Back to Journals » Cancer Management and Research » Volume 12
Ramucirumab, A Second-Line Option For Patients With Hepatocellular Carcinoma: A Review Of The Evidence
Authors De Luca E, Marino D, Di Maio M
Received 8 January 2020
Accepted for publication 27 April 2020
Published 20 May 2020 Volume 2020:12 Pages 3721—3729
DOI https://doi.org/10.2147/CMAR.S216220
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Emmanuele De Luca,1,2,* Donatella Marino,1,2,* Massimo Di Maio1,2
1Department of Oncology, University of Turin, Torino, Italy; 2Division of Medical Oncology, Ordine Mauriziano Hospital, Torino, Italy
*These authors contributed equally to this work
Correspondence: Massimo Di Maio
Division of Medical Oncology, Ordine Mauriziano Hospital, Via Magellano 1, Turin 10128, Italy
Tel +39 011 5082032
Fax +39 011 5085081
Email [email protected]
Abstract: Hepatocellular carcinoma (HCC) is the most frequent primary liver cancer and predominantly develops in patients with liver cirrhosis. In patients with advanced disease, such as extra-hepatic extension or portal vein involvement, and with intermediate disease unsuitable for locoregional therapies, systemic therapy is recommended, if liver function and performance status are adequate. Following a decade of negative Phase III trials since the approval of sorafenib, more recently several drugs have proven efficacy both in first line versus sorafenib (lenvatinib) or in second line versus placebo (regorafenib, cabozantinib, ramucirumab). In this review, we summarize the preclinical and clinical evidence supporting the use of ramucirumab, a recombinant IgG1 monoclonal antibody that specifically binds to Vascular Endothelial Growth Factor receptor 2 (VEGFR-2), in HCC. Following the results of the REACH trial, that was negative in the overall study population but identified a subgroup that could benefit from ramucirumab treatment, the REACH-2 trial was a randomized, placebo-controlled trial, designed to assess ramucirumab as second line in patients with alpha-fetoprotein (AFP) ≥ 400 ng/mL. The results of REACH-2 were published in February 2019, leading to Food and Drug Administration and European Medicines Agency approval of the drug as second-line agent for advanced HCC (after sorafenib) in patients with AFP ≥ 400 ng/mL. For the first time in the history of systemic treatments for HCC, a predictive factor of efficacy was identified. In this review, we also discuss the potential clinical development of systemic treatments in HCC, focusing on combination therapies with immunotherapy (following the recent results of the combination of atezolizumab and bevacizumab in the IMbrave 150 clinical trial) and treatment sequences as a way to maximize survival benefit.
Keywords: ramucirumab, hepatocellular carcinoma, VEGF, immunotherapy
Introduction
Primary liver cancer is among the leading causes of cancer-related death worldwide. Hepatocellular carcinoma (HCC) accounts for 80–90% of all primary liver cancers.1 HCC occurrence results from a complex interplay among genetic and non-genetic host factors, exposure to environmental carcinogens and viruses, and development of an underlying chronic liver disease, which, at its ultimate stage (ie cirrhosis), becomes a pro-carcinogenic field. HCC, especially in Western countries, is frequently associated with liver cirrhosis, a disease characterized by alteration of the normal anatomical structure of the liver, which then causes its malfunction until death; most cases of HCC (80–90%) occur on cirrhotic liver. Chronic infections with hepatitis B virus (HBV), hepatitis C virus (HCV), and alcohol abuse represent the most important risk factors. The risk is also greater in case of obesity (especially if complicated by the presence of diabetes), α-1-antitrypsin deficiency or in the case of non-alcoholic steatohepatitis (even in the absence of viral infection).2
Non-alcoholic steatohepatitis and metabolic syndrome, with or without cirrhosis, can promote HCC through different pathways. However, the leitmotif that is emerging from the literature is the role of inflammation in cancer, particularly HCC. Inflammation is the main factor that can lead to progression from non-alcoholic steatohepatitis to HCC. Insulin resistance and steatosis can provide inflammation, oxidative stress, and lipotoxicity. This condition evolves into hepatic necro-inflammation, fibrosis, cirrhosis and/or hepatocellular carcinoma.3 HCC diagnosis occurs frequently as advanced disease, due to the low specificity of the associated symptoms. Advanced disease precludes the possibility of loco-regional treatments such as surgery, ablative techniques and/or intra-arterial therapies.
