do not generally heal and may become scars. The most common complications are pain and scarring after a burn. Most patients require consultation from more than one physician, and skin grafts may be necessary to reduce the scarring in many patients.1
The aim of this study was to present the demographic data of patients with common and rarely seen chemical burns treated in our clinic, the type of chemical substance that caused the burn, the burn area and depth, the location of the accident, the first intervention made, the time to first admittance to hospital and to discuss these data in the light of information in literature.
Materials and methods
Patient selection
Approval for the study was granted by Diyarbakır Gazi Yaşargil Training and Research Hospital (6/7/2018-127) ethics committee; patient and guardian written informed consents were obtained in strict accordance with the principles set by Helsinki declaration. A retrospective evaluation was made of 19 patients (12 males, 7 females, mean age: 29.44 years) who were admitted for treatment of chemical burns in the Burns Unit of Healthcare Sciences University Diyarbakir Gazi Yaşargil Training and Research Hospital between 2014 and 2018. Initially, the records of 20 patients with chemical burns were examined but 1 case was excluded due to incomplete information. Thus, final evaluation in the study was made of 19 cases of chemical burns. All patients or their legal guardians provided informed consent for the use of their photographs and data. Samples of the chemical burns are shown in Figure 1.
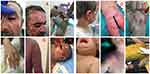 |
Figure 1 The examples of the chemical burns. |
Exclusion criteria
Of the 20 cases retrieved from the archives, 1 case was excluded from the study because of incomplete records.
Data collection
Data were retrieved from the hospital patient files and recorded on a data form for evaluation in this study. Demographic data included age and gender and clinical data included; the cause of the burn (type of chemical), burn percentage, burn degree, burn localization, length of hospital stay, type of first intervention performed, time from trauma to presentation at hospital, VAS score, type of anesthesia applied, ASA score, culture taken and the agent if there was production, laboratory values, the presence of additional pathology, and treatments applied.
Statistical analysis
Data obtained in the study were analyzed statistically using the hospital database Windows program. Descriptive statistical methods (mean, standard deviation, median, interquartile range values) were used in the data evaluation.
Results
The patients comprised 12 males and 7 females with a mean age of 29.44 years. The burns were evaluated as 2nd degree in 9 patients, 3rd degree in 5 patients and mixed 2nd–3rd degree in 5 patients (Table 1). The total body surface area (TBSA) affected was mean 7.55±6.59% (range, 2–30%). The localization of the burns was the lower extremity in 12 patients, upper extremity in 6, mixed in 2, the genital region in 1, the chest in 3 and the area around the eyes in 2 patients (Table 2, Figure 2)
 |
Table 1 Complementary information |
 |
Table 2 Burns areas |
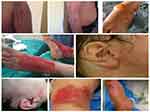 |
Figure 2 The examples of the acid burns. |
The time of presentation at hospital was recorded as on the 1st day after the burn in 6 patients, on the 2nd day in 7, the 5th day in 4, the 10th day in 1 and the 14th day in 1 (mean time to presentation at hospital 3.44±3.53 days, range: 1–14 days). The mean length of hospital stay was 5.5 days. Pain was assessed according to a VAS on presentation and routinely during the stay thereafter. The mean VAS value was 6.4. When the anesthesia types were examined, use of the sedo-analgesia method was seen to be predominant. As a result of the routine cultures taken, there was determined to be production in 3 patients and no productive agent in 16 patients (Table 1). The types of chemical burns of the patients are shown in Figures 3–5
 |
Figure 3 Distribution of chemical burns. |
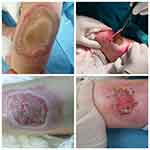 |
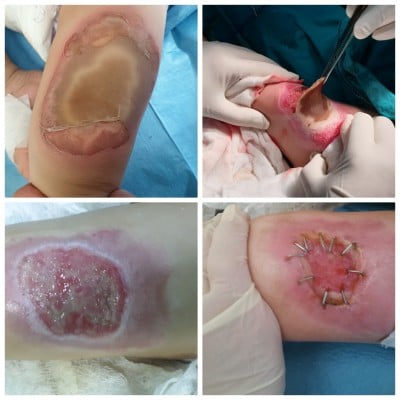
Figure 4 Alcaline burn with cement. Notes: 5% 3rd degree burn. Debridement under general anesthesia, 7th day after the operation, granulation formed. |
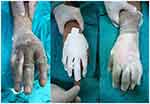 |
Figure 5 Cement burn. |
As the first intervention, cold water was applied to 5 patients, normal tap water to 2, ice to 1 and a fire extinguisher to 1 patient. No intervention was made to 10 (52.63%) patients (Table 3).
