Back to Journals » Cancer Management and Research » Volume 11
Radiotherapy for patients with completely resected pathologic IIIA(N2) non–small-cell lung cancer: a retrospective analysis
Authors Zhu Y, Fu L, Jing W, Kong L, Yu J
Received 6 December 2018
Accepted for publication 12 June 2019
Published 31 December 2019 Volume 2019:11 Pages 10901—10908
DOI https://doi.org/10.2147/CMAR.S197245
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Rituraj Purohit
Ying Zhu,1–3,* Lei Fu,2,* Wang Jing,2 Li Kong,2,4 Jinming Yu2,4
1Department of Clinical Medicine, Weifang Medical University, Weifang, Shandong Province, People’s Republic of China; 2Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University, Jinan, People’s Republic of China; 3Heze Medical College Affiliated Hospital, Heze, Shandong Province, People’s Republic of China; 4Shandong Academy of Medical Sciences, Jinan, Shandong Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jinming Yu
Department of Radiation Oncology, Shandong Cancer Hospital and Institute, Shandong First Medical University, 440 Jinan Road, Jinan 250117, Shandong Province, People’s Republic of China
Tel +86 05 318 798 4777
Fax +86 05 318 798 4079
Email [email protected]
Introduction: Adjuvant radiotherapy in non–small-cell lung cancer (NSCLC) remains controversial,Whether the mutation status of epidermal growth factor receptor (EGFR) will affect the recurrence and survival of patients with resected NSCLC is rarely reported. Our purpose is to study the effect of postoperative radiotherapy on patients with stage IIIA(N2) NSCLC with EGFR mutation.
Methods: Total of of 115 patients diagnosed with stage IIIA(N2) resected NSCLC were analyzed retrospectively. Their EGFR mutations were detected by real-time quantitative PCR and DNA sequencing technology together.
Results: At a median follow-up of 34.2 months for the postoperative adjuvant radiotherapy (PORT) group and 31.0 months for the non-PORT group, PORT group significantly improved progression free survival (PFS) and overall survival (OS). The median PFS and OS in the EGFR mutant group were not significantly longer than those in the EGFR wild-type group. The number of chemotherapy cycles, postoperative radiotherapy and the number of metastatic lymph nodes were independent factors influencing long-term survival.
Conclusion: Our retrospective analysis showed that PORT can improve survival of patients with stage IIIA(N2) NSCLC. EGFR-mutant group with stage IIIA(N2) NSCLC has a tendency of a higher survival than the wild-type EGFR group, but there was no significant difference for both groups. The EGFR mutation status was not associated with PFS or OS of stage IIIA(N2) NSCLC.
Keywords: non-small cell lung cancer, N2-stage, adjuvant radiotherapy, EGFR mutations
Introduction
Despite advances in treatment, lung cancer remains the leading cause of cancer-related deaths in humans. Approximately 25–30% of non-small-cell lung cancer (NSCLC) patients are diagnosed at a locally advanced stage (IIIA or IIIB), and postoperative 5-year survival rates range from 13% to 42.8%.1–3 Based on several prospective clinical trials that have validated the survival benefit of concurrent chemoradiotherapy over radiotherapy alone4 or chemotherapy followed by sequential radiotherapy for stage IIIA (N2) NSCLC.5,6 However, stage IIIA (N2) NSCLC patients have heterogeneous disease presentation,7 the value of postoperative adjuvant radiotherapy(PORT) for completely resected NSCLC remains controversial, as the effect on survival has been inconclusive.8–10 A secondary analysis of a prospective trial illustrated the benefit of adding PORT in pN2 disease regardless of chemotherapy use,11 this has been supported by multiple high-volume retrospective investigations.12 Furthermore, whether epidermal growth factor receptor (EGFR) mutation is associated with long-term survival in patients with Completely Resected Pathologic IIIA(N2) NSCLC is unclear. The purpose of this study was to explore the relationship between EGFR mutation status and long-term survival with combined chemoradiation.