In HCC patients with good liver function, prognosis is largely driven by tumor stage at time of diagnosis, with 5‐year survival exceeding 70% for early stage tumors, compared to a median survival of only 1‐2 years for those with intermediate to advanced stage tumors.4 The most acknowledged staging system for HCC has been the Barcelona Clinic Liver Cancer (BCLC) staging system, which classifies HCC into five stages—stage 0, A, B, C, and D—based on tumor burden, liver dysfunction, and patient performance status (PS).5 In case of advanced (BCLC C) HCC, such as patients with vascular invasion, distant metastases or tumor‐related symptoms, and for patients with intermediate disease, unsuitable for or refractory to locoregional therapies systemic therapy is the mainstay of treatment.
Tumor cells’ ability to metastasize depends on the formation of new blood vessels. Anti-angiogenesis is the prevention of the formation of new blood vessels, in order to inhibit the development and spread of cancer. The first and most important target is the Vascular Endothelial Growth Factor (VEGF), capable of stimulating the proliferation of endothelial cells and the “budding” of new vessels.5 Specific VEGF inhibitors have been produced, which have been approved and have entered in clinical practice.6,7 Blocking angiogenesis has become one of the key points in the systemic treatment of HCC. In cirrhotic liver and tumor tissue, overexpression of growth factors, cytokines, in particular platelet-derived growth factor (PDGF), transforming growth factor-β1 (TGF-β1), fibroblast growth factor (FGF) and VEGF directly promote fibrogenesis and angiogenesis. Another mechanism that stimulates neo-angiogenesis in cirrhotic tissue is the progressive increase of tissue hypoxia, indirectly caused by the changes of the liver anatomy.
These peculiar characteristics of HCC have long been exploited in clinic, both for diagnosis and for therapy, such as locoregional embolization-based techniques.
Ramucirumab
The inhibition of VEGF pathway at different levels (transmembrane receptors, or soluble ligands) and with different types of drugs such as monoclonal antibodies (MAb) or tyrosine-kinase inhibitors (TKI), has proven tumor growth inhibition, with significant clinical activity across several solid tumors types. Ramucirumab (IMC-1121B) is a fully human immunoglobulin G1 MAb and binds to the extracellular VEGF-binding domain of VEGFR-2, reducing endothelial cell permeability, migration and proliferation.8 Unlike bevacizumab, ramucirumab has a wider block profile, preventing the binding of all forms of VEGFs on VEGFR-2 and, with its high binding affinity (dissociation constant = 50 pM), probably favors a more complete block of angiogenesis. As for pharmacokinetics (PK), similar to PK profiles exhibited by other anti-receptor antibodies, ramucirumab shows a dose-dependent elimination, and nonlinear exposure consistent with saturable clearance. However, when ramucirumab is administered at doses higher than 8 mg/kg, clearance is saturated, leading to a high probability that all VEGFR-2s are blocked by the drug.
Recommended Phase II doses of 8 mg/kg every 2 weeks and 10 mg/kg every 3 weeks were chosen based on the results from two Phase I studies.9,10
Ramucirumab has achieved important results in several solid tumors. In REGARD study, ramucirumab, at the dose of 8 mg/Kg every 2 weeks, showed an overall survival (OS) improvement compared to placebo in second-line treatment of patients with advanced gastric and gastroesophageal junction adenocarcinomas and, at same dose and setting, in combination with paclitaxel compared to placebo in RAINBOW study.11,12 In REVEL phase III trial, at the dose of 10 mg/kg every 3 weeks, ramucirumab, administered in addition to docetaxel, has improved OS in second-line treatment of patients with advanced non-small-cell lung cancer.13
As we will further discuss, ramucirumab is currently the only drug that has proven efficacy in a biomarker-selected population of HCC patients. In this case, indeed, the predictive biomarker, serum AFP elevation, is a clinical biomarker that was already known to be associated with worse prognosis.
