Back to Journals » Therapeutics and Clinical Risk Management » Volume 12
Radiofrequency ablation versus resection for Barcelona clinic liver cancer very early/early stage hepatocellular carcinoma: a systematic review
Authors He Z, Xiang P, Gong J, Cheng N, Zhang W
Received 20 September 2015
Accepted for publication 4 January 2016
Published 23 February 2016 Volume 2016:12 Pages 295—303
DOI https://doi.org/10.2147/TCRM.S96760
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Deyun Wang
Zhen-Xin He,1 Pu Xiang,2 Jian-Ping Gong,1 Nan-Sheng Cheng,3 Wei Zhang4
1Department of Hepatobiliary Surgery, the Second Affiliated Hospital, Chongqing Medical University, Chongqing, 2State Key Laboratory of Biotherapy and Cancer Center, 3Department of Bile Duct Surgery, West China Hospital, Sichuan University, Chengdu, 4Department of Hepatobiliary Surgery, Yue Bei People’s Hospital, Shaoguan, Guangdong, People’s Republic of China
Aim: To compare the long-term survival outcomes of radiofrequency ablation and liver resection for single very early/early stage hepatocellular carcinoma (HCC).
Methods: The Cochrane Library (Issue 3, 2015), Embase (1974 to March 15, 2015), PubMed (1950 to March 15, 2015), Web of Science (1900 to March 15, 2015), and Chinese Biomedical Literature Database (1978 to March 15, 2015) were searched to identify relevant trials. Only trials that compared radiofrequency ablation and liver resection for single very early stage (≤2 cm) or early stage (≤3 cm) HCC according to the Barcelona clinic liver cancer (BCLC) staging system were considered for inclusion in this review. The primary outcomes that we analyzed were the 3-year and 5-year overall survival (OS) rates, and the secondary outcomes that we analyzed were the 3-year and 5-year disease-free survival (DFS) rates. Review Manager 5.3 was used to perform a cumulative meta-analysis. Possible publication bias was examined using a funnel plot. A random-effects model was applied to summarize the various outcomes.
Results: Six studies involving 947 patients were identified that compared radiofrequency ablation (n=528) to liver resection (n=419) for single BCLC very early HCC. In these six studies, the rates of 3-year OS, 5-year OS, 3-year DFS, and 5-year DFS were significantly lower in the radiofrequency ablation group than in the liver resection group (risk ratio [RR] =0.90, 95% confidence interval [CI]: 0.83–0.98, P=0.01; RR =0.84, 95% CI: 0.75–0.95, P=0.004; RR =0.77, 95% CI: 0.60–0.98, P=0.04; and RR =0.70, 95% CI: 0.52–0.94, P=0.02, respectively). Ten studies involving 2,501 patients were identified that compared radiofrequency ablation (n=1,476) to liver resection (n=1,025) for single BCLC early HCC. In these ten studies, the rates of 3-year OS, 5-year OS, 3-year DFS, and 5-year DFS were also significantly lower in the radiofrequency ablation group than in the liver resection group (RR =0.93, 95% CI: 0.88–0.98, P=0.003; RR =0.84, 95% CI: 0.75–0.94, P=0.002; RR =0.72, 95% CI: 0.58–0.89, P=0.002; and RR =0.47, 95% CI: 0.33–0.67, P<0.0001, respectively).
Conclusion: The long-term survival outcomes for patients with single BCLC very early/early stage HCC appear to be superior after liver resection compared to radiofrequency ablation.
