Back to Journals » Clinical and Experimental Gastroenterology » Volume 7
Radiofrequency ablation using BarRx for the endoscopic treatment of radiation proctopathy: a series of three cases
Authors Patel A, Pathak R , Deshpande V, Patel S, Wickremesinghe P, Vadada D
Received 20 April 2014
Accepted for publication 2 October 2014
Published 8 December 2014 Volume 2014:7 Pages 453—460
DOI https://doi.org/10.2147/CEG.S66534
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Andreas M. Kaiser
Anish Patel, Rahul Pathak, Vrushak Deshpande, Sunil H Patel, Prasanna C Wickremesinghe, Deepak Vadada
Department of Gastrointestinal Medicine, Richmond University Medical Center, Staten Island, NY, USA
Abstract: Radiation proctopathy is a complication of pelvic radiotherapy, which occurs in patients treated for carcinoma of the prostate, rectum, urinary bladder, cervix, uterus, and testes. If it presents within 6 weeks to 9 months after therapy, it is called acute radiation proctitis/proctopathy (ARP), and if it occurs 9 months to a year after treatment, it is classified as chronic radiation proctitis/proctopathy (CRP). CRP occurs in 5%–20% of patients receiving pelvic radiation, depending on the radiation dose and the presence or absence of chemotherapy. In many cases, CRP resolves spontaneously, but in some, it can lead to persistent rectal bleeding. Other symptoms of CRP include diarrhea, mucoid discharge, urgency, tenesmus, rectal pain, and fecal incontinence. Despite the availability of several therapies, many patients fail to respond, and continue to suffer in their quality of life. Radiofrequency ablation (RFA) is a newer endoscopic technique that uses radiofrequency energy to ablate tissue. This is an emerging way to treat radiation proctopathy and other mucosal telangiectasia. We present three cases of radiation proctopathy treated with RFA at our institute and review the literature on treatment modalities for CRP. We were also able to find 16 other cases of CRP that used RFA, and review their literature as well as literature on other treatment modalities.
Keywords: radiofrequency ablation, radiation proctopathy, BarRx, Halo catheter
Introduction
Radiation for treatment of locally advanced malignancies can lead to the development of radiation enteritis. This may present with neovascularization, ischemia, fibrosis, and even strictures, and occurs as a result of microvascular mucosal injury followed by ulceration, mucosal and serosal thickening, inflammatory cell infiltrates, and vascular sclerosis. These changes make it difficult to control the bleeding and fibrosis that occur in chronic radiation proctitis/proctopathy (CRP). Recently, Argon plasma coagulation (APC) has become one of the preferred modalities in the treatment of CRP, and many centers have reported success with treatment.1 Radiofrequency ablation (RFA) is a newer endoscopic technique that uses a catheter to deliver radiofrequency energy to ablate tissue, and in many centers has become a treatment of choice for early Barrett’s esophagus.2 The Halo® RFA system uses two different types of probes with a closely spaced arrangement of electrodes, which thermally ablate tissue. The depth (0.5–1 mm) is dependent on the power, density, and duration of contact. A generator connects to either a 360° Halo catheter or a Halo-90 catheter to provide a circumferential or more focused ablation.2 There have been some reports of the use of this treatment for patients with radiation proctopathy on whom other treatments like argon laser coagulation have failed. Only 16 such cases, at various institutions across the country, have been reported so far. We present cases of CRP treated with RFA and review literature on its management.
Case reports
Case 1
An 81-year-old male patient was treated for prostatic cancer with radiotherapy (total dose 71 Gy) 4 years ago. He presented with repeated episodes of rectal bleeding and a hematocrit of 22%. A rectosigmoidoscopy showed telangiectasia of his rectum, consistent with features of radiation proctopathy (Figure 1A).
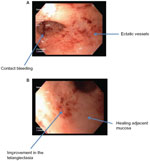  | Figure 1 Chronic radiation proctitis (case 1) on endoscopy first look (A) and second look (B). |
He went through one session of RFA and did very well after the treatment, with stabilization of his hematocrit (Figure 1B). The ablation started in the distal rectum (spanning approximately 5 cm), with a total procedure time of approximately 12 minutes. However, 2 months later, he developed deep vein thrombosis and had to be started on warfarin. Six weeks after this he developed a gastrointestinal bleed again.
