Back to Journals » OncoTargets and Therapy » Volume 7
Randomized double-blind trial of prophylactic topical Evozac® Calming Skin Spray for gefitinib-associated acne-like eruption
Authors Wang Y, Yang Y, Xu J, Yu J, Liu X, Gao R, Zhang L
Received 11 April 2014
Accepted for publication 9 June 2014
Published 10 July 2014 Volume 2014:7 Pages 1261—1266
DOI https://doi.org/10.2147/OTT.S65961
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Yalan Wang,* Yunpeng Yang,* Jinxia Xu, Juan Yu, Xia Liu, Ruizhen Gao, Li Zhang
State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, People's Republic of China
*These authors contributed equally to this work
Background: "Gefitinib" is a first-generation epidermal growth factor receptor tyrosine-kinase inhibitor. More than half of patients receiving gefitinib develop acne-like eruption. Evozac® Calming Skin Spray (Evaux Laboratoires, Évaux-les-Bains, France) is made of Évaux thermal spring water and commonly used for the treatment of dermatological toxicities caused by anti- epidermal growth factor receptor therapy. The aim of the study reported here was to test the effect of Evozac Calming Skin Spray on the prevention of rash in patients receiving gefitinib.
Methods: Non-small-cell lung cancer patients preparing to initiate gefitinib therapy were randomly assigned to apply Evozac Calming Skin Spray or physiological saline to the face three times a day. The treatment was started on the same day as initiation of gefitinib therapy and continued for 4 weeks.
Results: A total of 51 patients in the Evozac Calming Skin Spray group and 50 patients in the physiological saline group completed the study per the protocol. The number of facial lesions peaked at the end of 3 weeks in both groups. There were significantly fewer lesions in the Evozac Calming Skin Spray group than in the physiological saline group at the end of 1 week (0.25 versus [vs] 1.10, P=0.031) and 3 weeks (6.67 vs 12.26, P=0.022). Patients from the Evozac Calming Skin Spray group also developed fewer facial lesions at the end of 2 weeks and 4 weeks, however, the difference was not statistically significant. At the end of 4 weeks, fewer patients from the Evozac Calming Skin Spray group developed rash of grade 2 or greater severity (17.6% vs 36.0%, P=0.037), or experienced rash-associated symptoms (13.7% vs 34.0%, P=0.017).
Conclusion: Prophylactic treatment with Evozac Calming Skin Spray appears to decrease the number of facial lesions at the peak of the rash, reduce the incidence of grade 2 or more severe rash and relieve rash-associated symptoms.
Keywords: dermatological toxicities, facial rash lesions, rash severity, rash-associated symptoms
Introduction
Epidermal growth factor receptor (EGFR) has been well established as an important target for the treatment of several tumors, including lung, head and neck, colorectal, breast, and pancreatic cancer.1 EGFR can be inhibited by monoclonal antibodies generated against the ligand-binding domain of the receptor or small-molecule tyrosine kinase inhibitors (TKIs) that compete with the intracellular adenosine triphosphate binding domain of the receptor.2
Dermatologic toxic effects are the major side effects associated with EGFR inhibition. Common dermatological side effects include acneiform skin rash, pruritus, mucositis, xerosis, fissures, hyperpigmentation, nail changes, hair loss, and changes in colour.3–5 Incidences of these side effects range from 50% to 90%, and side effects of grade 3 or greater severity occur in 3% to 20% of patients receiving EGFR inhibition.6–13 Although a systematic review including 8,998 cancer patients receiving an EGFR inhibitor concluded that there were no reported deaths from dermatologic toxicities,14 the skin side effects could lead to dose modification or discontinuation of the treatment, and adversely affect patients’ quality of life.15–18
“Gefitinib” is a first-generation reversible EGFR-TKI widely used in the treatment of non-small-cell lung cancer (NSCLC) patients with EGFR mutations. More than half of patients receiving gefitinib develop dermatological side effects. Evozac® Calming Skin Spray (Evaux Laboratories, Évaux-les-Bains, France) is made of Évaux thermal spring water, which is naturally rich in lithium. It is commonly used to manage dermatological toxicities caused by chemotherapy, radiation therapy, and anti-EGFR therapy in several countries. It has been reported that Evozac Calming Skin Spray can relieve the dermatological toxicities of patients under anti-EGFR therapy.19 However, to the best of our knowledge, there has been no formal clinical trial designed to examine the role of Evozac Calming Skin Spray in managing skin side effects induced by EGFR inhibitors. Thus, the present trial was conducted to test the effect of Evozac Calming Skin Spray in the prevention of rash in patients receiving gefitinib (NCT01528488).