Keywords: radiofrequency ablation, liver resection, hepatocellular carcinoma, systematic review
Introduction
Hepatocellular carcinoma (HCC) ranks sixth in terms of the most common neoplasms.1–3 The age-adjusted overall incidence of HCC was ~16 cases per 100,000 individuals in 2008.1–3 HCC is highly prevalent in Asia, and its incidence is increasing in Europe and in the US.4 In recent decades, the development of diagnostic technology and the widespread screening of populations with a high risk of developing HCC have increased the detection of early stage HCC.5–7
Liver transplantation is regarded as the best curative approach for early stage HCC. However, only a small percentage of patients are offered this treatment due to high hospital costs and a shortage of liver donors.5–9 Liver resection is also a good surgical treatment for early stage HCC, and the 5-year survival rate of this procedure is >50%.6–8 However, some patients with HCC cannot undergo a liver resection due to either poor liver function or rejection. Many nonsurgical treatments have been proposed for these patients, such as radiofrequency ablation, microwave coagulation, and high-intensity focused ultrasound.10–14
The management of very early stage (Child-Pugh A, solitary ≤2 cm) and early stage (Child-Pugh A or B, solitary ≤3 cm) HCC, according to the Barcelona clinic liver cancer (BCLC) staging system, is controversial.15 Various systematic reviews and meta-analyses have confirmed the short-term efficacy of radiofrequency ablation for BCLC early stage HCC.16 The role of radiofrequency ablation in the management of BCLC very early stage HCC has not been systematically evaluated. The long-term survival outcome of patients with HCC is an important measure for the evaluation of various treatments.5–7 This systematic review compares the long-term survival outcomes of radiofrequency ablation versus liver resection for patients with single BCLC very early/early stage HCC.
Materials and methods
Study selection
The Cochrane Library (Issue 3, 2015), Embase (1974 to March 15, 2015), PubMed (1950 to March 15, 2015), Web of Science (1900 to March 15, 2015), and Chinese Biomedical Literature Database (1978 to March 15, 2015) were searched to identify trials that compared radiofrequency ablation with liver resection in the management of single BCLC very early/early stage HCC. The following keywords were used in these searches: catheter ablation, radiofrequency ablation, liver resection, hepatectomy, liver cancer, HCC, and liver neoplasm. The references of the relevant publications were also manually searched to identify any additional relevant clinical trials.
Criteria for inclusion and exclusion
The criteria for inclusion of the identified studies were as follows: 1) conformance to the BCLC staging classification: very early stage (Child-Pugh A, solitary ≤2 cm) or early stage (Child-Pugh A or B, solitary ≤3 cm); 2) comparison of the effects of radiofrequency ablation versus liver resection for single BCLC very early/early stage HCC, irrespective of etiology or the presence of liver cirrhosis or viral hepatitis; and 3) at least one outcome of interest reported.
The criteria for exclusion were as follows: 1) investigation of HCC nodules of >3 cm; 2) investigation of recurrent HCC; and 3) investigation of patients with cholangiocarcinomas or liver metastases.
Data extraction
The following data were extracted from each trial by two independent authors: 1) study design; 2) year of publication and authors; 3) number and characteristics of patients; and 4) outcome measures. The discrepancy between the two authors was resolved by discussion.
Outcomes
The primary outcomes measured in this review were the 3-year and 5-year overall survival (OS) rates. The secondary outcomes measured in this review were the 3-year and 5-year disease-free survival (DFS) rates.
Quality and publication bias assessment
The quality of each trial was evaluated using the GRADE system.17 Funnel plots were applied to evaluate publication bias.18 Both visual asymmetry and Egger’s linear regression test were used to examine the existence of publication bias.19
Statistical analysis
Review Manager 5.3 (RevMan; Cochrane Collaboration, Oxford, UK) was used to perform a meta-analysis. Statistical heterogeneity among trials was determined by the chi-square test.20 The risk ratio (RR) and 95% confidence interval (CI) were used to evaluate the treatment efficacy.20 A result was regarded as statistically significant if the P-value was <0.05.20 A random-effects model was used to summarize the various outcomes for conservative estimates. We conducted the meta-analysis and systematic review according to the Cochrane Handbook for Systematic Reviews of Interventions and Preferred Reporting Items for Systematic Reviews and Meta-Analyses.21,22
Results
Search results
A total of 4,830 records were identified by electronic searches of the Cochrane Library (n=322), Embase (n=1,847), PubMed (n=1,581), Web of Science (n=820), and Chinese Biomedical Literature Database (n=260) and a manual search of the reference lists of the included trials (n=6). We excluded 1,200 duplicated records and 3,612 irrelevant records by screening titles and abstracts. Twenty-four full-text articles were retrieved for further assessment. We excluded eleven articles for the reasons listed in Figure 1.