Repeat rectosigmoidoscopy showed a lot of improvement in the telangiectasia and his rectal mucosa, but a second session of RFA, 3 months after the initial one, was done to prevent further bleeding (Figure 2). The second ablation also started in the distal rectum, spanning 5 cm, with a total procedure time of 12 minutes.
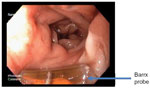  | Figure 2 Patient after second ablation with radiofrequency ablation with Barrx probe (case 1). |
His hematocrit has improved and has remained stable after the procedure. We have followed this patient up for 6 months since the first procedure. The procedure required a gastroscope for better maneuverability in the rectum to perform RFA, with a Halo-90 paddle demanding 12 J of energy. Another sigmoidoscopy done 6 months later showed marked improvement in the mucosal ectasia.
Case 2
An 83-year-old male patient with a history of prostate cancer treated with external-beam radiotherapy (total dose 74.4 Gy) in 2008 presented with rectal bleeding. His medical history was also significant for coronary artery disease with stent placement, requiring him to be on clopidogrel and aspirin. His hematocrit on presentation was 20%. Rectosigmoidoscopy showed rectal disease consistent with radiation proctopathy (Figure 3).
  | Figure 3 Radiation proctopathy (case 2). |
Focal RFA was performed, using a Halo-90 catheter at 12 J energy setting to ablate the telangiectasia in the rectum (Figure 4). The ablation started in the distal rectum (spanning approximately 7 cm), with a total procedure time of approximately 15 minutes.
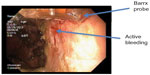  | Figure 4 Post-radiofrequency ablation with Barrx probe (case 2). |
Four months after his procedure there has been no bleeding, with his hematocrit stable at 30%–32%, and currently he is off clopidogrel but continues to take an aspirin daily.
Case 3
A third case, an 81-year-old male patient, with a history of prostatic cancer diagnosed in 2009, presented with rectal bleeding. He was treated with external-beam radiotherapy (total dose 77.3 Gy) in 2009 and presented with multiple episodes of rectal bleeding and a hematocrit of 21%. His medical history was significant for Parkinson’s disease and cerebrovascular accidents in 2006 and 2010, requiring him to be on aspirin. His rectosigmoidoscopy revealed significant telangiectasia consistent with CRP (Figure 5).
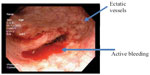  | Figure 5 Chronic radiation proctitis showing telangiectasia (case 3). |
He was treated with RFA using a Halo-90 catheter at 12 J energy setting. The ablation started in the distal rectum (spanning approximately 8 cm), with a total procedure time of approximately 20 minutes. The patient has done well since the procedure, with no further bleeding reported and a stable hematocrit of 28%–30% (Figure 6).
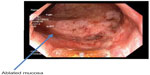  | Figure 6 Immediately post-radiofrequency ablation. |
Conclusion
RFA is an emerging technique that has been used in the treatment of Barrett’s esophagus for the last few years. Recent reports of its use for CRP have been promising, and we believe that in the future, RFA may become the preferred treatment. It is becoming more widely available, and it is easy to perform with minimal risks. More reports are needed with longer follow-up before it can be routinely recommended for CRP. Our cases have been treated primarily with RFA and have shown good results.
Discussion
Acute radiation proctitis (ARP) presents within 6 weeks of treatment and is characterized by diarrhea, urgency, tenesmus, and rectal bleeding. ARP is self-limited and caused by inflammation following radiation-induced injury to the mucosa and microvasculature. It usually resolves within 2–3 months.
CRP results from the mucosal microvascular damage from pelvic radiotherapy, and occurs in 5%–20% of patients, depending on the radiation dose and the presence or absence of chemotherapy.3 The term radiation proctopathy is a more appropriate term, although it may also extend into the sigmoid, in which case it could be called proctosigmoidopathy. Ischemia, fibrosis, neovascularization, and vascular sclerosis are the histological features seen on surgically resected specimens, which correlate with chronic ischemia, and is more severe in those who receive concurrent chemotherapy.3 CRP presents with bleeding, pain, tenesmus, diarrhea, and formation of strictures due to fibrosis. On endoscopic examination, CRP is characterized by erythema and telangiectasis, with the mucosa being edematous and friable. Continuous damage to the mucosa leads to rectal bleeding, often requiring blood transfusions.