Patients and methods
The study was designed as a single-center, randomized, double-blind, placebo-controlled trial, and approved by the institutional review board of Sun Yat-sen University Cancer Center. Informed consent was obtained from each participant.
Patient eligibility
Patients who met the following criteria were eligible to enroll in the study: ≥18 years old, histologically confirmed diagnosis of NSCLC, preparing to initiate gefitinib treatment, and normal hepatic and renal function. Study exclusion criteria were: Eastern Cooperative Oncology Group score ≥3; pregnancy; use of therapies within 4 weeks prior to enrollment that may induce similar skin reaction, such as cetuximab or sorafenib; use of anti-inflammatory or antibiotic drugs for other conditions; or any rash at the time of study registration.
Treatment
Eligible patients were randomly assigned to apply Evozac Calming Skin Spray or physiological saline. The topical treatment was started on the same day as initiation of gefitinib therapy and continued for 4 weeks. Patients were instructed to apply the Evozac Calming Skin Spray or physiological saline to the face three times a day, in the morning, at noon, and at bedtime. Patients were asked to record use of the study treatment, as well as that of gefitinib, in a study diary.
Evaluation
At baseline, a clinical history was obtained for all participants and a physical examination performed. Patients’ Eastern Cooperative Oncology Group performance score within 1 week of trial registration was also assessed. A blood draw for assessment of serum creatinine and total bilirubin levels was obtained. Finally, patients also underwent a complete skin examination by the participating oncologists, as well as standardized digital photography of the face.
The total number of patient face lesions was calculated at the end of Week 1, 2, 3, and 4. At the end of 4 weeks, each patient’s treating oncologist was to perform an evaluation, which included taking a history and performing a physical examination; an assessment of patient performance status; an assessment of rash-associated symptoms (itching, dry skin, pain, and irritation); and an assessment of rash severity, per the National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 3.0). In addition, digital photography utilizing the same standard poses as at baseline was also performed at Week 4. Further, because some preliminary studies indicated that the plasma concentration of gefitinib might be associated with the severity of the skin side effects, the plasma concentration of gefitinib was tested at Week 4. Plasma samples (2 mL) of the participants were collected on Day 28 and frozen at −80°C until required for analysis. The steady-state trough concentration was analyzed using a validated high-performance liquid chromatographic method with tandem mass spectrometry.
Statistical analysis
The primary endpoint was difference in total number of face lesions between Evozac Calming Skin Spray- and physiological saline-treated patients at completion of the study period (Week 4). Secondary endpoints were differences in total number of face lesions between Evozac Calming Skin Spray- and physiological saline-treated patients at the other follow-up intervals (Weeks 1–3), difference in rash severity evaluated according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (v 3.0) between the two arms at Week 4, and difference in rash-associated symptoms between the two arms at Week 4.
A sample size of 48 patients per group provided a 90% probability of detecting a lesion count difference of four between the two study arms and of thereby rejecting the null hypothesis of equal proportions with a P-value of 0.05 as a two-sided test. Assuming the dropout rate of <20%, it was determined that 59 patients should be enrolled in each arm. SPSS for Windows software (v 19.0; IBM Corporation, Armonk, NY, USA) was used for all data analysis. The normality of quantitative variables was analyzed using the Shapiro–Wilk test. Quantitative variables according with normal distribution were analyzed by independent-sample t-test; Quantitative variables departing from the normal distribution were analyzed using the Mann–Whitney U test. Pearson’s chi-squared test and Fisher’s exact test were used to test the difference in the distribution of categorical variables when appropriate. All significance levels reported refer to two-sided tests. A P-value of <0.05 was considered significant.
Results
Between December 2011 and July 2013, 118 NSCLC patients were randomly assigned to the Evozac Calming Skin Spray group (n=59) or physiological saline group (n=59) on the same day of initiation of gefitinib treatment. Details of patient attrition during the study are shown in Figure 1. In total, 51 patients in the Evozac Calming Skin Spray group and 50 patients in the physiological saline group completed the study per protocol; the baseline characteristics of these patients are listed in Table 1. There was no significant difference in the baseline characteristics between the two arms.
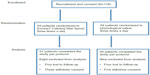  | Figure 1 Study enrollment, randomization, and attrition data. |
As shown in Figure 2, the number of lesions increased rapidly over the first 3 weeks of the study then began to decrease. Table 2 lists the total number of patient face lesions at the end of Weeks 1, 2, 3, and 4. At the end of Week 1 and Week 3, the total number of facial lesions in the Evozac Calming Skin Spray group was significantly fewer than that of the physiological saline group. Patients in the Evozac Calming Skin Spray group also developed fewer facial lesions compared with patients in the physiological saline group at the end of Week 2 and Week 4. However, the difference was not statistically significant.
