Back to Journals » Open Access Journal of Clinical Trials » Volume 14
Randomized Clinical Trial Comparing Insulin Fast Dissolving Films versus Control Group for Anosmic Patients for Improving Their Health and Social Qualities of Life
Authors Mohamad SA , Sayed SM, Sadek AA, Badawi AM
Received 13 September 2022
Accepted for publication 8 December 2022
Published 23 December 2022 Volume 2022:14 Pages 25—33
DOI https://doi.org/10.2147/OAJCT.S389489
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Soad A Mohamad,1 Sara Mesbah Sayed,2 Ahmed A Sadek,3 Ahmed M Badawi3
1Department of Pharmaceutics and Clinical Pharmacy, Faculty of Pharmacy, Deraya University, Minya, Egypt; 2Department of Occupational Medicine and Public Health, Faculty of Medicine, Minia University, Minya, Egypt; 3Department of Otorhinolaryngology, Faculty of Medicine, Minia University, Minia, Egypt
Correspondence: Soad A Mohamad, Department of Pharmaceutics and Clinical Pharmacy, Faculty of Pharmacy, Deraya University, Minia, 61519, Egypt, Tel +1003621641, Email [email protected]
Background: Olfactory anomalies are the most common diseases among post-COVID-19 disorders. Only 15% of patients completed their prescribed treatment plans, even though several different treatment strategies were recommended; this had a detrimental effect on the patients’ physical, social, and emotional wellbeing.
Purpose: The aim of this study was approving intranasal fast-dissolving insulin films as the treatment of choice for anosmia in comparison to the control group, and the innovative treatment for anosmic post-COVID-19 is assessed in terms of the patients’ health-related quality of life (HRQoL).
Methods: For therapy and evaluation, a randomized clinical trial with forty adult anosmic post-viral patients was performed. The recruited participants were recruited between October 1 and March 8 of 2021 based on predetermined criteria. A validated smell assessment questionnaire concerning the participants’ olfactory, physical, and psychological outcomes was given to them. Recruited patients were randomly subdivided into two groups: intervention and control group. Intervention was treated with insulin intranasal films, while control group took plain films (placebo).
Results: The physical, emotional, and social health quality of life were significantly (p-value < 0.0001) improved after 4 consecutive weeks of treatment with the intervention group compared to the control group. The data were analyzed statistically with the aid of GraphPad Prism 9.1.0.
Conclusion: The lowest HRQoLs, which significantly impact their quality of life, are found in post-COVID-19 anosmic patients treated with insulin films. It is advised to employ this new intervention (insulin films) as the main therapy approach and to gather additional industry data for its development and dissemination. Problems with self-hygiene, eating, sense of danger and emotional satisfaction were significantly enhanced with insulin intervention versus placebo.
Keywords: anosmia, post-COVID syndromes, insulin, health-related quality of life, intranasal fast dissolving film, treatment outcomes
Introduction
Health-related aspects should advance swiftly along with the expansion of patient aspirations. Patients are now unhappy with inadequate treatments. Today’s therapies should address situations, practices, and policies at the community level that have an impact on the population’s perceptions of their health and functional status. Since patients’ lifestyles are significantly impacted by olfactory dysfunctions, olfactory abnormalities have been identified as one of the most prevalent post-COVID-19 symptoms affecting HRQoLs (health-related quality of life).1 Additionally, 20% of the general population, particularly those with rhinitis, exhibit this impairment, and 60% of people over 65 have trouble with smell.2
The quality of life for individuals with decreased smell following COVID-19 infection was much lower, according to earlier research. Daily actions are directly impacted by that.3 Additionally, anosmia has been demonstrated to dramatically lower HRQOL, resulting in melancholy, exhaustion, disturbed sleep, and a lack of sexual desire.4 The diagnosis and treatment of Smell disorder should therefore be prioritized.
Despite the fact that many individuals can restore their smell ability with time and spontaneous recovery rates as high as 30% at one year have been described,5 there is still a strong need for fast and effective treatment.
Currently, the most popular treatments for anosmia are systemic steroids, intranasal steroids, olfactory training, alpha-lipoic acid, intranasal vitamin A, zinc, and omega-3 fatty acids. When compared to the control group, both local and systemic steroids exhibit a slight improvement in olfactory abnormalities6 that failed to satisfy patients.