Methods and materials
Patient selection
One hundred and fifteen stage with pathological stage IIIA(N2) NSCLC were surgically treated at the Shandong Cancer Hospital and Institute (Jinan, China) between March 2011 and December 2015 were retrospectively reviewed. Histology and fluorodeoxyglucose positron emission tomography computed tomography (FDG-PET/CT) confirmed the staging of all patients. The median follow-up time was 34.2 months (range 3.5–42.7 months).
Each patient received postoperative adjuvant chemotherapy. The inclusion criteria for the postoperative radiotherapy and chemotherapy group (PORCT) groups and the postoperative chemotherapy group (POCT) groups were the same, as follows: complete surgical resection through either lobectomy or pneumonectomy; systematic nodal dissection, a minimum of three N2 stations sampled or complete dissection (one of which must be the subcarinal station); and histologically proven NSCLC of stage pT1-3N2M0 (according to the TNM classification in the UICC 7th ed).13,14 Patients who received neoadjuvant therapy (chemotherapy and/or radiotherapy), showed evidence of metastatic disease or presented with previous malignancy were excluded. Patients with EGFR mutations receiving tyrosine kinase inhibitor (TKI) treatment after surgery were also excluded. Then, the EGFR mutational states of the two groups were compared.
Ethical approval
The study was approved by the Ethics Committee of Shandong Cancer Hospital and Institute. All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Written informed consent was obtained from all patients before their participation in this clinical research, and their data are used for future research.
Grouping
Of the 115 patients, the 15 (13%) patients who composed group A received PORCT and had EGFR mutations, the 31 (27%) patients who composed group B received PORCT and had wild-type EGFR, the 21 (18%) patients who composed group C received POCT alone and had EGFR mutations, and the 48 (42%) patients who composed group D received POCT alone and had wild-type EGFR.
EGFR mutation detection
The test specimens were collected during surgery; EGFR mutations were detected by fluorescence quantitative polymerase chain reaction (PCR), and the positive specimens were confirmed by gene sequencing. Exons 18, 19, 20 and 21 of the EGFR gene were tested in 115 surgical specimens.
Patient evaluation
The pretreatment assessments typically included clinical evaluations, blood tests, CT scans of the chest, bronchoscopy, ultrasound or abdominal CT, brain magnetic resonance imaging (MRI), and bone scans. The patients were followed up every 3 months for 2 years after surgery and then followed up every 6 to 12 months. The regular follow-up evaluations included a physical examination, determination of World Health Organization (WHO) Performance Status (PS), biological assessment, chest X-ray, chest CT scan, bronchoscopy, abdominal ultrasound or CT scan, and bone scan.
Disease recurrence at the surgical margin, ipsilateral hilum, and/or mediastinum was considered a local-regional failure.14 All other sites of failure, including the supraclavicular zone, contralateral hilum and distant organs, were considered distant metastasis.15,16
Surgery
In our analysis, positron emission tomography (PET)-CT scans were used as part of the routine preoperative work-up. All thoracic surgeons performed thoracotomy and mediastinal lymphadenectomy in a similar manner. All patients underwent systematic and complete mediastinal lymph node dissection or sampling. Systematic sampling was defined as the routine removal of at least one lymph node from each of the lowest levels. The lymph nodes at levels 5, 6 and 7 (at least) were sampled during the left thoracotomy or dissected completely, while those at levels 4 and 7 were sampled during the right thoracotomy. A total of 3~20 N2 lymph nodes were taken, with an average of 6 lymph nodes.
Postoperative chemotherapy
Platinum-based chemotherapy was administered with a median of four cycles and was given with radiotherapy or alone. A total of 63 patients received gemcitabine (1,000 mg/m2 intravenously on days 1 and 8) and cisplatin (40 mg/m2 intravenously on days 1–3) for a median of four cycles (range, two to five); 52 patients received pemetrexed (500 mg/m2 intravenously on day 1) and cisplatin (40 mg/m2 intravenously on days 1–3) for a median of four cycles (range, two to five). Platinum-based chemotherapy was administered with a median of four cycles and was given with radiotherapy or alone.