Clinical Evidence of Ramucirumab Use in HCC
In the early clinical development of ramucirumab, some patients with HCC were enrolled, among patients with other types of tumors, in dose-finding studies10 with preliminary signs of activity, including a patient showing a long-lasting disease stabilization. Subsequently, ramucirumab was assessed as first-line therapy specifically in patients with advanced HCC in a non-randomized, phase II trial.11 Among the forty-two subjects enrolled, median progression-free survival (PFS) was 4.0 months (95% CI, 2.6–5.7), objective response rate (ORR) was 9.5% (95% CI, 2.7–22.6; namely, 4/42 patients showed a partial response), and median OS was 12.0 months (95% CI, 6.1–19.7). This study also included patients with Child-Pugh B cirrhosis (26.2%); as expected, in line with the results of other drugs in the same setting,12 worse hepatic function was associated with lower survival (median OS was 4.4 months [95% CI, 0.5–9.0] in Child-Pugh B patients vs 18.0 months [95% CI, 6.1–23.5] in Child-Pugh A patients). Ramucirumab was described by investigators as generally well tolerated by patients. The most common treatment-related grade ≥3 adverse events (AEs) were hypertension (14%), gastrointestinal hemorrhage and infusion-related reactions (7% each), and fatigue (5%). One treatment-related death occurred, due to esophageal varices hemorrhage. As shown in similar analyses testing the association between adverse events and efficacy of other targeted drugs, the development of hypertension was associated with longer PFS and OS (4.2 months and 23.1 months respectively), vs a median PFS of 3.1 months and a median OS of 6.1 months for those who did not develop hypertension.
These encouraging data from phase I and phase II trials led to the design of the REACH study, a randomized, multicenter, double blind phase III trial that evaluated ramucirumab vs placebo as second line treatment in patients with advanced HCC, pretreated with sorafenib (Table 1).13 Main inclusion criteria were: diagnosis of HCC BCLC stage B (not amenable for further locoregional treatment) or stage C, Child-Pugh A liver disease, performance status 0 or 1 according to Eastern Cooperative Oncology Group (ECOG) scale. Initially, patients with Child-Pugh B cirrhosis were eligible but, following a safety warning, the protocol was amended and the 79 Child-Pugh B patients already enrolled were excluded from the analysis. Patients with previous or current hepatic encephalopathy, clinically meaningful ascites, high bleeding risk from esophageal or gastric varices, arterial thrombotic event within 6 months before randomization and uncontrolled arterial hypertension were excluded. Overall, 565 patients were enrolled from 154 centers worldwide and were assigned, in a 1:1 ratio, to ramucirumab 8 mg/kg or placebo every 2 weeks until disease progression, unacceptable toxicity, or withdrawal of consent. Randomization was stratified by geographic region (North and South America, Europe, or East Asia) and cause of liver disease (hepatitis B, hepatitis C, or other). The primary endpoint of the study, OS in the intention-to-treat (ITT) population, was not met (Table 2); in detail, median OS in the ramucirumab group was 9.2 months (95% CI 8.1–10.6) compared with 7.6 months (6.0–9.3) in the placebo group (Hazard Ratio [HR] 0.87 [95% CI 0.72–1.05]; p=0.14). Statistically significant differences favoring ramucirumab were observed in secondary endpoints, although of limited clinical magnitude (Table 2). Namely, patients assigned to ramucirumab achieved better time-to-progression (TTP) (median 3.5 [95% CI 2.8–4.5] vs 2.6 months [1.6–2.8]; p<0.0001), ORR (7% [95% CI 4.6–10.7] vs <1% [95% CI 0.2–2.5]; p<0.0001) and disease-control-rate (DCR) (56% [95% CI 50.4–61.8] vs 46% [95% CI 40–51.6]; p=0.011). As for toxicity, ramucirumab was associated with a higher proportion of dose reductions/omissions and discontinuation: nevertheless, the general safety profile was defined manageable and predictable by investigators. Most importantly, in the prespecified subgroup analyses, patients with a baseline AFP concentration of 400 ng/mL or greater had a statistically significant improvement in the outcome, both in OS (median OS 7.8 vs 4.2 months, HR 0.67, 95% CI 0.51–0.90, p=0.006) and in PFS (median PFS 2.7 months vs 1.5 months). This subgroup also experienced a benefit from ramucirumab treatment in terms of time to deterioration of symptoms measured by the Functional Assessment of Cancer Therapy - Hepatobiliary Symptom Index (FHSI-8) (HR 0.690; p=0.054) and time to deterioration of performance status (HR 0.642; p=0.057).14 Conversely, no benefit was observed in the subgroup of patients with baseline AFP <400 ng/mL (median OS 10.1 months with ramucirumab versus 11.8 months with placebo).