Olfactory training recovers as a treatment for anosmic patient showed slow improvement (>6 months) and was not significantly different from control group in comparison.7
Since post-traumatic anosmia responded better to zinc sulphate treatment than post-viral anosmia, it was discovered that zinc treatment was related to the pathology of anosmia.8 One of the medications used to treat olfactory abnormalities, especially anosmia, was alpha-lipoic acid. We still need further clinical research to prove that -lipoic acid may be beneficial in young individuals with olfactory loss following upper respiratory tract infection, according to certain reports.9
Recently revised publications recommended insulin as a local treatment for people with post-viral anosmia. Insulin, the treatment of concern, was recommended by Rezaeian A at 201010 and Falkowski B and his coworkers at 2020.11 Growth factor hormone was found to directly interact with insulin to enhance smell perception. Due to insulin effects on the nitric oxide cycle as a phosphodiesterase enzyme inhibitor (cGMP), growth factors (GFs) such as cyclic adenylate monophosphate (cAMP) and cyclic guanylate monophosphate (cGMP) are increased. Several studies have demonstrated that growth factors (GFs) stimulate the olfactory epithelium; consequently, a decline in GF levels can result in sensory deprivation.
In April 2021, the intranasal insulin rapid dissolving films were studied to create the sole dosage form that could facilitate and alter doses for safe insulin intranasal delivery.12 They attach to the application site with the help of fast hydration and are extremely thin and easily positioned on the mucosal tissue. The film releases the drug content for absorption into the desired tissue after it has disintegrated.
Sniffin’ and olfactory discrimination tests were used to define and clinically assess the intervention in this investigation to confirm its safety and efficacy for scent restoration.13 According to the clinical study’s findings, the intervention group’s olfactory detection scores and olfactory discrimination values significantly improved over time compared to the placebo group within four weeks. Because there is currently no intervention on the market that has been approved and could be considered standard care for people with post-viral anosmia, interventions in all prior research have been compared to controls or placebos. We now have a well-designed, evidence-based medication dosage form for the treatment of anosmia. In addition to a Phase I clinical trial for safety and efficacy, our team also developed a study for dosage form and dose estimate. The outcomes of the previous trial12 validated the efficacy of the novel therapy. The current study’s objective is to assess the effects of insulin fast dissolving film on anosmic patients in terms of their health and social circumstances (HRQOLs).
Patients and Methods
At the Department of Otorhinolaryngology, Faculty of Medicine, outpatient clinic, a randomized clinical trial was carried out between October 1 and March 8 of 2021 to compare the HRQOLs of anosmic patients treated with insulin film post- and pre-treatment to those of a control group. Staff at the pharmacy faculty produced, assessed, and characterized the intranasal fast dissolving films. All individuals who visited the outpatient clinic with a protest of subjective anosmia were invited to participate in the study. Patients accepted for sharing have been asked to fill in a well-designed questionnaire with detailed medical history under the supervision of a clinical pharmacist and an ENT specialist. A standardized ear, nose, and throat examination (including nasal endoscopy) and an evaluation of smell loss threshold were performed.
Forty-eight patients accepted sharing in study, which were distributed randomly into two groups of study. The first group served as the control group and was given intranasal film only. Sample size was calculated using Andrew Fisher’s Formula. Insulin FDF was adapted for the second group (experimental group) (40 IU). Three times a week, one film was administered into each nostril. An ENT specialist was the one who used the intervention. With the aid of the Demo® program, randomization was created.
Patient Criteria
Only post-viral anosmic patients that lasted more than two months, both sexes, aged 18–55 years old were enrolled in the study. Participants must agree to share and sign the consent form. Patients with known chronic conditions or disabilities (physical or mental) that affect QOL have been excluded from the study.
Smell Threshold
The Smell threshold score was calculated as a sum of scores from three tests: threshold, discrimination, and identification subtests. The reported Sniffin’ Sticks test was used to investigate quantitative olfactory performance as discussed by our team in 202112. Only patients with anosmia (the total score of identified bottles ≤16) were enrolled in the study. Nine concentrations of butanol were prepared and stored in amber stoppered bottles and numbered from 1 to 9 in ascending order of concentration. Each patient was asked to try to identify the smell with each nostril. Three distinct fragrances were used in three different amber stoppered vials for the discrimination test. Each time, the patient successfully distinguished between the three bottles’ various aromas; they were given one point. The simplest test is the smell identification test, which involves giving a patient a single bottle and asking them to name and describe the smell within. The final “TDI score” was the sum of scores for Threshold, Discrimination, and Identification subtests, with a range between 1 and 48 points.14 Each test was performed three times, and one point was scored each time the odor at the lowest concentration was found. Pre- and post-treatment tests were administered to each participant (intervention and control group).