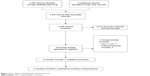  | Figure 1 Flow diagram showing study selection process. |
Description of studies
Thirteen studies published between 2008 and 2015 fulfilled the inclusion criteria, including two randomized controlled trials (RCTs) and eleven nonrandomized studies (NRS).23–35 Three studies compared radiofrequency ablation with liver resection for BCLC very early stage HCC,25,29,30 seven studies compared radiofrequency ablation with liver resection for BCLC early stage HCC,23,24,31–35 and three studies compared radiofrequency ablation with liver resection for both BCLC very early stage and early stage HCC.26–28 A total of six studies including 947 patients (528 radiofrequency ablations and 419 liver resections) compared radiofrequency ablation with liver resection for BCLC very early stage HCC.25–30 Ten studies with a total of 2,501 patients (1,476 radiofrequency ablations and 1,025 liver resections) compared radiofrequency ablation with liver resection for BCLC early stage HCC.23–25,29–35 The characteristics and quality of the included trials are presented in Tables 1 and 2.
Effects of interventions
Radiofrequency ablation versus liver resection for single BCLC very early stage HCC
The outcome measures data are presented in Table 3.
Three-year OS rate: Five studies25–27,29,30 reported this outcome. Patients in the radiofrequency ablation group had significantly lower 3-year OS rate than those in the liver resection group (RR =0.90, 95% CI: 0.83–0.98, P=0.01; heterogeneity: I2=54%, P=0.07; Figure 2A).
Five-year OS rate: Five studies25,26,28–30 reported this outcome. Patients in the radiofrequency ablation group had significantly lower 5-year OS rate than those in the liver resection group (RR =0.84, 95% CI: 0.75–0.95, P=0.004; heterogeneity: I2=50%, P=0.09; Figure 2B).
Three-year DFS rate: Four studies26,27,29,30 reported this outcome. Patients in the radiofrequency ablation group had significantly lower 3-year DFS rate than those in the liver resection group (RR =0.77, 95% CI: 0.60–0.98, P=0.04; heterogeneity: I2=69%, P=0.02; Figure 2C).
Five-year DFS rate: Four studies26,28–30 reported this outcome. Patients in the radiofrequency ablation group had significantly lower 5-year DFS rate than those in the liver resection group (RR =0.70, 95% CI: 0.52–0.94, P=0.02; heterogeneity: I2=52%, P=0.10; Figure 2D).
Radiofrequency ablation versus liver resection for single BCLC early stage HCC
The outcome measures data are presented in Table 4.
Three-year OS rate: Ten studies23,24,26–28,31–35 reported this outcome. Patients in the radiofrequency ablation group had significantly lower 3-year OS rate than those in the liver resection group (RR =0.93, 95% CI: 0.88–0.98, P=0.003; heterogeneity: I2=59%, P=0.008; Figure 3A).
Five-year OS rate: Seven studies23,26,28,31–33,35 reported this outcome. Patients in the radiofrequency ablation group had significantly lower 5-year OS rate than those in the liver resection group (RR =0.84, 95% CI: 0.75–0.94, P=0.002; heterogeneity: I2=76%, P=0.0004; Figure 3B).
Three-year DFS rate: Eight studies 24,26–28,31,32,34,35 reported this outcome. Patients in the radiofrequency ablation group had significantly lower 3-year DFS rate than those in the liver resection group (RR =0.72, 95% CI: 0.58–0.89, P=0.002; heterogeneity: I2=83%, P<0.00001; Figure 3C).
Five-year DFS rate: Six studies26,28,31–33,35 reported this outcome. Patients in the radiofrequency ablation group had significantly lower 5-year DFS rate than those in the liver resection group (RR =0.47, 95% CI: 0.33–0.67, P<0.0001; heterogeneity: I2=77%, P=0.0005; Figure 3D).
Quality of evidence and publication bias
Only one study had a moderate quality,23 and the quality of all other studies varied from low to very low (Tables 1 and 2). Thus, the current quality of evidence is considered very low. A funnel plot of the 3-year OS rate is presented in Figure 4. Neither visual asymmetry nor Egger’s linear regression test (P=0.15) of the funnel plot indicated publication bias.
  | Figure 4 Funnel plot of the 3-year disease-free survival rate. |
Discussion
This systematic review comprehensively collected the available long-term survival outcomes for radiotherapy versus liver resection for single BCLC very early/early stage HCC. The results indicated that liver resection led to significantly better long-term survival outcomes than radiofrequency ablation in the management of single BCLC very early/early stage HCC.