Review of literature for treatment of radiation proctopathy
ARP is self-limiting and usually resolves after discontinuing radiotherapy. Patients with persistent symptoms may be treated with butyrate enemas.4
CRP may also resolve spontaneously within 6 months and may not require any treatment if the symptoms are mild, with minimal bleeding.5 Patients with severe symptoms have a worse prognosis and require medical or surgical management. Examples of available treatment options can range from less invasive modalities such as sulfasalazine, aminosalicylates, topical sucralfate, hyperbaric oxygen, metronidazole, and formaldehyde to more invasive procedures such as laser therapy, cryoablation, electrocoagulation, APC, and RFA are shown in Table 1. However, there have been no randomized controlled trials that can conclusively recommend one treatment over another, and thus the focus is on relieving the primary symptom.
  | Table 1 Summary table of treatment options |
Patients with CRP may develop strictures, due to fibrosis, and thus may cause mild obstructive symptoms and sometimes even complete obstruction. Stool softeners may be used for mild symptoms; however, longer strictures and those causing obstructive symptoms may need a balloon or bougie dilatation. Patients with severe strictures, intractable pain, and uncontrolled bleeding should undergo surgery. Prior adhesions in the pelvis and other radiation damage make it technically difficult. In one review of 48 patients who had been referred for severe refractory radiotherapy complications that had failed initial treatment, surgery was required. Complications such as fistula formation, permanent diversion, severe radiation enteritis, and distal colonic strictures are more likely to occur in severe radiation proctitis.6 These patients may need total rectal resection with an ileocecal pouch for good functional outcome.6–8
Patients with ulcerative symptoms have been treated using a wide range of modalities, with varying symptomatic relief reported, but none have shown to be universally successful.
Sulfasalazine and aminosalicylates can be given either orally or as an enema preparation, and has shown to be effective in some reports; however, other studies have not shown the same success.9 Rectal prednisolone enemas when added to oral sulfasalazine improve the symptoms of CRP as compared with sucralfate enemas.10
Corticosteroid enemas on their own have not been studied in a controlled manner or shown to improve the symptoms of CRP.
Topical sucralfate has shown to improve the symptoms of proctosigmoiditis and CRP in many reports. Kochhar et al randomized 37 patients with proctosigmoiditis to a 4-week course of oral sulfasalazine (3.0 g/d) plus prednisolone enemas (20 mg twice daily) or sucralfate enemas alone (2.0 g twice daily).11 Although patients in both groups improved, outcomes with sucralfate enemas were superior.11
Only one case showed improvement of symptoms with estrogen in CRP, although it has been used (with or without progesterone) for obscure gastrointestinal bleeding in patients with angiodysplasia.12
Hyperbaric oxygen has been described in several observational studies to improve the symptoms in patients with CRP.13–15 A controlled trial, which included 120 patients with refractory radiation proctitis, showed a significantly higher rate of clinical improvement in the intervention group (89% versus 63%). The study also showed the intervention group required less treatment for other symptoms; however, hyperbaric oxygen therapy remains limited to specialized centers.15
Case reports have shown short-chain fatty acids and pentosan polysulfate to be effective in the treatment of radiation proctopathy,16,17 but not in controlled trials.18,19
Metronidazole has been studied with the concurrent use of mesalamine and betamethasone in 60 patients with rectal bleeding and diarrhea. Those assigned treatment with mesalamine plus betamethasone enemas and metronidazole (400 mg orally three times daily) had a significantly lower incidence of rectal bleeding, mucosal ulcers, diarrhea, and edema at 4 weeks, 3 months, and 12 months compared with those not receiving metronidazole.20
Formaldehyde helps to stop bleeding in patients with radiation proctitis.21,22 It was shown that 4% formalin achieved hemostasis after one or two applications via sigmoidoscopy. However, the incidence of strictures and morbidity after formaldehyde applications was higher.23
Vitamin E (400 international units three times daily) and vitamin C (500 mg three times daily) were associated with improvement in diarrhea and urgency in a small, uncontrolled study with a high dropout rate involving ten patients.24
In a pilot, placebo-controlled trial involving 18 patients with CRP, Vitamin A was shown to have a better outcome than a placebo group (defined as a reduction in two or more symptoms by at least two points on a validated scale).25 More studies are needed before it can be routinely recommended.