 |
Table 1 Main Characteristics of the REACH and REACH-2 Trials |
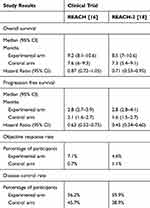 |
Table 2 Main Results of the REACH and REACH-2 Randomized Trials |
Following the results of the REACH trial in the subgroup with AFP≥ 400 ng/mL, a population-enriched phase III trial was designed.15 The REACH-2 trial had similar design and inclusion criteria of the REACH trial, except for the selection according to higher levels of AFP (Table 1). This second study was conducted in 92 centers worldwide and enrolled 292 patients, previously treated with first-line sorafenib, who were randomly allocated in a 2:1 ratio to ramucirumab (197 subjects) or placebo (95 subjects). Patients were stratified according to macrovascular invasion (yes vs no), ECOG performance status (0 vs 1), geographic region (Japan vs rest of Asia vs other regions). The primary endpoint of the study was OS, secondary objectives included PFS, ORR measured by Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 and safety.
In the efficacy analysis, treatment with ramucirumab was associated with a better OS compared to placebo: namely, median OS was 8.5 (95% CI 7.0–10.6) for patients assigned to ramucirumab, vs 7.3 months (95% CI 5.4–9.1) for patients assigned to placebo (HR for death 0.719, 95% CI 0.531–0.949; p=0.0199) (Table 2). Most subgroups showed improvement from the experimental treatment, independently of the subsequent treatment lines. AFP remained a strong negative prognostic factor for OS, even beyond the threshold of 400 ng/mL; for every 10-fold increase of AFP, the hazard of death would increase by 53.6%.
Secondary endpoints were improved as well (Table 2); median PFS was 1.6 months (95% CI 1.5–2.7) in the placebo arm and 2.8 months (95% CI 2.8–4.1) in the ramucirumab arm (HR 0.452, 95% CI 0.339–0.603; p<0.0001), and DCR was 38.9% (95% CI 29.1–48.8.) and 59.9% (95% CI 53.1–66.7) respectively (p=0.0006). Objective responses were infrequent in both groups (5% vs 1%), resulting in no statistically significant difference (p=0.1697).
The experimental treatment was associated with a slightly higher incidence of discontinuation because of any adverse event (11% vs 3%), dose reductions (9% vs 2%), delays (6% vs 3%) and dose omissions (29% vs 11%). As for the previous clinical experiences of ramucirumab in HCC, the most frequent treatment-emergent adverse events of any grade were fatigue (27%), peripheral edema (25%), hypertension (25%) and decreased appetite (23%), most of which were grade 1–2. Hypertension and hyponatremia were the only grade 3 or worse treatment-emergent adverse events that were noted in 5% or more of patients and at higher frequencies in the ramucirumab group than in the placebo group. As for quality of life, median time to deterioration of FHSI-8 scores (3.7 months vs 2.8 months, HR 0.799, 95% CI 0.545–1.171, p= 0.238) and median time to deterioration of ECOG performance status (HR 1.082, 95% CI 0.639–1.832, p=0.77) did not differ between groups.
In order to strengthen the results of ramucirumab treatment in the subgroup of patients with high AFP with a larger population, an analysis including patients with AFP ≥ 400 ng/mL from both the REACH and REACH-2 trials was presented in 201816 and then included in the final publication of the REACH-2 trial. In a population of 542 patients (292 patients from the REACH-2 and 250 patients from the REACH trial), 316 were treated with ramucirumab and 226 with placebo. Results of this pooled analysis of the 2 trials are consistent with the individual studies, with a significant improvement in survival favoring ramucirumab (median OS 8.1 months (95% CI 6.9–9.3) vs 5.0 months (95% CI 4.3–6.1); HR 0.694 (95% CI 0.571–0.842); p=0.0002). Furthermore, improvements in PFS (median PFS 2.8 months vs 1.5 months; HR 0.572; p<0.0001), ORR (5.4% vs 0.9%; p=0.0040), and DCR (56.3% vs 37.2% p<0.0001) were confirmed. The analysis of patient-reported outcomes in the same pooled population showed that ramucirumab significantly delayed time to deterioration as measured by FHSI-8 (median 3.3 months for ramucirumab vs 1.9 months for placebo, HR 95% 0.725 [0.559–0.941]) for several disease-related symptoms such as back pain (HR 0.668), weight loss (HR 0.699) and pain items (HR 0.769).17
Other Options for Second Line Treatment in HCC
The results above described with ramucirumab are part of a rich scenario of clinical trials performed in the setting of advanced HCC in recent years. In detail, several phase III trials conducted after the approval of sorafenib, testing new drugs both as first-line compared to sorafenib or as second-line for patients who have failed sorafenib, yielded dismal results, and for almost a decade, sorafenib remained the only available treatment for advanced HCC patients.14,18-27 Luckily, in the past 3 years, several phase III trials have positively changed the therapeutic scenario.