Postoperative radiotherapy
Of the 115 patients, 46 received adjuvant PORT. Radiation was delivered with 6 to 8 MV X-rays at 1.8–2Gy per fraction, 5 days per week, for a total dose ranging from 48Gy to 54Gy and a median dose of 50Gy. The clinical target volume (CTV) for treatment generally included the mediastinum and ipsilateral portal (based on a postoperative CT scan of the anatomical markers). The planning target volume (PTV) was defined as the CTV plus 0.5–0.8 cm margins. The tumor bed was included only if invasion of the parietal pleura was documented in the operative report. The exact location of the visual field boundaries varied from case to case and depended on the postoperative displacement of the mediastinal structures. The respective 99% PTVs had to be covered by the 95% prescription dose, and the 95% PTVs had to be covered by the 100% prescription dose14 The dose limits for the peripheral normal organs were as follows: the maximum dose for the spinal cord was less than 45Gy; the mean lung dose was less than 15Gy, and less than 25% of the lung volume received 20Gy (V20); and the mean cardiac dose was less than 30Gy.
Statistical analyses
All statistical analyses were conducted using SPSS for Windows, version 20.0 (IBM Corporation, Armonk, NY, USA). OS was measured from the day of surgery to the date of death from any cause or the last follow-up. Progression free survival (PFS) was calculated from the day of surgery to the date of confirmed progression or death from any progression. If the complete survival time of a patient was impossible to obtain or if the disease did not progress, the patient status was assumed at the last known survival and/or contact date.
The PFS and OS rates were estimated using the Kaplan-Meier method, and log-rank tests were used for the univariate analysis. Variables that showed significant associations in the univariate analysis (p<0.05) were included in a multivariate stepwise backward Cox regression model to validate their independent prognostic values. Differences were assumed to be significant when a p-value of <0.05 was achieved.
Results
Patient characteristics
A total of 115 patients were enrolled in the trial between May 2011 and July 2015, comprising 80 (69.0%) males and 35 (31.0%) females with a median age of 60 years (range 31–70 years). A total of 61 (53%) patients had a performance score (PS) of 0, a total of 47 patients (41%) had a PS of 1, and a total of 7(6%) had a PS of 2. In total, 54 (47%) patients never smoked, and 61 (53%) patients either previously smoked or were current smokers. A total of 37 (32%) patients were stage T1N2, a total of 66 (57%) patients were stage T2N2, and another 12 (11%) patients were stage T3N2. The predominant histological type was adenocarcinoma (65%), with a small representation of squamous carcinoma (30%). A total of 36 (31%) patients had EGFR mutations, and 79 (69%) patients had wild-type EGFR. In total, 74 (64%) patients received chemotherapy for more than 4 cycles. The characteristics of the patients are shown in Table 1.
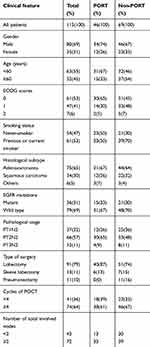 |
Table 1 Baseline characteristics of the 115 patients |
Analysis of predictors for survival
The prognostic factors of all patients are summarized in Table 2. In univariate analysis, smoking status, EGFR mutation status, number of chemotherapy cycles (>4 vs ≤4), number of total involved nodes (<2 vs ≥2), and planned radiotherapy had predictive value for both OS and PFS.
 |
Table 2 Univariate analysis of PFS and OS according to baseline characteristics |
In the multivariate analysis of all patients, as shown in Table 3, the number of chemotherapy cycles (≥4 vs <4), the total number of lymph nodes involved (<2 vs ≥2), and planned radiotherapy had significant effects on OS and PFS.