The Nd:YAG laser penetrates to a depth of 5 mm, is well absorbed by tissue, and is a noncontact method.26,27 Three case series reported the use of Nd:YAG laser in CRP. One had a total of 19 patients and reported efficacy of 66%–100% with no complications.28–30 In another series reported, there were a total of 23 patients with CRP treated using a potassium titanyl phosphate filter with the Nd:YAG laser. There was symptomatic improvement in 65%, but two patients developed rectal ulcers.31 The Nd:YAG laser is expensive, has a lot of complications, and therefore its use has decreased over time.
Cryoablation is a noncontact method of tissue ablation by application of extreme cold temperatures to a targeted area. Cryoablation has the benefit of uniform treatment of larger surface areas and ease of targeted application. Kantsevoy et al achieved a 100% bleeding resolution by using cryotherapy in seven patients with radiation proctopathy.32 Hou et al recently reported a prospective series of ten patients with hemorrhagic CRP. Each patient underwent a single session of cryotherapy and showed 80% symptomatic improvement, 70% endoscopic improvement, and a 37% decrease in rectal telangiectasis density.33 Cryoablation has not been compared with other therapeutic modalities for CRP, and remains experimental at this time. One of the risks of the procedure is overdistension of the bowel and perforation.33 Cryotherapy requires the maintenance of liquid nitrogen or carbon dioxide, which is difficult to perform in smaller practices. The unit is less mobile compared with RFA, and the depth of ablation is more. The Halo® RFA system has a depth that is predetermined by the power, density, and duration of contact. The rapidly expanding gas requires constant elimination to prevent overdistension or bowel perforation.
Bipolar electrocoagulation (BiCap) and heater probe are widely available and relatively inexpensive compared with lasers and cryotherapy. They are contact probes and are useful in delivering focal, directed therapy to actively bleeding mucosa. However, the disadvantage is char formation at the probe tip. In a randomized prospective trial by Jensen et al, a total of 21 patients were treated either by a heater probe (n=9) or by a bipolar electrocoagulation probe (n=12). A mean of four sessions was required for either probe. In the 12 months of endoscopic treatment compared with 12 months of medical therapy, the bleeding episodes diminished significantly for both the bipolar probe and the heater probe groups. The hematocrit improved as the rate of bleeding decreased in both groups.34 There were no significant complications. However, for these modalities, the depth of coagulation is dependent on the energy setting (1, 3, 5, 7, and 9 watts), duration (2, 6, 10, and 14 seconds), and force applied (0, 50, and 100 g).35
APC uses high-frequency energy transmitted to tissue by ionized gas. In APC, the laser current is conducted through argon gas to the tissue from the tip of a monopolar electrode. The coagulation depth is variable from 0.8 mm to 3.0 mm, based upon the generator settings, gas flow rate, time applied, and distance from the tissue.36 Silva et al treated 28 patients with CRP. After a median of 2.9 sessions (range 1–8), scheduled 4 weeks apart, most of the patients had less bleeding and anemia. The improvement was by 1.2 gm/dL and 1.9 gm/dL of hemoglobin in those who presented with anemia. Other than some postprocedure rectal pain and cramps, there were no significant complications.37 APC has been shown to control bleeding that could not be managed by other methods.38,39
All of these modalities, including APC, have reported stricture formation as a complication.40
The Halo® RFA system uses two different types of probes with a closely spaced arrangement of electrodes, which thermally ablate tissue. The depth (0.5–1 mm) is dependent on the power, density, and duration of contact. A generator connects to either a 360° Halo catheter or a Halo-90 catheter to provide a circumferential or more focused ablation.2
Zhou et al have reported the use of RFA in three patients with CRP. Electrocautery and APC had failed in two of the three patients.41 The three patients studied required one or two sessions to achieve resolution of bleeding. After 19 months of follow-up, there was no ulceration or stricture formation reported. These authors analyzed the tissue structure before and after ablation using endoscopic optical coherence tomography. They noted squamous epithelialization of the ablated tissue and resolution of the ectatic vessels. Nikfarjam et al reported successfully treating a case of CRP refractory to APC therapy, using a Halo-90 RFA over three sessions.42 Follow-up at 6 months showed minimal residual mucosal abnormalities, and the patient was symptom free. Eddi and DePasquale recently reported another case of CRP treated with RFA after multiple sessions of APC to control bleeding. This was an 81-year-old patient with prostate cancer treated with radiation therapy who required a right hemicolectomy due to uncontrolled bleeding of colonic angioectasias. After only one session with RFA, there was no further bleeding, and a repeat sigmoidoscopy a month later showed normal rectal mucosa.43 A recent European collaborative study reported on eleven patients with CRP treated with RFA.44 The patients (three female and eight male) were aged 63–87. Medical history included prostate cancer,11 bladder cancer,3 and cervical cancer.5 Three patients were treated with aspirin, two with anticoagulants. Five patients had received previous APC treatment (one to five sessions), one had bipolar coagulation (one session), and two patients had laser therapy (one to five sessions). RFA was performed with the Halo-90 ablation catheter and the HaloFlex system (Covidien), with a power of 12–15 J/cm2. The patients received a mean number of 2.2 RFA sessions (range 1–4). No early complications were recorded. All patients reported an improvement of symptoms and a significant increase in the hemoglobin count from a mean of 7.1±0.6 g/dL to 10.5±1.1 g/dL (P<0.001). One patient was referred for additional APC treatment. One patient developed a fibrotic narrowing of the rectum, with no sign of obstruction.