 |
Table 3 The causes of chemical burns |
When the location where the burn trauma happened was examined, the majority were seen to have been in the home and there was also a high rate in the workplace (Table 4).
 |
Table 4 Place of occurrence of the event |
Of the patients, 84.21% (n=16) received only burn wound dressing by using Alloplastic Dermis Equivalent + Collagen + Antibiotic Baktiras, whereas 15.79% (n=3) received burn wound debridement and reconstruction with auto skin grafts. Intermingled allo and auto skin grafts were applied in 3 patients with cement burns (alkaline burns).
Microorganisms growing in the injury cultures of the patients: Staphylococcus aureus in 2 patients, following Staphylococcus epidermidis in 1 patient. Systemic antibiotics used included cefotaxime, trimethoprim and sulfamethoxazole. All the patients were discharged from the hospital.
Discussion
In this study, in addition to the agents of chemical burns previously frequently reported in literature, evaluation was also made of the physiopathological injury of uncommon and different chemical burns in patients who presented at our Burns Unit, and the first interventions and treatments applied are discussed.
Chemical burns formed from contact with acid or alkaline chemical substances are generally seen as a result of workplace accidents or oral intake by children.2 The substances commonly included in these products are hydrochloric acid, phosphoric acid, sulfuric acid, hydrofluoric acid, sodium hydroxide and potassium hydroxide. Acid burns result from chemicals with a low pH and are usually less severe than alkali burns.
The common use of chemical substances in many areas of life has increased the possibility of exposure and burns. Insufficient personal protection in the workplace and lack of knowledge about chemicals cause an increase in these types of burns. Lack of knowledge and experience increases exposure in children in particular even more. There is a cluster effect in children aged over 2 years and below 5 years.3 Small children are very active and tend to explore their surroundings, but they do not have sufficient cognitive skills to understand the caustic and burn potential of these substances. Leaving chemicals such as cleaning materials and personal care products at random in the home increases the possibility of contact by children. Of the current cases, 10 presented because of burns caused in the home by accidental or incorrect use of acetone, white vinegar, IL-33 wart medication, hydroquinone (Expigment 4%) and drain cleaner. The remaining 9 cases were workplace accidents or accidents in the external environment (Table 4).
The mean age of the patients in the current study was 29.4 years, which was higher due to the exclusion of oral and inhaled burns. In addition, the higher age can be attributed to the insufficient knowledge of adults in Turkey about the use of chemical substances.
Less frequently seen causes of chemical burns are exposure to metals, phenols, calcium oxide, alcohol, solvents and acetic acid.