Assessment of HRQoL in Anosmic Patients in Intervention and Control Group
Patients recruited to share in this study learned the aim of study, accept sharing, and assigned consent. With the assistance of an ENT physician and a clinical pharmacist, patients were asked to complete a questionnaire prior to being prescribed treatment. In accordance with the EQ-5D and SF-6D criteria, the questionnaire was created and reviewed by the research team.15 Subjects in the two arms of the study filled in the questionnaire twice, at zero time and after four weeks of treatment. The questionnaire included three parts. The first part is designed to assess and evaluate the importance of smell in various categories of patient life. The section included questions about the impact of smell loss on their life patterns and whether there are changes in other sensors (such as listening, vision, touch, and taste) because of the change in smell sensory function. The second section of the questionnaire was created to estimate the effects of smell loss on patients’ everyday routines, including personal cleanliness, eating, and drinking, perception of fire and smoke, and emotional contentment, in addition to inquiries on the patient’s health before, during, and after the anosmic episode. Questions about wellbeing covered topics like depression, a lack of overall wellbeing, irritability, and asthmatic reactions. A question about the significance of characteristics of research participants’ health-related quality of life was asked at the conclusion. The importance of physical health, financial security, work life, partnership, friendship, and emotional stability were all addressed directly in closed-ended questions with a Yes/No response option. Which of these objects was most impacted by the lack of fragrance was the goal of this section.
Statistical Analysis
Statistical analysis and data visualization were performed using GraphPad Prism 9.1.0 (GraphPad Software, Inc., La Jolla, CA) and SPSS version 25 (IBM Corp., Armonk, NY). Continuous data is presented as mean ± standard deviation, whereas categorical data is presented as absolute numbers (%). The Chi Square test is used to compare two or more proportions. P-value shows significant results when <0.0001.
Results
The study began with forty-eight participants, but only forty patients completed the study. Five participants were satisfied by smell restoration before the study’s end and did not adhere to the final two doses, and three others did not follow instructions and were given local steroids during the study, patients’ flow chart is illustrated in Figure 1.
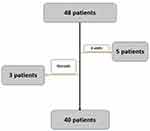 |
Figure 1 Flow chart of patients. |
The demographics of the full sample are shown in Table 1. The 18–30 age group had the highest percentage of participants in the study. Females were more sensitive to smell loss than males, and urban areas more so than rural ones in seeking smell restoration.
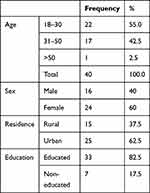 |
Table 1 Demographic Characteristics of the Studied Patients |
A comparison between intervention group (insulin group) and Placebo on their demographics is shown in Table 2, which shows no significant differences between them in age, sex, and education (p-values 0.3, 0.1, and 0.6 respectively). This implies that the variations in outcomes only apply to distinct interventions.
 |
Table 2 Demographics of the Control and Intervention Groups |
The threshold of smell loss in the participants in the two groups showed no significant differences (p-value 0.6) at the beginning of the study between the two groups, as shown in Table 3. From the table, it was clear that all the participants were anosmic and the majority were of TDI = 3.
 |
Table 3 Threshold of Smell Loss in the Two Groups |
As shown in Table 4, the importance of the smell sensor was clear among the participants, as it was assumed that the smell sense is a prominent issue in the participants’ lives and its loss has a negative impact on their HRQoLs.
 |
Table 4 Importance of Smell Sensors for the Participant of Study |
The consequences of smell loss on patients’ daily practices such as personal hygiene, food, and drink, perception of fire/smoke, and emotional satisfaction are illustrated in Figure 2. The participants in the two groups complained due to loss of smell. It is obvious that the daily practices were negatively impacted by smell loss and changed significantly with the intervention group.
 |
Figure 2 Consequences of smell loss on patients’ daily practices before and after 4 weeks of treatment with insulin intranasal FDF. |
HRQOLs changed extensively in the intervention group, as shown in Table 5. The HRQOL parameters differed in response after 4 weeks of treatment, but it was significant (<0.0001).