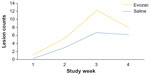  | Figure 2 Mean number of facial lesions in Evozac® Calming Skin Spray (Evaux Laboratories, Évaux-les-Bains, France) group and physiological saline group at each of the four study time points. |
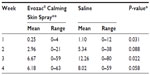  | Table 2 Total number of patient facial lesions |
With regard to rash severity, in the Evozac Calming Skin Spray group, 21 patients were diagnosed with grade 1 rash, eight with grade 2 rash, and one with grade 3 rash at the end of 4 weeks, while, in the physiological saline group, 18 patients were diagnosed with grade 1 rash, 16 with grade 2 rash, and two with grade 3 rash. In total, rash of grade 2 or greater severity occurred in 17.6% (9/51) of the Evozac Calming Skin Spray-treated patients and in 36.0% (18/50) of the physiological saline-exposed patients, and the difference reached statistical significance (P=0.037).
In addition, an assessment of rash-associated symptoms (itch, dry skin, pain, and irritation) was performed at the end of Week 4. In the Evozac Calming Skin Spray-treated group and physiological saline-exposed group, 13.7% (7/51) and 34.0% (17/50) of patients experienced one or more rash-associated symptoms, respectively. This difference was of statistical significance (P=0.017).
The steady-state trough concentration of gefitinib was available for 43 patients in the Evozac Calming Skin Spray group and 35 patients in the physiological saline group. The concentration was comparable between the Evozac Calming Skin Spray group (mean 172.4, median 159.4, range 47.8–433.0 ng/mL) and the physiological saline group (mean 170.2, median 145.1, range 51.8–391.8 ng/mL) (P=0.533).
Discussion
To the best of our knowledge, the trial reported here is the first clinical study to test the effectiveness of Evozac Calming Skin Spray for the management of dermatological toxicities caused by gefitinib. Designed as a randomized, double-blind, placebo-controlled trial, this study sought as its primary endpoint to determine whether Evozac Calming Skin Spray could reduce the number of facial lesions at the end of 4 weeks. Evozac Calming Skin Spray did not appear to decrease the number of facial lesions compared with placebo at the end of 4 weeks.
However, despite the fact that the primary endpoint was not reached, the results of the study have generated some useful findings. Evozac Calming Skin Spray did reduce the total number of facial lesions at the end of Week 1 and 3. Considering that the lesion counts peaked at the end of 3 weeks, Evozac Calming Skin Spray seemed to decrease the number of facial lesions at the peak of the rash. In addition, at the end of 4 weeks, a decrease in the incidence of grade 2 or more severe rashes in patients assigned to the Evozac Calming Skin Spray arm was noted. Further, fewer patients from the Evozac Calming Skin Spray arm suffered from rash-associated symptoms (itch, dry skin, hurting and irritation) than patients treated with placebo. In view of these points, patients could benefit from treatment with Evozac Calming Skin Spray.
The underlying mechanism responsible for why Evozac Calming Skin Spray can help manage the dermatological toxicities associated with gefitinib remains unclear. One reasonable explanation is that the Evozac Calming Skin Spray contains rich lithium (2.20 mg/L). The pathogenesis of the EGFR-TKI-induced rash involves abnormalities in the follicular epithelium together with inflammation. Lithium may have anti-inflammatory effects on keratinocytes by increasing expression of interleukin 10 and decreasing expression of Toll-like receptors 2 and 4.20 Topical agents containing lithium have been widely used for the treatment of inflammatory dermatitis, especially seborrheic dermatitis, and significantly improve patient symptoms.21–24 In addition, activation of the neurokinin-1 receptor by substance P is associated with pruritus and other symptoms of EGFR-TKI-induced rash.25 Lithium could inhibit the effect stimulated by substance P26 and relieve the symptoms of rash.
Our study has some limitations. Since only patients receiving gefitinib were enrolled in the study, the effect of Evozac Calming Skin Spray on dermatological toxicities associated with other EGFR-TKIs or anti-EGFR antibodies is uncertain. Considering that the pathogenesis of skin toxicities during use of anti-EGFR antibodies or TKIs is thought to be similar, Evozac Calming Skin Spray might also be effective for these. However, further trials should be undertaken to validate the efficacy of Evozac Calming Skin Spray for the treatment of dermatological toxicities caused by other EGFR inhibition therapies. In addition, although assessment of rash-associated symptoms was performed at the end of 4 weeks, patient quality of life was not systematically evaluated in the study. Finally, the number of patients enrolled in the study was relatively small, and large-scale trials are needed to confirm the findings in the future.