In several sources, chemicals are classified as acid, alkaline, organic and inorganic components. The most common substances in chemicals are acid in character. Acids affect proteins through denaturization and coagulation. These properties prevent penetration of the acidic substance to deeper tissues. As a result of the cellular dehydration and protein denaturization and coagulation that develops after exposure to an acidic substance, there is less fluid loss and edema, thereby creating a characteristic dry surface for acid burns.2
One type of acidic burns is from IL-33, which is used in Turkey in the treatment of warts in a mixed weak acidic solution. No burn case related to this was encountered. It is possible that throughout the world there are no burns related to this drug which is included in combined acid types for warts in Turkey. No reports of this type of burn were found. This drug is common in homes in Turkey as it is used in the treatment of warts caused by the Verruca vulgaris virus, and it is therefore a drug with a greater possibility for accidents. As the bottle is small with a safety cap, burns are not often encountered. However, small local burns are formed around the treatment area which do not require presentation at hospital. As the burn associated with this drug is acidic in character, it is a deep burn with protein denaturization and coagulation necrosis and is slow to heal. There is a high likelihood of a requirement for grafting and of scar formation. The first intervention requires irrigation with a large amount of water.
Although the content varies according to the type of fruit, white vinegar is formed of amino acid, potassium hydrate, aldehyde, propionic acid, 4–5% acetic acid, 1% alcohol, pectin and fruit flavoring. It is a sour fruit juice frequently used in Turkey in salads and pickling. It is used in the home most often in autumn when pickles are prepared for winter. Severe burns and even death can be caused by the alcohol content and especially because the skin is thin.4 The content of 4–5% acetic acid causes burns of the skin and esophagus in newborn infants and children. When these chemicals are examined, these burns are seen to have the same characteristics as acid burns. The first intervention and treatment is similar to that for acid burns (Table 3).
Alkaline components cause soaping and liquefaction necrosis on the skin surface epithelium and they penetrate to deeper tissues.2
Despite the increase in new technologies, the opening of an airbag in a traffic accident releases nitrogen, carbon monoxide, carbon dioxide, ammonia and various hydrocarbons, which cause inhaler and aerosol complications, and the sodium hydroxide within the bag causes alkaline burns.5,6 Patients brought to the emergency department (ED) with this type of trauma must be evaluated in respect of corrosive alkaline burns and inhaler complications in addition to cervical and chest trauma. The current study cases with airbag burns were unfortunately not applied with the necessary first interventions before arrival at ED and this increased the wound depth in these patients.
Lengthy contact with wet cement can cause severe burns as alkaline burns similar to airbag burns. Wet cement destroys the skin in 3 ways, firstly with allergic dermatitis through a reaction with hexavalent chromate ions, secondly by wear from the fine aggregate in cement and thirdly by alkaline burns as it has a pH of 12.5.7 In contrast to thermal burns, these types of burns have an insidious onset. Cement entering from a tear in a boot that is not noticed continues to corrode and deepen skin necrosis. A few hours after exposure, the first symptoms emerge of a burning feeling, pain, redness and vesicular symptoms. After 12–48 hrs, a full thickness burn is formed.8 To prevent cement burns, dirty clothes must be removed and the skin must be immediately washed with plenty of water. The first treatment should be started at the site of the accident.
Retrospective studies have shown that a high rate (75% or higher) of cement burns are full thickness burns requiring wound excision and grafting.9 In a study by Lewis et al7, 51% of patients were unaware of the risk of cement burns and had taken no precautions. The current study patients were building laborers and, as in the Lewis et al study, were unaware of the risks of these chemicals, and the burn was noticed the following morning. Most of the current study patients had no knowledge about the first interventions for these types of substances. When the patients were admitted to hospital, debridement was performed followed by dressings with materials equivalent to alloplastic skin and healing was obtained without any requirement for grafting.
Organic solvents cause wounds through dissolution of the lipid membrane which leads to impaired physiological processes. Inorganic solvents cause wounds through a denaturisation mechanism.7
Acetone, which is an organic solvent, was another chemical in this study for which no case was found in literature. Impairment of the skin barrier through treatment with diethyl ether, acetone and water causes dry skin, including increased transepidermal water loss and decreased hydration of the stratum corneum.10,11 Following induction of dry skin, various pruritogens and factors related to pruritogens become unstable in cutaneous cells.12,13 Acetone solvent is frequently used in the home as nail polish remover and glue solvent. Adults leaving these around after use increases the risk of young children in particular coming into contact with these chemicals. In the first intervention after the accident for this type of burn, decontamination of the chemical with fast-flowing water is extremely important. As infants and children have thin skin, the skin becomes dry and irritated. After impairment of the skin barrier, skin inflammation and necrosis develop.