There are several HCC staging systems (eg, Child-Pugh, MELD, TNM, Okuda, CLIP, JIS, and BCLC),5–9 of which the BCLC staging system is the most commonly used.15 The BCLC staging system is recommended by both the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL).6,7 According to the current AASLD and EASL guidelines, liver transplantation, liver resection, and radiofrequency ablation are the recommended treatment choices for single BCLC very early/early stage HCC.6,7 There is little evidence available on the optimal management of single BCLC very early/early stage HCC.25–30 The AASLD and EASL guidelines are based on several retrospective cohort studies.6,7 The National Comprehensive Cancer Network and the Asian Pacific Association for the Study of the Liver do not mention the management of single BCLC very early stage HCC.8,9 Due to the shortcomings of liver transplantation, radiofrequency ablation and liver resection are commonly used to treat single BCLC very early/early stage HCC.24–35
Currently, the management of single BCLC very early/early stage HCC is controversial. With a 5-year OS rate of >50%, liver resection is generally considered the preferred first-line treatment.6,7 Liver resection offers the possibility of the curative excision of the entire tumor and microscopic tumor thrombi.36 Alternatively, radiofrequency ablation is a minimally invasive technique that can be performed using a percutaneous or laparoscopic approach.37 Many systematic reviews have demonstrated comparable short-term survival outcomes, lower morbidity, and shorter hospitalization in the radiofrequency ablation group when compared to the liver resection group.16 However, the quality of published systematic reviews on this topic is poor due to the low quality of evidence and high clinical heterogeneity of the included studies.16
Compared to previous systematic reviews, the major advantages of this systematic review are the use of the BCLC staging system and the evaluation of long-term survival outcomes. The findings of this review suggest that liver resection results in significantly better survival outcomes than radiofrequency ablation in the management of single BCLC very early/early stage HCC. The improvement in survival outcomes over time after liver resection may be associated with an improved understanding of liver anatomy, improved perioperative care, and increased surgical experience. Furthermore, radiofrequency ablation has some disadvantages. Radiofrequency ablation causes thermal injury and is therefore not suitable for the treatment of HCC located close to other organs (eg, gallbladder, colon, or kidney). In addition, the heat-sink effect of radiofrequency ablation complicates the complete ablation of HCC adjacent to large vessels.
Our review is subject to the following limitations. First, only two RCTs with sample sizes were included in the meta-analysis; the other studies were all NRS. Second, much of the data in this review were from NRS, and the Jadad scores of those NRS were very low; therefore, the quality of evidence is considered very low. Third, most of the included studies were conducted in Eastern countries, and thus, the results of this review are only applicable to Eastern populations.
Conclusion
In summary, this review encompasses all currently available evidence regarding the comparison of radiofrequency ablation with liver resection for single BCLC very early/early stage HCC. Based on this evidence, liver resection appears to be superior to radiofrequency ablation in terms of long-term survival outcomes. Future high-quality RCTs from Western countries are necessary to confirm our findings.
Acknowledgments
We would like to thank Jian Hu and Yao Cheng, who assisted with the development of the review.