 |
Table 3 Multivariate analyses of PFS and OS according to baseline characteristics |
The median PFS of the PORCT groups was significantly longer than that of non-PORCT groups (group A vs C: 20.0 vs 12.0 months, p=0.023; group A vs D: 20.0 vs 14.0, p=0.002; group B vs C: 18.0 vs 12.0, p=0.021; group B vs D: 18.0 vs 14.0, p=0.002). The median PFS of the EGFR mutant groups was not significantly longer than that of EGFR wild-type groups (group A vs B: 20.0 vs 18.0, p=0.314; group C vs D: 12.0 vs 14.0, p=0.813) (Figure 1). The median OS of the PORCT groups was longer than that of the non-PORCT groups (group A vs C: 32.0 vs 20.0 months, p=0.007; group A vs D: 32.0 vs 20.0, p<0.001; Gro group up B vs C: 28.0 vs 20.0, p=0.017; group B vs D: 28.0 vs 20.0, p<0.001); these differences were statistically significant. The median OS of the EGFR mutant groups was not significantly longer than that of the EGFR wild-type groups (group A vs B:32.0 vs 28.0, p=0.166; group C vs D: p=0.927) (Figure 2).
 |
Figure 1 Kaplan-Meier progression-free survival curve. |
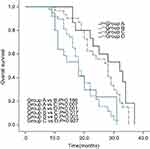 |
Figure 2 Kaplan-Meier overall survival curve. |
Compared with that of patients who underwent other types of surgery (mainly lobectomy and bilobectomy), the overall survival rate of patients who underwent pneumonectomy was lower, both in the group that received PORCT and in the group that did not receive PORCT.
Discussion
Patients who underwent complete removal of stage IIIA (N2) NSCLC are at risk of local and distant recurrence7 Adjuvant chemotherapy has been accepted as a part of the standard postoperative treatment of patients with NSCLC, including those with N2 disease.17–20 In recent years, with the significant improvement of technology, the simulation, planning and delivery of radiotherapy have been optimized. These improved techniques are useful for adjuvant radiotherapy in patients with completely resected NSCLC. Zou et al20 believe that radiotherapy combined with chemotherapy can improve the survival rate of patients. One of the most representative articles in the previous study was that from Lally et al21, who retrospectively analyzed the treatment results of NSCLC patients over 21 years old in the US SEER database from 1988 to 2002. After excluding patients with stage I or IV disease, with an unclear disease stage, and with N3 stage disease and surgical patients who died 4 months later, a total of 7545 patients were included in the analysis; 47% of the patients received postoperative radiotherapy. The results showed that, compared to no radiotherapy, the use of radiotherapy after surgery increased the 5-year overall survival rate from 20% to 27% for patients with N2 disease and reduced the risk of death by 14.5% (p=0.007), which was statistically significant. A randomized trial from Mayer et al evaluated the role of adjuvant radiotherapy in 155 patients with completely resected stage IB to IIIA NSCLC. All patients underwent modern conformal radiation treatment, which administers 50Gy in pN0 disease and 56Gy in pN1 and pN2 disease. The results showed a significant increase in terms of local control with PORT compared to without PORT.22 However, there have been many previous studies in which the results have not been satisfactory. It has also been shown that PORT causes an increase in the number of deaths unrelated to cancer. Radiation toxicity, in the form of pneumonia, esophagitis and cardiotoxicity, is the leading cause of treatment-related mortality. This is currently believed to be due to old-fashioned radiotherapy equipment, techniques and scheduling.23 At present, with the improvements made to radiotherapy equipment and personnel technology, the number of deaths caused by radiotherapy and unrelated to cancer has been greatly reduced.
In this study, postoperative radiotherapy, younger age, lobectomy, smaller tumor diameter, and lower number of positive lymph nodes were all suggested as independent factors for better prognosis for patients who underwent removal after N2 radical surgery. Due to the large number of samples analyzed by Lally et al21, their conclusions deserve our attention, suggesting that postoperative radiotherapy can improve the survival rate of N2 patients. Recently, Corso et al24 searched the National Cancer Database to find 30,552 stage II~IIIA NSCLC patients who received postoperative treatment. The 5-year OS was significantly improved in the N2 patients, and postoperative radiotherapy had a significant impact on the OS. Patients who received 45~54Gy had a greater benefit than patients who received other doses; the 5-year OS for patients who received 45~54Gy was 38%, but patients who received higher than 54Gy had the same 5-year survival as those who did not receive PORT. The range of surgical excision, extent of lymph node dissection and dose of radiotherapy affected the curative effect of postoperative radiotherapy. The RTOG standard recommends that radiation therapy be started within 9 weeks of surgery, but the precise timing remains to be studied. Our results are consistent with the abovementioned results. Furthermore, we also analyzed the effect of EGFR mutation status on patient survival. EGFR mutation status was not an independent factor that influenced long-term survival, which is consistent with the results of previous studies.25,26 We hypothesized that EGFR activation mutations cause tumor cells to proliferate faster than normal. On the one hand, the cells with EGFR mutations are highly sensitive to treatment (including EGFR-TKIs, chemotherapy, and radiotherapy), and the treatment shows high efficacy. On the other hand, EGFR mutations make the tumor cells highly invasive and prone to metastasis, and metastases may rapidly develop once the disease progresses, thus offsetting the survival advantage.