Some chemicals cause thermal burns due to the different chemical reactions formed and exacerbation other than in acidic and basic conditions, and because of the high heat generated. The best examples of this are cyanoacrylate, liquid oxygen, hot bitumen and hydroquinone-like medical drugs. Recent publications have shown that there has been an increase in skin burns in addition to eye burns of cyanoacrylate and other strong superglues.14–20 Cyanoacrylate [CH2C(CN) CO2R] is a monomer formed as a result of the reaction of formaldehyde with alkaline cyanoacetate.20 When this molecule is in contact with hydroxyl groups (–OH) (eg, water), it is reduced to exothermic polymerization.18 In the presence of cotton fibers, which are abundant in cellulose and hydroxyl (–OH) groups, the abovementioned exothermic polymerization is accelerated, and when this is in the form of downward contact, it also causes a rapid and strong reaction which may cause thermal burns.17,18,21 When there is contact at temperatures higher than 40°C, skin damage forms and when temperatures exceed 80°C, full thickness burns occur within a second. Kelemen et al22 reported full thickness burns in 3 cases with a mean age of 14 years. In the same study, a mean temperature of 68° C was measured after contact of 4 different brands of glue with fabric. The 2 cases in the current study with cyanoacrylate burns were aged 2 and 5 years and in both cases full thickness burns formed.
Oxygen can be in liquid form and in this form can be more effectively transported and stored in large amounts. Liquid oxygen is widely used for medical and industrial purposes. It is used as an oxidant for liquid fuels in the propulsion systems of aircraft and submarines.23 Due to its cryogenic property, if liquid oxygen comes into contact with the skin, it causes numbness, severe skin irritation and frostbite. If it is ignited, it causes severe thermal burns. There is very little information in medical literature about the emergency treatment for liquid frozen burns. In the application of first aid specifically for contact with liquid oxygen, the clothes that have been wet or splashed with liquid oxygen must be removed immediately. It is also necessary to wash the contaminated skin immediately with soap or mild detergent and water.24 In the current study, a 45-year-old male working on an industrial site developed 2nd- and 3rd-degree burns on 4–5% of both calves as a result of leakage from an oxygen tube. However, the patient only applied tap water to the burned region for 15 mins. When the patient was hospitalized, debridement was performed followed by dressings with materials equivalent to alloplastic skin and healing was obtained in 10–12 days without any requirement for grafting.
Hot bitumen burns are relatively rare and the majority are seen in the paving and roofing industries. The term bitumen is used to mean mineral products formed by raw petrol and asphalt oil, and long-chain petrol and coal or fossil hydrocarbons.25 Direct contact with the skin when temperature transfer continues when adhering causes full thickness burns. Complete removal of the bitumen can result in suboptimal wound healing in bitumen burns and can increase the infection potential. In literature, the importance has been emphasized of early cooling and the use of Medi-Soll adhesive remover or liquid solvents such as petrol-based creams for the removal of bitumen.26 However, treatment recommendations are based to a great extent on reports of small surface area burns.27,28 The patient in the current study with bitumen burns applied cold water as soon as the bitumen made contact with the skin and then removed it with vaseline when it had dried and presented at the hospital the next day. These procedures applied by the patient were compatible with the recommendations in literature of cooling then removal with oil.
Hydroquinone prevents the transformation of DOPA to melanin by tyrosinase inhibition. Although it has been used in treatment at varying concentrations for more than 50 years, it is used most often at 2–5%. Commonly seen short-term side effects are irritation, erythema, stinging and irritant or contact dermatitis. In the mid and long term, milia and exogenous ochronosis may develop.29–31 When literature was examined, no case was found of burn forming after long-term use of hydroquinone. Therefore, the case in the current study can be considered to be the first case in literature of the rare chemical burn of hydroquinone. This chemical burn that formed was potently acidic in character and caused clotting necrosis. In the first intervention, irrigation is required with continuously strong flowing water.