 |
Table 5 HRQOL Parameters Before and After Treatment with Insulin Intranasal FDF |
Discussion
As we may feel, love, and hate; in addition, it governs our emotions and behaviors; the value of healthy sensors is closely tied to our soul. Following the COVID-19 crisis, one of these sensors that is highly valued is smell. The effects of COVID-19 on smell have been investigated in numerous investigations as Mullol J and his coworkers16 and Parma V and his coworkers at 2020,17 However, there has been little discussion of the impact of loss of smell on patient life. Smell loss etiologies are many, like sinonasal disorders,18 traumatic,19 or viral20 that is the most common type. Insulin fast dissolving film was reported as an effective treatment of post-COVID anosmia12 The impact on HRQoLs has yet to be determined. In this study, we evaluated the impact of anosmia treatment with intranasal insulin FDF on patients’ quality of life. It is widely acknowledged that smell loss has a negative impact on patient life, particularly appetite and activities such as cooking, physical activities, and emotional desires.3,21 The subjects of the study were anosmic (TDI = 1,2,3,4, and 5) for more than 2 months, and they accepted sharing in the study. In addition to the serious drawbacks of smell loss on the identification of smells in life activities, the recognition of risks like smoke or fire shows a significant sign in the study group that was reported before by Mullol et al.22 This problem resolved significantly after 4 weeks of treatment with insulin FDF.
Emotional stability and bad relationships with food and drinks were among the serious qualities affected by smell loss. These outcomes were well reported previously.23,24 These qualities significantly changed after treatment with the new intervention of insulin (p value <0.0001).
The community and social activities of anosmic individuals are significantly impacted by smell problems. Smell dysfunction in the research groups had an impact on a variety of characteristics, including the capacity to work, engage in leisure activities, and socialize with friends. These results are supported by previously published material.25,26 These activities significantly improved after treatment with Insulin FDF. Insulin that directly affects growth factor hormone in the olfactory region could strongly increase the smell perception in case of anosmia.
In brief, this study’s limitations were its single-center design, which limited its generalizability and made it challenging to choose appropriate articles and extract data. The study’s many strengths, on the other hand, included a clearly defined purpose, inclusion and exclusion criteria, acceptable procedures for combining study findings, balanced patient characteristics as shown by P-values, and descriptions of patient characteristics, interventions, and outcomes.
Conclusion
The patient’s health and social quality of life are negatively impacted by anosmia, a severe olfactory impairment. With disappointing outcomes, numerous therapies are used to treat this problem. This literature endorsed the use of insulin FDF to treat anosmia. The new technique showed a notable increase in patients’ quality of life and sense of smell.
Abbreviations
FDF, fast dissolving film; Qol, quality of life; HRQOL, health-related quality of life.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval and Consent to Participate
The study protocol was complied with the Declaration of Helsinki statement and approved by the ethics committee of Minia University, Faculty of Pharmacy, Egypt (ethical approval number is 60/2019). The study was identified in clinicaltrials.gov with ID number NCT04657809.
Consent for Publication
Written informed consent was obtained from the participants for sharing in the study and for publication.
Acknowledgment
The authors acknowledge Dr Heba F Mansour, professor of pharmaceutics, Department of Pharmaceutics, Faculty of Pharmacy, Minia University, Minia, Egypt, for her kind contribution in designing insulin films and sharing ideas in idea preparation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors declare that they have no sources of funding for the research.
Disclosure
The authors declare that they have no competing interests.
References
1. Alenazi AA, Alqahtani AS, Sultan AE, Alhazmi HO, Alduhfeeri TM. Impact of covid related anosmia on patients quality of life at hail region. Eur J Mol Clin Med. 2022;9(3):721–729.
2. Mozzanica F, Gerardo R, Albera A, et al. Quality of life impairment and its assessment in patients with olfactory dysfunction; 2019.
3. Elkholi SMA, Abdelwahab MK, Abdelhafeez M. Impact of the smell loss on the quality of life and adopted coping strategies in COVID-19 patients. Eur Arch Otorhinolaryngol. 2021;278(9):3307–3314. doi:10.1007/s00405-020-06575-7
4. Bachert C, Hellings PW, Mullol J, et al. Dupilumab improves health‐related quality of life in patients with chronic rhinosinusitis with nasal polyposis. Allergy. 2020;75(1):148–157. doi:10.1111/all.13984
5. Pellegrino R, Mainland JD, Kelly CE, Parker JK, Hummel T. Prevalence and correlates of parosmia and phantosmia among smell disorders. Chem Senses. 2021;46. doi:10.1093/chemse/bjab046
6. Kim DH, Kim SW, Kang M, Hwang SH. Efficacy of topical steroids for the treatment of olfactory disorders caused by COVID‐19: a systematic review and meta‐analysis. Clin Otolaryngol. 2022;47:509–515. doi:10.1111/coa.13933
7. Jiang RS, Twu CW, Liang KL. The effect of olfactory training on odor identification in patients with traumatic anosmia. Paper presented at: International forum of allergy & rhinology; 2019.