In the RESORCE study, regorafenib - an oral multi target tyrosine-kinase inhibitor - was tested versus placebo after sorafenib failure in patients with a BCLC stage B or C HCC not amenable to locoregional treatments, and with good liver function (Child-Pugh A).28 As a key inclusion criterion, all enrolled patients needed to be sorafenib-tolerant, meaning that they must have received ≥400 mg of sorafenib daily for at least 20 of the 28 days preceding discontinuation. After a median follow-up of 7 months, the benefit of regorafenib treatment was consistent for primary and secondary endpoints and in all prespecified subgroup analyses. Namely, median OS was 10.6 months with regorafenib vs 7.8 months with placebo (HR 0.63, 95% CI 0.50–0.79, p<0.0001). In addition, median TTP by modified RECIST was 3.2 months with regorafenib and 1.5 months with placebo and more patients in the experimental arm experienced disease control (DCR 65% vs 36%, p<0.0001). All the efficacy results have been confirmed according to RECIST 1.1. In the sequential treatment of sorafenib followed by regorafenib, at least in this selected population of patients who were sorafenib-tolerant and eligible for a second-line treatment, the survival benefit was independent of the pattern of the disease progression during prior sorafenib treatment and of their last sorafenib dose (800 mg/day or <800 mg/day).29 Based on these results, regorafenib was the first drug to be approved by regulatory agencies as second-line treatment for HCC patients following first-line sorafenib.
Cabozantinib, another oral TKI, was evaluated in the CELESTIAL trial, a Phase III, double-blind, placebo-controlled trial in patients with HCC who had received prior sorafenib therapy.30 As for patients enrolled in the RESORCE trial, preserved liver function (Child–Pugh A) was required, but patients could have received up to two previous systemic treatments including sorafenib, regardless of previous sorafenib tolerance. In a population of 707 patients (470 assigned to cabozantinib and 237 to placebo), the trial reached its primary endpoint of improving survival: namely, median OS was 10.2 months in the cabozantinib group and 8.0 months in the placebo group (HR for death 0.76, 95% CI 0.63–0.92, p=0.005). In the subgroup of patients who had received only sorafenib as previous systemic therapy, median OS was 11.3 months with cabozantinib and 7.2 months with placebo (HR for death, 0.70, 95% CI 0.55–0.88). Also secondary endpoints favored the experimental arm; cabozantinib yielded a DCR of 64% as compared with 33% in the placebo group. Median PFS was 5.2 months in the cabozantinib group and 1.9 months in the placebo group (HR 0.44, 95% CI 0.36–0.52, p<0.001).
Future Directions
The treatment of advanced hepatocellular carcinoma has experienced a significant revolution with the introduction of sorafenib since 2007. After the introduction of sorafenib in clinical practice, subsequent trials on new target drugs did not lead to new therapeutic changes in the following decade, due to their low antitumor activity and high incidence of side effects. In the era of immunotherapy and following promising results of initial phase I/II trials, anti-PD-1/PD-L1 checkpoints inhibitors are being investigated for HCC treatment.31 During 2019, the results of two highly awaited phase III trials, those of anti-PD-1 Mab as first (nivolumab) or second line (pembrolizumab), failed to reach their primary endpoints. The Checkmate 459 trial32 randomized 743 patients to receive first-line treatment with sorafenib (n=372) or with nivolumab (n=371). Although median OS was longer in the experimental arm with nivolumab (16.4 months [95% CI 13.9–18.4] vs 14.7 months [95% CI 11.9–17.2] for sorafenib), and it was associated with more favorable safety profile and better ORR, this difference did not meet the prespecified threshold of statistical significance and the study has to be considered formally negative (HR 0.85 [95% CI: 0.72–1.02]; p= 0.0752).