As in all burns, the first aid to be applied to a chemical burn is important in respect of monitoring the burn. The first intervention required is to remove the chemical and clean the patient. In the treatment of burns formed with chemical agents, the burned area must be washed with plenty of water.32,33 The earlier that neutralization with water is started, then the less severe the damage will be. In the washing procedure, saline or tap water can be used. Irrigation should be made gently with a high volume of water at low pressure. The duration of washing should be at least 30 mins and this can be extended to 1 hr in severe cases. During the irrigation, necrotic tissues and any foreign bodies that are present must be cleaned. It was observed in the current study that most of the patients had insufficient knowledge on this subject. While 10 patients had made no intervention, 5 patients had applied cold water for a short time, 1 patient had applied ice and 2 patients had correctly applied tap water but not for a sufficiently long period. Therefore, it was determined that in these patients, although the burn area was small, the depth of the burn was greater.
In extensive and moderate chemical burns, the first hours are of vital importance. Therefore, the patient should contact the nearest health care institution immediately. In these types of burns, metabolic effects can emerge in addition to the skin burn. For small burns not to become infected, the patient should present at a health care facility on the same day for antiseptic intervention and treatment. For outpatients with moderate and small burns, the first intervention is generally delayed. The reason for infection usually originates from skin flora. The most common agent is Staphylococcus. Wound infection or increased wound depth is often seen in delayed cases.34
Some studies have reported that neutralization of alkaline burns with weak acids applied immediately after the trauma is more effective than neutralization with water.35 However, it is known that when washing with neutralizing substances the healing process can be negatively affected by a thermal trauma forming additional to the chemical burn injury because of the excessive heat created as a result of the reaction between the acid and the neutralizing substance.35 It would therefore be more appropriate to apply neutralization treatment only to some selected cases. The types of burns in the current study and the first interventions to be made are summarized in Table 1.
If chemical burns are not treated, they can cause short-term, long-term and life-long health problems.36,37 Cutaneous wounds and the clinical status of the patient must be rapidly evaluated and treatment must be provided immediately. Appropriate early management is very important for the reduction of patient morbidity.
Conclusion
Chemical burn injuries represent a small proportion of total burn injuries. However, they are a specific type of injury requiring immediate initial intervention and treatment management. It is especially important to draw attention to the importance of prevention in working environments and in home environments. Patients should be treated by specialist physicians and consult a burn center as soon as possible. The first intervention is very important in such burns. With the exception of some particular chemicals, the gold standard initial treatment is washing with abundant water. Non-sterile and water-soluble new chemical neutralizers should also be kept in mind. In delayed cases, wound depth and infection rate increase.
In this study, the importance of the first intervention, the importance of early presentation at hospital and the treatment applied have been discussed in light of the information in literature.
Limitation
There were few cases in this study as these were patients with uncommon chemical burns. Further clinical studies with a high number of patients will provide a more advanced perspective on the results obtained. In addition, as the intensive care unit of our burns unit has not yet entered into operation, no evaluation was made of patients with large burns (over 20%) who needed intensive care. Future articles are planned to encompass a wider area and more patients after intensive care.
Acknowledgments
The authors thank Nurse Medeni Akbalık, who helped with the data collection process, and Specialist Doctor Ünal Öztürk, who helped with the calculation and interpretation of statistics. No funding was used toward the development of this study and there are no financial interests or conflicts of interests related to this work.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
All the authors have no conflicts of interests to declare in this work.