Author contributions
He Z-X and Zhang W designed the study. Xiang P and Gong J-P performed the literature research and collected the data. Cheng N-S analyzed the data. He Z-X and Zhang W wrote the paper. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. | ||
Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379(9822):1245–1255. | ||
de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56(suppl 1):S75–S87. | ||
El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127. | ||
Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236. | ||
Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. | ||
European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. | ||
Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int. 2010;4(2):439–474. | ||
National Comprehensive Cancer Network [webpage on the Internet]. NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers. Version 2 [updated March 2015]. The NCCN Guideline. Available from: http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed January 12, 2016. | ||
Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243(3):321–328. | ||
Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57(4):794–802. | ||
Sato M, Watanabe Y, Ueda S, et al. Microwave coagulation therapy for hepatocellular carcinoma. Gastroenterology. 1996;110(5):1507–1514. | ||
Seki T, Nonaka T, Kubota Y, Mizuno T, Sameshima Y. Ultrasonically guided percutaneous ethanol injection therapy for hepatocellular carcinoma. Am J Gastroenterol. 1989;84(11):1400–1407. | ||
Ng KK, Poon RT, Chan SC, et al. High-intensity focused ultrasound for hepatocellular carcinoma: a single-center experience. Ann Surg. 2011;253(5):981–987. | ||
Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–338. | ||
Wang Y, Luo Q, Li Y, Deng S, Li X, Wei S. A systematic assessment of the quality of systematic reviews/meta-analyses in radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. J Evid Based Med. 2014;7(2):103–120. | ||
Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–382. | ||
Sterne JAC, Egger M, Moher D, editors [homepage on the Internet]. Chapter 10: addressing reporting biases. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from: www.cochrane-handbook.org. Accessed January 12, 2016. | ||
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. | ||
Deeks JJ, Higgins JPT, Altman DG, editors [homepage on the Internet]. Chapter 9: analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from: www.cochrane-handbook.org. Accessed January 12, 2016. | ||
Higgins JPT, Green S, editors [homepage on the Internet]. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from: www.cochrane-handbook.org. Accessed January 12, 2016. | ||
Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. | ||
Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252(6):903–912. | ||
Fang Y, Chen W, Liang X, et al. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29(1):193–200. | ||
Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47(1):82–89. | ||
Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56(2):412–418. | ||
Pompili M, Saviano A, de Matthaeis N, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol. 2013;59(1):89–97. | ||
Imai K, Beppu T, Chikamoto A, et al. Comparison between hepatic resection and radiofrequency ablation as first-line treatment for solitary small-sized hepatocellular carcinoma of 3cm or less. Hepatol Res. 2013;43(8):853–864. | ||
Zhou Z, Lei J, Li B, et al. Liver resection and radiofrequency ablation of very early hepatocellular carcinoma cases (single nodule <2 cm): a single-center study. Eur J Gastroenterol Hepatol. 2014;26(3):339–344. | ||
Liu PH, Hsu CY, Hsia CY, et al. Surgical resection versus radiofrequency ablation for single hepatocellular carcinoma ≤2 cm in a propensity score model. Ann Surg. Epub 2015 Mar 13. | ||
Hiraoka A, Horiike N, Yamashita Y, et al. Efficacy of radiofrequency ablation therapy compared to surgical resection in 164 patients in Japan with single hepatocellular carcinoma smaller than 3 cm, along with report of complications. Hepatogastroenterology. 2008;55(88):2171–2174. | ||
Wong KM, Yeh ML, Chuang SC, et al. Survival comparison between surgical resection and percutaneous radiofrequency ablation for patients in Barcelona Clinic Liver Cancer early stage hepatocellular carcinoma. Indian J Gastroenterol. 2013;32(4):253–257. | ||
Yang HJ, Lee JH, Lee DH, et al. Small single-nodule hepatocellular carcinoma: comparison of transarterial chemoembolization, radiofrequency ablation, and hepatic resection by using inverse probability weighting. Radiology. 2014;271(3):909–918. | ||
Kim JM, Kang TW, Kwon CH, et al. Single hepatocellular carcinoma ≤3 cm in left lateral segment: liver resection or radiofrequency ablation? World J Gastroenterol. 2014;20(14):4059–4065. | ||
Kang TW, Kim JM, Rhim H, et al. Small Hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection-propensity score analyses of long-term outcomes. Radiology. 2015;275(3):908–919. | ||
Vivarelli M, Guglielmi A, Ruzzenente A, et al. Surgical resection versus percutaneous radiofrequency ablation in the treatment of hepatocellular carcinoma on cirrhotic liver. Ann Surg. 2004;240(1):102–107. | ||
Machi J, Uchida S, Sumida K, et al. Ultrasound-guided radiofrequency thermal ablation of liver tumors: percutaneous, laparoscopic, and open surgical approaches. J Gastrointest Surg. 2001;5(5):477–489. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