In this study, the multifactor analysis showed that the number of chemotherapy cycles and the number of metastatic lymph nodes were independent factors that influenced long-term survival. This study suggests that postoperative radiotherapy and chemotherapy were beneficial to stage IIIA (N2) NSCLC with multistation metastasis. In terms of reducing the recurrence rate and improving the disease-free survival rate, postoperative radiotherapy was of positive value to N2 stage multistation metastasis.
Conclusions
Our analysis shows that in patients with NSCLC classified as N2 nodal disease, OS and PFS could be improved by adding PORT to chemotherapy and administering chemotherapy for more than four cycles. Our analysis shows that in patients with stage IIIA (N2) NSCLC, the OS and PFS can be improved by combining chemotherapy with adjuvant radiotherapy and administering chemotherapy for more than 4 cycles. PORT can improve the survival rate and local relapse-free survival rate in patients with N2-positive multistation lymph nodes. The EGFR mutation status was not associated with long-term survival. Therefore, the efficacy of chemotherapy combined with radiotherapy indicates that this treatment method should be widely used in the clinic. However, due to our small sample size, further evaluations of patients with N2 non-small-cell lung cancer undergoing surgical resection with EGFR mutations are required for clinical research and exploration.
Abbreviation list
NSCLC, non-small cell lung cancer; PORT, Postoperative radiotherapy; OS, overall survival; EGFR, epidermal growth factor receptor mutation; ROCT, Postoperative chemotherapy; TKI, tyrosine kinase inhibitor; PCR, polymerase chain reaction; CT, computed tomography; MRI, magnetic resonance imaging; WHO, World Health Organization; PS, performance status; CTV, clinical target volume; PTV, planning target volume; FDG-PET/CT, fluorodeoxyglucose positron emission tomography computed tomography.
Acknowledgment
The authors thank Dong Guo for the support, revision and helpful suggestions.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111(6):1710–1717. doi:10.1378/chest.111.6.1710
2. Goya T, Asamura H, Yoshimura H, et al. Prognosis of 6644 resected non-small cell lung cancers in Japan: a Japanese lung cancer registry study. Lung Cancer. 2005;50(2):227–234. doi:10.1016/j.lungcan.2005.05.021
3. Sawabata N, Miyaoka E, Asamura H, et al. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol. 2011;6(7):1229–1235. doi:10.1097/JTO.0b013e318219aae2
4. Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med. 1990;323(14):940–945. doi:10.1056/NEJM199010043231403
5. Dillman RO, Herndon J, Seagren SL, Eaton WL
6. Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol. 2005;23(25):5883–5891. doi:10.1200/JCO.2005.55.405
7. Lei T, Xu XL, Chen W, Xu YP, Mao WM. Adjuvant chemotherapy plus radiotherapy is superior to chemotherapy following surgical treatment of stage IIIA N2 non-small-cell lung cancer. Onco Targets Ther. 2016;9:921–928. doi:10.2147/OTT.S95517
8. PORT Meta-analysis Trialists Group. Postoperative radiotherapy in non-small-cell lung cancer: systematic review and meta-analysis of individual patient data from nine randomised controlled trials. Lancet. 1998;352(9124):257–263.
9. Group PM-aT. Postoperative radiotherapy for non-small cell lung cancer. Cochrane Database Syst Rev. 2005;9(2):CD002142.