References
1. Maghsoudi H, Gabraely N. Epidemiology and outcome of 121 cases of chemical burn in East Azarbaijan province, Iran. Injury. 2008;39(9):1042–1046. doi:10.1016/j.injury.2008.03.019
2. Wiesner N, Dutescu RM, Uthoff D, Kottek A, Reim M, Schrage N. First aid therapy for corrosive chemical eye burns: results of a 30-year longitudinal study with two different decontamination concepts.Graefes Arch Clin Exp Ophthalmol. 2019;30. doi:10.1007/s00417-019-0435
3. D’Cruz R, Pang TCY, Harvey JG, Holland AJA. Chemical burns in children: aetiology and prevention. Burns. 2015;30:569–572.
4. Brayer C, Micheau P, Bony C, Laurent T, Pilorget H, Samperiz S. Brûlure néonatale accidentelle à l’isopropanol. Archives de Pédiatrie. 2004;11(8):932–935. doi:10.1016/j.arcped.2004.04.023
5. Mouzakes J, Koltai PJ, Kuhar S, Bernstein DS, Wing P, Salsberg E. The impact of airbags and seat belts on the incidence and severity of maxillofacial injuries in automobile accidents in New York State. Arch Otolaryngol Head Neck Surg. 2001;127(10):1189–1193.
6. Suhr MAA, Kreusch T. Burn injuries resulting from (accidental) airbag inflation. J Cranio-Maxillofacial Surg. 2004;32(1):35–37.
7. Lewis PM, Ennis O, Kashif A, Dickson WA. Wet cement remains a poorly recognised cause of full-thickness skin burns. Injury. 2004;35(10):982–985. doi:10.1016/j.injury.2003.09.010
8. Mehta RK, Handfield-Jones S, Bracegirdle J, Hall PN. Cement dermatitis and chemical burns. Clin Exp Dermatol. 2002;27(4):260–263.
9. Alam M, Moynagh M, Lawlor C. Cement burns: the dublin national burns unit experience. J Burns Wounds. 2007;24(7):4.
10. Grubauer G, Feingold KR, Harris RM, Elias PM. Lipid content and lipid type as determinants of the epidermal permeability barrier. J Lipid Res. 1989;30(1):89–96.
11. Tominaga M, Ozawa S, Tengara S, Ogawa H, Takamori K. Intraepidermal nerve fibers increase in dry skin of acetone-treated mice. J Dermatol Sci. 2007;48:103–111. doi:10.1016/j.jdermsci.2007.06.003
12. Tominaga M, Takamori K. Sensitization of Itch Signaling: Itch Sensitization—Nerve Growth Factor, Semaphorins. Frontiers in Neuroscience; 2014. Chapter 17.
13. Kumari V, Babina M, Hazzan T, Worm M. Thymic stromal lymphopoietin induction by skin irritation is independent of tumour necrosis factor-alpha, but supported by interleukin-1. Br J Dermatol. 2015;172(4):951–960. doi:10.1111/bjd.13465
14. Akelma H, Tarıkçı Kılıç E, Kaçar CK, et al. Accidental full thickness burns by super glue. Ann Med Health Sci Res. 2017;7:70–71.
15. Clarke TFE. Cyanoacrylate glue burn in a child–lessons to be learned. J Plast Reconstr Aesthet Surg. 2011;64(7):170–173. doi:10.1016/j.bjps.2011.03.009
16. Acar U, Tök Ö, Kocaoğlu FA, Acar MA, Örnek F. Göz acil servisine travma ile başvuran hastalarin demografik ve epidemiyolojik verileri. MN Ophthalmology. 2015;16(1):47–50.
17. Bélanger RE, Marcotte M-E, Bégin F. Burns and beauty nails. Paediatr Child Health. 2013;18(3):125–126. doi:10.1093/pch/18.3.125
18. Clarke TFE. Cyanoacrylate glue burn in a child – lessons to be learned. J Plast Reconstructive Aesthetic Surg. 2011;64(7):70–73. doi:10.1016/j.bjps.2011.03.009
19. Takeru M, Keisuke N, Hiroyuki I, Takuya K, Arito F, Toshinori K. Burn caused by a cyanoacrylate adhesive agent: a case report. No title. Jpn J Burn Injur. 2003;29(49):53.