The phase III KEYNOTE-240 trial involved 413 HCC patients who were randomly assigned to receive pembrolizumab or best supportive care as second‐line treatment following sorafenib.33 OS and PFS were co-primary endpoints of this study. After a median follow-up of 13.8 months, pembrolizumab numerically improved OS (HR: 0.78; one sided p=0.0238) and PFS (HR: 0.78; one sided p=0.0209) with a manageable toxicity profile; however, these differences did not reach the prespecified boundaries of statistical significance, and even this trial has to be considered formally negative. Thus, the results of these two trials shed a doubtful light on the otherwise promising role of checkpoint inhibitors in HCC, at least as single agent anti-PD1 MAb. Nevertheless, both nivolumab and pembrolizumab received FDA approval as second-line treatment after sorafenib, based on results from Phase 1–2 trials and these drugs are still approved in clinical practice, based on the clinical benefit confirmed in these Phase 3 studies.
In the recent past, mutations specific to cellular components and genes associated with HCC have been better documented and in the next future it will be important to understand and develop custom-made therapeutic targets for HCC which will hopefully allow improved survival.34
Molecular studies of HCC have determined abnormal activation of different signaling pathways, which illustrate key targets for novel molecular therapies. Overall, combination therapies that would provide a synergistic effect, with acceptable drug toxicity, are new directions for the upcoming treatments of HCC. As already mentioned above, angiogenesis is one the hallmarks of cancer, it leads to formation of new blood vessels to bring blood and therefore nourishment to cancer cells, and very often it is a disorganized network of blood vessels.37 This “pathological process” is involved in a inflammatory process in which VEGF also plays an important role as immunosuppressive molecule with recruitment immunosuppressive regulatory T cells’ (Treg) into the tumor. Furthermore in tumor vascular endothelium there is an increase of expression of adhesion molecules for various immune cells, whose downregulation is mediated by angiogenetic factors including VEGF. So, VEGF inhibition enhances local antitumor immunity by reducing accumulation of Treg and leads to an increase of number of immune cells, in particular CD8+ T cells. This explains the role of VEGF-A in escaping antitumor immunity and the link between angiogenesis and immunosuppression in cancer progression.38
Under the clinical point of view, the addition of and anti-angiogenic agent might be the key to enhance the activity of checkpoint inhibitors in HCC. Recently, in a randomized cohort of the Phase 1b GO30140 study the addition of bevacizumab to the anti-PDL1 MAb atezolizumab prolonged PFS compared to atezolizumab monotherapy in advanced, untreated HCC patients.35 This combination has been evaluated in an open-label, multicenter phase III trial, whose results have recently been presented. The IMbrave150 (NCT03434379) randomized patients with locally advanced or metastatic and/or unresectable HCC to receive first-line treatment with the combination of atezolizumab and bevacizumab versus sorafenib. This study met its co-primary endpoints. With median follow-up of 8.6 months, median OS with the atezolizumab combination was not estimable compared to 13.2 months with sorafenib (HR 0.58; 95% CI, 0.42–0.79; p = 0.0006). Median PFS with the combination was 6.8 months (95% CI, 5.7–8.3) versus 4.5 (95% CI, 4.0–5.6) with sorafenib (HR 0.59 (95% CI, 0.47–0.76; p<0.0001). The ORR with the respective treatments was 27% versus 12% (p< 0.0001). Atezolizumab/bevacizumab delayed deterioration of quality of life compared to sorafenib, with the potential to be a practice-changing treatment in the first-line setting for patients with unresectable HCC.36
The immune-modulatory characteristics of biological drugs and target therapies represent and will represent the basis and rationale to conduct new clinical trials.
The strategic importance of the association between immunotherapeutic and antiangiogenic drugs opens new important horizons and, with the introduction of new molecules, will make more challenging the choice of the right therapeutic sequence in the near future. The first interesting results of studies evaluating in advanced HCC the combination of anti-PD1 and anti-PD-L1 with other immune checkpoint inhibitors, antiangiogenic drugs and multitarget tyrosine kinase inhibitors are a sign of the important phase of translational and clinical research that has been developed in this field. In the coming years, this progress may be moved to the earlier stages of the disease to downstage unresectable HCC or to improve prognosis in patients with high risk of recurrence.