8. Aiba MS, Mori J, Matsumoto K, Tomiyama K, Okuda F, Nakai Y. Tsunemasa. Effect of zinc sulfate on sensorineural olfactory disorder. Acta Otolaryngol. 1998;118(538):202–204. doi:10.1080/00016489850182936
9. Hummel T, Heilmann S, Hüttenbriuk KB. Lipoic acid in the treatment of smell dysfunction following viral infection of the upper respiratory tract. Laryngoscope. 2002;112(11):2076–2080. doi:10.1097/00005537-200211000-00031
10. Rezaeian A. Effect of intranasal insulin on olfactory recovery in patients with hyposmia: a randomized clinical trial. Otolaryngology. 2018;158(6):1134–1139. doi:10.1177/0194599818764624
11. Falkowski B, Duda‐Sobczak A, Araszkiewicz A, et al. Insulin resistance is associated with impaired olfactory function in adult patients with type 1 diabetes: a cross‐sectional study. Diabetes Metab Res Rev. 2020;36(6):e3307. doi:10.1002/dmrr.3307
12. Mohamad SA, Badawi AM, Mansour HF. Insulin fast-dissolving film for intranasal delivery via olfactory region, a promising approach for the treatment of anosmia in COVID-19 patients: design, in-vitro characterization and clinical evaluation. Int J Pharm. 2021;601:120600. doi:10.1016/j.ijpharm.2021.120600
13. Min HJ, Kim SM, Han DH, Kim KS. The sniffing bead system, an olfactory dysfunction screening tool for geriatric subjects: a cross-sectional study. BMC Geriatr. 2021;21(1):1–8. doi:10.1186/s12877-020-01871-7
14. Oleszkiewicz A, Schriever VA, Croy I, Hähner A, Hummel T. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol. 2019;276(3):719–728. doi:10.1007/s00405-018-5248-1
15. Brazier J, Roberts J, Tsuchiya A, Busschbach J. A comparison of the EQ‐5D and SF‐6D across seven patient groups. Health Econ. 2004;13(9):873–884. doi:10.1002/hec.866
16. Mullol J, Alobid I, Mariño-Sánchez F, et al. The loss of smell and taste in the COVID-19 outbreak: a tale of many countries. Curr Allergy Asthma Rep. 2020;20(10):1–5. doi:10.1007/s11882-020-00961-1
17. Parma V, Ohla K, Veldhuizen MG, et al. More than smell—COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses. 2020;45(7):609–622. doi:10.1093/chemse/bjaa041
18. Wolfensberger M, Hummel T. Anti-inflammatory and surgical therapy of olfactory disorders related to sino-nasal disease. Chem Senses. 2002;27(7):617–622. doi:10.1093/chemse/27.7.617
19. Zang Y, Hähner A, Negoias S, Lakner T, Hummel T. Apparently minor head trauma can lead to anosmia: a case report. ORL. 2021;83(1):2–6. doi:10.1159/000511442
20. de Melo GD, Lazarini F, Levallois S, et al. COVID-19–related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 2021;13(596):eabf8396. doi:10.1126/scitranslmed.abf8396
21. Tchekmedyian R, Lundberg M, Buchheit KM, et al. Loss of smell in patients with aspirin‐exacerbated respiratory disease impacts mental health and quality of life. Clin Exp Allergy. 2022;52:1414–1421. doi:10.1111/cea.14157
22. Mullol J, Alobid I, Mariño-Sánchez F, et al. Furthering the understanding of olfaction, prevalence of loss of smell and risk factors: a population-based survey (OLFACAT study). BMJ open. 2012;2(6):e001256. doi:10.1136/bmjopen-2012-001256
23. Varga E, Breslin P, Cowart B. The impact of chemosensory dysfunction on quality of life. Chem Senses. 2000;25:654.
24. Blomqvist EH, Brämerson A, Stjärne P, Nordin S. Consequences of olfactory loss and adopted coping strategies. Rhinology. 2004;42(4):189–194.
25. Hummel T, Nordin S. Olfactory disorders and their consequences for quality of life. Acta Otolaryngol. 2005;125(2):116–121. doi:10.1080/00016480410022787
26. Erskine SE, Philpott CM. An unmet need: patients with smell and taste disorders. Clin Otolaryngol. 2020;45(2):197–203. doi:10.1111/coa.13484
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