20. Hettiaratchy S, Dziewulski P. Pathophysiology and types of burns. BMJ. 2004;328(7453):1427–1429. doi:10.1136/bmj.328.7445.934
21. Jamnadas-Khoda B, Khan MAA, Thomas GPL, Ghosh SJ. Histoacryl glue: a burning issue. Burns. 2011;37(1):1–3. doi:10.1016/j.burns.2010.09.005
22. Kelemen N, Karagergou E, Jones SL, Morritt. AN. Full thickness burns caused by cyanoacrylate nail glue: a case series. Burns. 2016;42(4):51–54. doi:10.1016/j.burns.2015.11.009
23. Oda T, Pasquarello A. Noncollinear magnetism in liquid oxygen: a first-principles molecular dynamics study. Phys Rev B. 2004;70(13):134402. doi:10.1103/PhysRevB.70.134402
24. Heggers MC, McCauley JP, Phillips RL, Robson LG. Cold-induced injury: frostbite. In Herndon DN, editor. Total Burn Care London. 1996;408–414.
25. Kartik Logishetty M, Asuku ME, Stjepanovic Z. A fistful of tar. Interesting Case. Available from: www.ePlasty.com.
26. Demling RH, Buerstatte WR, Perea A. Management of hot tar bums. J Trauma Inj Infect Crit Care. 1980. doi:10.1097/00005373-198003000-00009
27. Baruchin AM, Schraf S, Rosenberg L, Sagi AA. Hot bitumen burns: 92 hospitalized patients. Burns. 1997;23(5):438–441.
28. Bose B, Tredget T. Treatment of hot tar burns. Can Med Assoc J. 1982;127(1):21–22.
29. Jimbow K, Obata H, Pathak MA, Fitzpatrick TB. Mechanism of depigmentation by hydroquinone. J Invest Dermatol. 1974;62(4):436–449. doi:10.1111/1523-1747.ep12701679
30. Rendon M, Berneburg M, Arellano I, Picardo M. Treatment of melasma. J Am Acad Dermatol. 2006;54(5):272–281. doi:10.1016/j.jaad.2005.12.039
31. Prignano F, Ortonne J-P, Buggiani G, Lotti T. Therapeutical approaches in melasma. Dermatol Clin. 2007;25(3):337–342. doi:10.1016/j.det.2007.04.006
32. Barret JP. No TitlePrinciples and Practice of Burn Surgery. Vol. 281. New York: Marcel Dekker; 2005:9.
33. Baradaran-Rafii A, Eslani M, Haq Z, Shirzadeh E, Huvard MJ, Djalilian AR. Current and upcoming therapies for ocular surface chemical injuries. Ocul Surf. 2017;15(1):48–64. doi:10.1016/j.jtos.2016.09.002
34. Zor F, Ersöz N, Külahçı Y, Kapı E, Bozkurt M. Gold standards for primary care of burn management. Dicle Med J. 2009;36(3):219–225.
35. Andrews K, Mowlavi A, Milner SM. The treatment of alkaline burns of the skin by neutralization. Plast Reconstr Surg. 2003;111(6):1918–1921. doi:10.1097/01.PRS.0000058953.16695.A7
36. Malisiewicz B, Meissner M, Kaufmann R, Valesky E. Physikalische und chemische notfälle in der dermatologie. Der Hautarzt. 2018;69(5):376–383. doi:10.1007/s00105-018-4137-2
37. Stone R, Shanmugasundaran S, Christine JK, Lauren HM, Nicholas EC, Ryan MC. Advancements in regenerative strategies through the continuum of burn care. Front Pharmacol. 2018;9(9):672. doi:10.3389/fphar.2018.00672
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