RFA covers a broader surface area compared with APC and electrocautery. Applying pressure to the probe results in some coaptive coagulation before the application of power to ablate the tissue, leading to a more superficial burn in less time. This helps shorten treatment duration even in extensive disease. Squamous reepithelialization, which occurs after RFA, minimizes the bleeding, fibrosis, and stricture formation.45–47 This is due to the superficial depth of ablation (0.5–1 mm). Even with two ablations in the same location, the greatest depth in the colon and rectum was limited to the muscularis propria.48 This was less than with APC (less than 3 mm) and contact thermal methods (variable), and therefore much safer.49
These studies show that RFA could prove to be a useful method for refractory CRP. Our experience shows that it could even be used as the primary treatment modality for CRP. Still considered a newer method, we need more experience and longer follow-up times before recommending it as a primary treatment.
Patients with strictures, intractable pain, and uncontrolled bleeding should undergo surgery. Prior adhesions in the pelvis and other radiation damage make it technically difficult. In one review of 48 patients who had been referred for severe refractory radiotherapy complications that had failed initial treatment, surgery was required. Complications such as fistula formation, permanent diversion, severe radiation enteritis, and distal colonic strictures are more likely to occur in severe radiation proctitis.6 These patients may need total rectal resection with an ileocecal pouch for good functional outcome.6–8
Disclosure
The authors report no conflicts of interest in this work.
References
Villavicencio R, Rex D, Rahmani E. Efficacy and complications of argon plasma coagulation for hematochezia related to radiation proctopathy. Gastrointest Endosc. 2002;55:70–74. | |
Akiyama J, Triadafilopoulos G. Endoscopic ablation therapy of Barrett’s esophagus. Minerva Gastroenterol Dietol. 2010;56:405–420. | |
Pilepich MV, Krall JM, Sause WT, et al. Correlation of radiotherapeutic parameters and treatment related morbidity in carcinoma of the prostate–analysis of RTOG study 75-06. Int J Radiat Oncol Biol Phys. 1987;13:351–357. | |
Vernia P, Fracasso PL, Casale V, et al. Topical butyrate for acute radiation proctitis: randomised, crossover trial. Lancet. 2000;356:1232–1235. | |
Gilinsky NH, Burns DG, Barbezat GO, Levin W, Myers HS, Marks IN. The natural history of radiation-induced proctosigmoiditis: an analysis of 88 patients. Q J Med. 1983;52:40–53. | |
Turina M, Mulhall AM, Mahid SS, Yashar C, Galandiuk S. Frequency and surgical management of chronic complications related to pelvic radiation. Arch Surg. 2008;143:46–52; discussion. | |
Marks G, Mohiudden M. The surgical management of the radiation-injured intestine. Surg Clin North Am. 1983;63:81–96. | |
von Flue MO, Degen LP, Beglinger C, Harder FH. The ileocecal reservoir for rectal replacement in complicated radiation proctitis. Am J Surg. 1996;172:335–340. | |
Goldstein F, Khoury J, Thornton JJ. Treatment of chronic radiation enteritis and colitis with salicylazosulfapyridine and systemic corticosteroids. A pilot study. Am J Gastroenterol. 1976;65:201–208. | |
Kochhar R, Patel F, Dhar A, et al. Radiation-induced proctosigmoiditis. Prospective, randomized, double-blind controlled trial of oral sulfasalazine plus rectal steroids versus rectal sucralfate. Dig Dis Sci. 1991;36:103–107. | |