Conclusion
Prophylactic treatment with Evozac Calming Skin Spray appears to decrease the number of facial lesions at the peak of the rash, reduce the incidence of grade 2 or more severe rash and relieve rash-associated symptoms. Prophylactic Evozac Calming Skin Spray treatment with gefitinib initiation is reasonable.
Disclosure
The study was funded by Evaux Laboratories. The authors report no other conflicts of interest in this work.
References
Balagula Y, Garbe C, Myskowski P, et al. Clinical presentation and management of dermatological toxicities of epidermal growth factor receptor inhibitors. Int J Dermatol. 2011;50(2):129–146. | |
Arteaga CL. The epidermal growth factor receptor: from mutant oncogene in nonhuman cancers to therapeutic target in human neoplasia. J Clin Oncol. 2001;19(Suppl 18):32S–40S. | |
Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol. 2006;55(4):657–670. | |
Segaert S, Van Cutsem E. Clinical signs, pathophysiology and management of skin toxicity during therapy with epidermal growth factor receptor inhibitors. Ann Oncol. 2005;16(9):1425–1433. | |
Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6(10):803–812. | |
Potthoff K, Hofheinz R, Hassel JC, et al. Interdisciplinary management of EGFR-inhibitor-induced skin reactions: a German expert opinion. Ann Oncol. 2011;22(3):524–535. | |
Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351(4):337–345. | |
Saltz LB, Meropol NJ, Loehrer PJ Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22(7):1201–1208. | |
Van Cutsem E, Peeters M, Siena S, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25(13):1658–1664. | |
Soulieres D, Senzer NN, Vokes EE, Hidalgo M, Agarwala SS, Siu LL. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22(1):77–85. | |
Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA. 2003;290(16):2149–2158. | |
Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. | |
Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30(10):1122–1128. | |
Jatoi A, Nguyen PL. Do patients die from rashes from epidermal growth factor receptor inhibitors? A systematic review to help counsel patients about holding therapy. Oncologist. 2008;13(11):1201–1204. | |
Robert C, Soria JC, Spatz A, et al. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol. 2005;6(7):491–500. | |
Lacouture ME. The growing importance of skin toxicity in EGFR inhibitor therapy. Oncology (Williston Park). 2009;23(2):194–196. | |
Osio A, Mateurs C, Soria JC, et al. Cutaneous side-effects in patients on long-term treatment with epidermal growth factor receptor inhibitors. Br J Dermatol. 2009;161(3):515–521. | |
Joshi SS, Ortiz S, Witherspoon JN, et al. Effects of epidermal growth factor receptor inhibitor-induced dermatologic toxicities on quality of life. Cancer. 2010;116(16):3916–3923. | |
Chanez JF. The neurogenic component of cutaneous toxicities induced by chemotherapy – new solutions. Eur Oncol. 2010;6(1):28–30. | |
Ballanger F, Tenaud I, Volteau C, Khammari A, Dréno B. Anti-inflammatory effects of lithium gluconate on keratinocytes: a possible explanation for efficiency in seborrhoeic dermatitis. Arch Dermatol Res. 2008;300(5):215–223. | |
Dreno B, Chosidow O, Revuz J, Moyse D; Study Investigator Group. Lithium gluconate 8% vs ketoconazole 2% in the treatment of seborrhoeic dermatitis: a multicentre, randomized study. Br J Dermatol. 2003;148(6):1230–1236. | |
Langtry JA, Rowland Payne CM, Staughton RC, Stewart JC, Horrobin DF. Topical lithium succinate ointment (Efalith) in the treatment of AIDS-related seborrhoeic dermatitis. Clin Exp Dermatol. 1997;22(5):216–219. | |
Dreno B, Moyse D. Lithium gluconate in the treatment of seborrhoeic dermatitis: a multicenter, randomised, double-blind study versus placebo. Eur J Dermatol. 2002;12(6):549–552. | |
Cuelenaere C, De Bersaques J, Kint A. Use of topical lithium succinate in the treatment of seborrhoeic dermatitis. Dermatology. 1992;184:194–197. | |
Santini D, Vincenzi B, Guida FM, et al. Aprepitant for management of severe pruritus related to biological cancer treatments: a pilot study. Lancet Oncol. 2012;13(10):1020–1024. | |
Boisnic S, Branchet MC, Chanez JF. Evaluation of the inhibition of human sebocytes proliferation stimulated by substance P and corticotropin-releasing hormone by mineral constituents in Evaux thermal spring water. Nouv Dermatol. 2004;23:569–575. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