Although the combination therapeutic strategy (biological and target drugs, immunotherapy) is imposing on the therapeutic horizon of HCC and aware of the clinical potential that these drugs have, it is also necessary to consider the fragility of the clinical conditions with liver dysfunction of these patients, making the management of side effects even more problematic. The next challenge will therefore be, in the context of a more personalized medicine, to offer these patients the best therapeutic strategy, possibly safer.
Conclusions
Based on the results obtained in the two randomized trials REACH and REACH-2 in the HCC population with high AFP values, on May 10th, 2019 the FDA approved ramucirumab as single agent as second-line treatment for HCC in patients who have an AFP ≥ 400 ng/mL pretreated with sorafenib.
Several questions remain open. Still controversial is the question whether the results of the REACH-2 study are only an epiphenomenon of a still poorly biochemical and molecular characterized tumor. Furthermore, the exclusion from the same study and other clinical trials of patients at high risk of bleeding (high risk varices) does not allow to fully translate these results to daily clinical practice. Patients with high risk varices should be treated before starting systemic treatment in clinical practice. Finally, the absolute gain in median survival is disappointingly modest (1.2 months), also considering the potential costs associated with drug administration, both in terms of clinical and financial toxicity. The authors tried to interpret this marginal OS improvement due to the long median OS reached by the placebo group, probably to be attributed to AFP levels that on average, although high due to inclusion criteria, were lower than those in the “high-AFP” subgroup of the previous REACH study. Consequently, patients were considered to be at “lower prognostic risk” compared to the “high-AFP” subgroup of the REACH trial.
In conclusion, ramucirumab represents a new therapeutic option for advanced HCC (after treatment with sorafenib) with AFP ≥400 ng/mL. Further works will need to better investigate the predictive role of AFP, its impact in the natural history of HCC and in the response to the systemic treatment.
The treatment landscape for patients with HCC is therefore expanding and further therapeutic options will soon become available, providing future clinical trials a clinical and translational imprint.
With the increased knowledge of liver cancer biology and genomics, the hope is to go towards precision medicine, in a cancer that is characterized by a poor prognosis and limited responsiveness to systemic therapy.
Disclosure
MDM has acted as consultant or participated in advisory boards, receiving fees, from Bristol Myers Squibb, Merck Sharp & Dohme, Roche, AstraZeneca, Pfizer, Takeda, Eisai. Other authors declare no conflicts of interest in this work.
References
1. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
2. Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149(5):1226–1239. (). doi:10.1053/j.gastro.2015.05.061
3. Lee TY, Wu JC, Yu SH, Lin JT, Wu MS, Wu CY. The occurrence of hepatocellular carcinoma in different risk stratifications of clinically noncirrhotic nonalcoholic fatty liver disease. Int J Cancer. 2017;141(7):1307–1314. doi:10.1002/ijc.30784
4. Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatol. 2018;68(2):723–750. doi:10.1002/hep.29913
5. Marrero JA, Fontana RJ, Barrat A, et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatol. 2005;41(4):707–716. doi:10.1002/hep.20636
6. Zhu Z, Bohlen P, Witte L. Clinical development of angiogenesis inhibitors to vascular endothelial growth factor and its receptors as cancer therapeutics. Curr Cancer Drug Targets. 2002;2(2):135–156. doi:10.2174/1568009023333881
7. Witte L, Hicklin DJ, Zhu Z, et al. Monoclonal antibodies targeting the VEGF receptor-2 (Flk1/KDR) as an anti-angiogenic therapeutic strategy. Cancer Metastasis Rev. 1998;17(2):155–161. doi:10.1023/A:1006094117427
8. Miao HQ, Hu K, Jimenez X, et al. Potent neutralization of VEGF biological activities with a fully human antibody Fab fragment directed against VEGF receptor 2. Biochem Biophys Res Commun. 2006;345(1):438–445. doi:10.1016/j.bbrc.2006.04.119
9. Spratlin JL, Cohen RB, Eadens M, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28(5):780–787. doi:10.1200/JCO.2009.23.7537
10. Chiorean EG, Hurwitz HI, Cohen RB, et al. Phase I study of every 2- or 3-week dosing of ramucirumab, a human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2 in patients with advanced solid tumors. Ann Oncol. 2015;26(6):1230–1237. doi:10.1093/annonc/mdv144
11. Zhu AX, Finn RS, Mulcahy M, et al. A phase II and biomarker study of ramucirumab, a human monoclonal antibody targeting the VEGF receptor-2, as first-line monotherapy in patients with advanced hepatocellular cancer. Clin Cancer Res. 2013;19(23):6614–6623. doi:10.1158/1078-0432.CCR-13-1442
12. Marrero JA, Kudo M, Venook AP, et al. Observational registry of sorafenib use in clinical practice across child-pugh subgroups: the GIDEON study. J Hepatol. 2016;65(6):1140–1147. doi:10.1016/j.jhep.2016.07.020
13. Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859–870. doi:10.1016/S1470-2045(15)00050-9
14. Chau I, Peck-Radosavljevic M, Borg C, et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib: patient-focused outcome results from the randomised phase III REACH study. Eur J Cancer. 2017;81:17–25. doi:10.1016/j.ejca.2017.05.001
15. Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi:10.1016/S1470-2045(18)30937-9
16. Zhu A, Finn R, Galle P, et al. LBA-001Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated alpha-fetoprotein (AFP) following first-line sorafenib: pooled efficacy and safety across two global randomized phase 3 studies (REACH-2 and REACH). Ann Oncol. 2018;29(suppl_5):mdy208–mdy208.