Kochhar R, Sriram PV, Sharma SC, Goel RC, Patel F. Natural history of late radiation proctosigmoiditis treated with topical sucralfate suspension. Dig Dis Sci. 1999;44:973–978. | |
Wurzer H, Schafhalter-Zoppoth I, Brandstatter G, Stranzl H. Hormonal therapy in chronic radiation colitis. Am J Gastroenterol. 1998;93:2536–2538. | |
Dall’Era MA, Hampson NB, Hsi RA, Madsen B, Corman JM. Hyperbaric oxygen therapy for radiation induced proctopathy in men treated for prostate cancer. J Urol. 2006;176:87–90. | |
Craighead P, Shea-Budgell MA, Nation J, et al. Hyperbaric oxygen therapy for late radiation tissue injury in gynecologic malignancies. Curr Oncol. 2011;18:220–227. | |
Clarke RE, Tenorio LM, Hussey JR, et al. Hyperbaric oxygen treatment of chronic refractory radiation proctitis: a randomized and controlled double-blind crossover trial with long-term follow-up. Int J Radiat Oncol Biol Phys. 2008;72:134–143. | |
al-Sabbagh R, Sinicrope FA, Sellin JH, Shen Y, Roubein L. Evaluation of short-chain fatty acid enemas: treatment of radiation proctitis. Am J Gastroenterol. 1996;91:1814–1816. | |
Grigsby PW, Pilepich MV, Parsons CL. Preliminary results of a phase I/II study of sodium pentosanpolysulfate in the treatment of chronic radiation-induced proctitis. Am J Clin Oncol. 1990;13:28–31. | |
Pilepich MV, Paulus R, St Clair W, Brasacchio RA, Rostock R, Miller RC. Phase III study of pentosanpolysulfate (PPS) in treatment of gastrointestinal tract sequelae of radiotherapy. Am J Clin Oncol. 2006;29:132–137. | |
Talley NA, Chen F, King D, Jones M, Talley NJ. Short-chain fatty acids in the treatment of radiation proctitis: a randomized, double-blind, placebo-controlled, cross-over pilot trial. Dis Colon Rectum. 1997;40:1046–1050. | |
Cavcic J, Turcic J, Martinac P, et al. Metronidazole in the treatment of chronic radiation proctitis: clinical trial. Croat Med J. 2000;41:314–318. | |
Parikh S, Hughes C, Salvati EP, et al. Treatment of hemorrhagic radiation proctitis with 4 percent formalin. Dis Colon Rectum. 2003;46:596–600. | |
Haas EM, Bailey HR, Farragher I. Application of 10 percent formalin for the treatment of radiation-induced hemorrhagic proctitis. Dis Colon Rectum. 2007;50:213–217. | |
de Parades V, Etienney I, Bauer P, et al. Formalin application in the treatment of chronic radiation-induced hemorrhagic proctitis – an effective but not risk-free procedure: a prospective study of 33 patients. Dis Colon Rectum. 2005;48:15351541. | |
Kennedy M, Bruninga K, Mutlu EA, Losurdo J, Choudhary S, Keshavarzian A. Successful and sustained treatment of chronic radiation proctitis with antioxidant vitamins E and C. Am J Gastroenterol. 2001;96:1080–1084. | |
Ehrenpreis ED, Jani A, Levitsky J, Ahn J, Hong J. A prospective, randomized, double-blind, placebo-controlled trial of retinol palmitate (vitamin A) for symptomatic chronic radiation proctopathy. Dis Colon Rectum. 2005;48:1–8. | |
Swaroop VS, Gostout CJ. Endoscopic treatment of chronic radiation proctopathy. J Clin Gastroenterol. 1998;27:36–40. | |
Wilson SA, Rex DK. Endoscopic treatment of chronic radiation proctopathy. Curr Opin Gastroenterol. 2006;22:536–540. | |
Ventrucci M, Di Simone MP, Giulietti P, De Luca G. Efficacy and safety of Nd: YAG laser for the treatment of bleeding from radiation proctocolitis. Dig Liver Dis. 2001;33:230–233. | |
Barbatzas C, Spencer GM, Thorpe SM, Sargeant LR, Bown SG. Nd: YAG laser treatment for bleeding from radiation proctitis. Endoscopy. 1996;28:497–500. | |
Leuchter RS, Petrilli ES, Dwyer RM, Hacker NF, Castaldo TW, Lagasse LD. Nd: YAG laser therapy of rectosigmoid bleeding due to radiation injury. Obstet Gynecol. 1982;59:65S–67S. | |