10. Burdett S, Rydzewska L, Tierney JF, Fisher DJ, Group PM-aT. A closer look at the effects of postoperative radiotherapy by stage and nodal status: updated results of an individual participant data meta-analysis in non-small-cell lung cancer. Lung Cancer. 2013;80(3):350–352. doi:10.1016/j.lungcan.2013.02.005
11. Douillard JY, Rosell R, De Lena M, et al. Impact of postoperative radiation therapy on survival in patients with complete resection and stage I, II, or IIIA non-small-cell lung cancer treated with adjuvant chemotherapy: the adjuvant Navelbine International Trialist Association (ANITA) Randomized Trial. Int J Radiat Oncol Biol Phys. 2008;72(3):695–701. doi:10.1016/j.ijrobp.2008.01.044
12. Robinson CG, Patel AP, Bradley JD, et al. Postoperative radiotherapy for pathologic N2 non-small-cell lung cancer treated with adjuvant chemotherapy: a review of the National Cancer Data Base. J Clin Oncol. 2015;33(8):870–876. doi:10.1200/JCO.2014.58.5380
13. Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136(1):260–271. doi:10.1378/chest.08-0978
14. Feng W, Zhang Q, Fu XL, et al. The emerging outcome of postoperative radiotherapy for stage IIIA(N2) non-small cell lung cancer patients: based on the three-dimensional conformal radiotherapy technique and institutional standard clinical target volume. BMC Cancer. 2015;15:348. doi:10.1186/s12885-015-1584-3
15. Trodella L, Granone P, Valente S, et al. Adjuvant radiotherapy in non-small cell lung cancer with pathological stage I: definitive results of a phase III randomized trial. Radiother Oncol. 2002;62(1):11–19.
16. Higgins KA, Chino JP, Berry M, et al. Local failure in resected N1 lung cancer: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 2012;83(2):727–733. doi:10.1016/j.ijrobp.2011.07.018
17. Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon J-P, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350(4):351–360. doi:10.1056/NEJMoa031644
18. Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med. 2005;352(25):2589–2597. doi:10.1056/NEJMoa043623
19. Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol. 2006;7(9):719–727. doi:10.1016/S1470-2045(06)70804-X
20. Zou B, Xu Y, Li T, et al. A multicenter retrospective analysis of survival outcome following postoperative chemoradiotherapy in non-small-cell lung cancer patients with N2 nodal disease. Int J Radiat Oncol Biol Phys. 2010;77(2):321–328. doi:10.1016/j.ijrobp.2009.05.044
21. Lally BE, Zelterman D, Colasanto JM, Haffty BG, Detterbeck FC, Wilson LD. Postoperative radiotherapy for stage II or III non-small-cell lung cancer using the surveillance, epidemiology, and end results database. J Clin Oncol. 2006;24(19):2998–3006. doi:10.1200/JCO.2005.04.6110
22. Mayer R, Smolle-Juettner FM, Szolar D, et al. Postoperative radiotherapy in radically resected non-small cell lung cancer. Chest. 1997;112(4):954–959. doi:10.1378/chest.112.4.954
23. Bekelman JE, Rosenzweig KE, Bach PB, Schrag D. Trends in the use of postoperative radiotherapy for resected non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2006;66(2):492–499. doi:10.1016/j.ijrobp.2006.04.032
24. Corso CD, Rutter CE, Wilson LD, Kim AW, Decker RH, Husain ZA. Re-evaluation of the role of postoperative radiotherapy and the impact of radiation dose for non-small-cell lung cancer using the National Cancer Database. J Thorac Oncol. 2015;10(1):148–155. doi:10.1097/JTO.0000000000000406
25. Koh Y, Jang B, Jeon YK, et al. EGFR gene copy number gain is related to high tumor SUV and frequent relapse after adjuvant chemotherapy in resected lung adenocarcinoma. Jpn J Clin Oncol. 2011;41(4):548–554. doi:10.1093/jjco/hyq248
26. Ragusa M, Vannucci J, Ludovini V, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcome in resected non-small cell lung cancer patients. Am J Clin Oncol. 2014;37(4):343–349. doi:10.1097/COC.0b013e31827a7e7a
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