17. Zhu AX, Gable J, Bowman L, et al. 622PDRamucirumab as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated alpha-fetoprotein (AFP) following first-line sorafenib: patient reported outcome results across two phase III studies (REACH-2 and REACH). Ann Oncol. 2018;29(suppl_8). doi:10.1093/annonc/mdx807.
18. Abou-Alfa GK, Niedzwieski D, Knox JJ, et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J Clin Oncol. 2016;34(15_suppl):4003.
19. Abou-Alfa GK, Qin S, Ryoo BY, et al. Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann Oncol. 2018;29(6):1402–1408. doi:10.1093/annonc/mdy101
20. Brizzi MP, Pignataro D, Tampellini M, Scagliotti GV, Di Maio M. Systemic treatment of hepatocellular carcinoma: why so many failures in the development of new drugs? Expert Rev Anticancer Ther. 2016;16(10):1053–1062. doi:10.1080/14737140.2016.1227706
21. Cainap C, Qin S, Huang WT, et al. Linifanib versus sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33(2):172–179. doi:10.1200/JCO.2013.54.3298
22. Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31(32):4067–4075. doi:10.1200/JCO.2012.45.8372
23. Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31(28):3517–3524. doi:10.1200/JCO.2012.48.4410
24. Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31(28):3509–3516. doi:10.1200/JCO.2012.47.3009
25. Rimassa L, Assenat E, Peck-Radosavljevic M, et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): a final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018;19(5):682–693. doi:10.1016/S1470-2045(18)30146-3
26. Zhu AX, Kudo M, Assenat E, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA. 2014;312(1):57–67. doi:10.1001/jama.2014.7189
27. Zhu AX, Rosmorduc O, Evans TR, et al. SEARCH: a phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33(6):559–566. doi:10.1200/JCO.2013.53.7746
28. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi:10.1016/S0140-6736(16)32453-9
29. Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69(2):353–358. doi:10.1016/j.jhep.2018.04.010
30. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi:10.1056/NEJMoa1717002
31. Chang Lee R, Tebbutt N. Systemic treatment of advanced hepatocellular cancer: new hope on the horizon. Expert Rev Anticancer Ther. 2019;19(4):343–353. doi:10.1080/14737140.2019.1585245
32. Yau T, Park JW, Finn RS, et al. LBA38_PRCheckMate 459: A randomized, multi-center phase III study of nivolumab (NIVO) vs sorafenib (SOR) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019;30(Supplement_5):v874–v875. doi:10.1093/annonc/mdz394.029
33. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. doi:10.1200/JCO.19.01307
34. Vivekanandan P, Singh OV. High-dimensional biology to comprehend hepatocellular carcinoma. Expert Rev Proteomics. 2008;5(1):45–60. doi:10.1586/14789450.5.1.45
35. Lee M, Ryoo B-Y, Hsu C-H, et al. LBA39Randomised efficacy and safety results for atezolizumab (Atezo) + bevacizumab (Bev) in patients (pts) with previously untreated, unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30(Supplement_5):v875.
36. Cheng A-L, Qin S, Ikeda M, et al. LBA3IMbrave150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30(Supplement_9):ix186–ix187. doi:10.1093/annonc/mdz446.002
37. Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. 2011;11(10):702–711.
38. Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70(15):6171–6180.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