Taylor JG, Disario JA, Bjorkman DJ. KTP laser therapy for bleeding from chronic radiation proctopathy. Gastrointest Endosc. 2000;52:353–357. | |
Kantsevoy SV, Cruz-Correa MR, Vaughn CA, Jagannath SB, Pasricha PJ, Kalloo AN. Endoscopic cryotherapy for the treatment of bleeding mucosal vascular lesions of the GI tract: a pilot study. Gastrointest Endosc. 2003;57:403–406. | |
Hou JK, Abudayyeh S, Shaib Y. Treatment of chronic radiation proctitis with cryoablation. Gastrointest Endosc. 2011;73:383–389. | |
Jensen DM, Machicado GA, Cheng S, Jensen ME, Jutabha R. A randomized prospective study of endoscopic bipolar electrocoagulation and heater probe treatment of chronic rectal bleeding from radiation telangiectasia. Gastrointest Endosc. 1997;45:20–25. | |
Laine L. Determination of the optimal technique for bipolar electrocoagulation treatment. An experimental evaluation of the BICAP and Gold probes. Gastroenterology. 1991;100:107–112. | |
Farin G, Grund KE. Technology of argon plasma coagulation with particular regard to endoscopic applications. Endosc Surg Allied Technol. 1994;2:71–77. | |
Silva RA, Correia AJ, Dias LM, Viana HL, Viana RL. Argon plasma coagulation therapy for hemorrhagic radiation proctosigmoiditis. Gastrointest Endosc. 1999;50:221–224. | |
Tjandra JJ, Sengupta S. Argon plasma coagulation is an effective treatment for refractory hemorrhagic radiation proctitis. Dis Colon Rectum. 2001;44:1759–1765; discussion 71. | |
Taieb S, Rolachon A, Cenni JC, et al. Effective use of argon plasma coagulation in the treatment of severe radiation proctitis. Dis Colon Rectum. 2001;44:1766–1771. | |
Rustagi T, Mashimo H. Endoscopic management of chronic radiation proctitis. World J Gastroenterol. 2011;17:4554–4562. | |
Zhou C, Adler DC, Becker L, et al. Effective treatment of chronic radiation proctitis using radiofrequency ablation. Therap Adv Gastroenterol. 2009;2:149–156. | |
Nikfarjam M, Faulx A, Laughinghouse M, Marks JM. Feasibility of radiofrequency ablation for the treatment of chronic radiation proctitis. Surg Innov. 2010;17:92–94. | |
Eddi R, Depasquale JR. Radiofrequency ablation for the treatment of radiation proctitis: a case report and review of literature. Therap Adv Gastroenterol. 2013;6:69–76. | |
Dray X, Carlino A, Wengrower D, et al. Mo1619 radiofrequency ablation treatment of radiation proctitis: results from an European collaborative study. Gastrointest Endosc. 2013;77:AB448. | |
Sharma VK, Wang KK, Overholt BF, et al. Balloon-based, circumferential, endoscopic radiofrequency ablation of Barrett’s esophagus: 1-year follow-up of 100 patients. Gastrointest Endosc. 2007;65:185–195. | |
Ganz RA, Utley DS, Stern RA, Jackson J, Batts KP, Termin P. Complete ablation of esophageal epithelium with a balloon-based bipolar electrode: a phased evaluation in the porcine and in the human esophagus. Gastrointest Endosc. 2004;60:1002–1010. | |
Dunkin BJ, Martinez J, Bejarano PA, et al. Thin-layer ablation of human esophageal epithelium using a bipolar radiofrequency balloon device. Surg Endosc. 2006;20:125–130. | |
Trunzo JA, McGee MF, Poulose BK, et al. A feasibility and dosimetric evaluation of endoscopic radiofrequency ablation for human colonic and rectal epithelium in a treat and resect trial. Surg Endosc. 2011;25:491–496. | |
Smith CD, Bejarano PA, Melvin WS, Patti MG, Muthusamy R, Dunkin BJ. Endoscopic ablation of intestinal metaplasia containing high-grade dysplasia in esophagectomy patients using a balloon-based ablation system. Surg Endosc. 2007;21:560–569. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
