Back to Journals » International Journal of General Medicine » Volume 11
Pyomyositis in the setting of complicated diverticulitis: case report
Authors Sun J , Kashan DL, Auguste JM, Chendrasekhar A
Received 11 May 2017
Accepted for publication 20 October 2017
Published 22 December 2017 Volume 2018:11 Pages 11—14
DOI https://doi.org/10.2147/IJGM.S141581
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
James Sun,1 David Leor Kashan,1 Jolita Marie Auguste,1 Akella Chendrasekhar2
1Department of Surgery, State University of New York, Downstate Medical Center, Brooklyn, NY, USA; 2Department of Surgery, Richmond University Medical Center, Staten Island, NY, USA
Abstract: Pyomyositis is typically thought of as a disease of the tropics. However, it is becoming more prevalent in temperate regions, and may be underdiagnosed. Here, pyomyositis is encountered as a complication of perforated diverticulitis, which has not been previously reported. A 61-year-old Caucasian man initially presented in respiratory distress and was diagnosed with respiratory failure due to COPD exacerbation. The patient was taking high-dose prednisone, 60 mg daily for the past 2 years. Initially, he was afebrile, normotensive, tachycardic to 178 beats/minute and tachypneic to 28 breaths/minute, requiring noninvasive ventilation to maintain oxygenation. Blood tests revealed leukocytosis of 16.7×103/μL, and blood cultures grew Escherichia coli. Broad-spectrum antibiotics were started but leukocytosis and bacteremia persisted on repeated tests. On the seventh hospital day, a CT scan of the abdomen was performed for complaints of abdominal pain, and the patient was diagnosed with Hinchey stage 3 diverticulitis. A Hartmann’s procedure was performed with intraoperative findings of purulent peritonitis. Intraoperative cultures grew E. coli and vancomycin-resistant Enterococcus faecium. The patient continued to have leukocytosis of 15.1×103/μL despite surgical therapy. He began to complain of left lower extremity pain, and a CT scan on hospital day 24 revealed gluteal intramuscular abscesses, which were percutaneously drained. Persistent symptoms prompted another CT scan on hospital day 28, which revealed additional intramuscular abscesses in the vastus lateralis muscle, which was also drained, with subsequent resolution of pain and normalization of inflammatory markers. This is the first case demonstrating pyomyositis as a complication of diverticulitis. While the mechanism of pyomyositis may not be unique, it is important to recognize the potential complications of frequently encountered diseases. In this critically ill and immunosuppressed patient, there was delayed diagnosis of both diverticulitis and pyomyositis, but the patient quickly improved once the diseases were recognized and treated.
Keywords: abscess, immunosuppression, steroid use, COPD, intramuscular infection
Introduction
Pyomyositis is defined as an acute, primary, intramusuclar infection that is hematogenously disseminated from transient bacteremia.1,2 This condition was first described by Scriba as a disease of the tropics.1–4 It was nearly 100 years before the first reported case in North America by Levin et al and its prevalence has been increasing.5 To the author’s knowledge, it has only been associated with a surgical disease of the abdomen in a report described by Ea et al as a complication of laparoscopy.6 We submit a case of pyomyositis as an adverse outcome of complicated diverticulitis to underscore the importance of this disease as a consideration in a surgical patient.
Case report
A 61-year-old Caucasian male initially presented with COPD exacerbation with delirium, visual hallucinations and altered mental status. The patient had been taking high-dose prednisone 60 mg daily for 2 years for COPD. On presentation, the patient was afebrile with a temperature of 98.3°F, normotensive with a blood pressure of 140/85, tachycardic to 178 beats/minute and tachypneic to 28 breaths/minute, with oxygen saturation of 98% on bilevel positive airway pressure ventilation. Blood tests were significant for leukocytosis of 16.7×103/μL, with a left shift of 89.1% and a lactic acid of 5.8 mmol/L. He was admitted to the medical service for respiratory failure secondary to COPD exacerbation. The patient was treated with piperacillin/tazobactam and vancomycin empirically. Leukocytosis improved and blood cultures from admission grew Escherichia coli resistant to ampicillin and levofloxacin, confirmed on repeated blood culture.
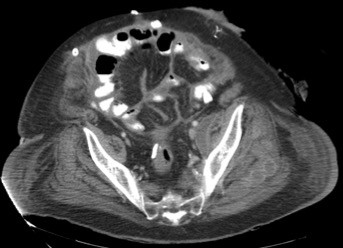
On the fifth hospital day, the source of bacteremia was not identified and routine evaluations of the urinary tract and lungs failed to show evidence of infection. The patient had complaints of left flank pain; however, with improved leukocytosis and no other sources of infection, work-up was deferred. On the seventh hospital day, persistent abdominal pain prompted CT imaging of the abdomen, which revealed a 14×3.7×7.2 cm abscess along the medial wall of the left psoas muscle (Figure 1) and 2 additional abscesses at the L5–S1 level; 5.4×3.6 cm in the midline and 3.4×2.3 cm left of midline, and intramuscular air in the left gluteal muscles. The patient was diagnosed with Hinchey stage 3 complicated diverticulitis and the surgery service was consulted. A Hartmann’s procedure and abdominal washout was performed, with intraoperative findings of purulent peritonitis. The patient was taken back to the operating room for two additional abdominal washouts during the following week.
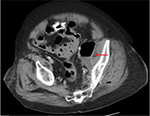  | Figure 1 Initial CT-scan showing abscess with air–fluid level abutting left psoas muscle, indicated by red arrow. |
Cultures of the peritoneal fluid from the initial procedure revealed E. coli and vancomycin-resistant Enterococcus faecium (VRE). Post-operatively, leukocytosis persisted, reaching a peak of 29.2×103/μL and nadir of 15.1×103/μL despite antibiotic therapy. We subsequently repeated a CT-scan on hospital day 24, which was consistent with multiple intramuscular abscesses in the gluteal muscles for which he underwent percutaneous drainage (Figure 2).
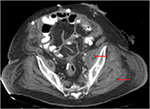  | Figure 2 Repeat CT-scan showing abscesses in the left gluteal muscles, indicated by red arrows. |
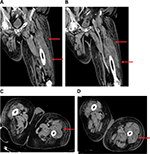
Following drainage, leukocytosis remained the same, and the patient began complaining of left lower extremity pain, which was edematous and erythematous on physical examination. After deep venous thrombosis was ruled out, a CT scan was performed on hospital day 28, which showed a 10×20×3 cm intramuscular abscess in the vastus lateralis muscle, again treated with percutaneous drainage (Figure 3A–D). Following drainage, cultures were negative, and ultrasound confirmed resolution of abscesses. The patient’s symptoms resolved, leukocytosis normalized to 7.7×103/μL and the patient was discharged to a rehabilitation facility on hospital day 34.
Written informed consent was obtained from the patient and family members to use anonymized information from the medical record, including, but not limited to, images and laboratory values. The patient and family granted permission for the publication of all anonymized information.
Discussion
Pyomyositis is primarily caused by Staphylococcus aureus, accounting for up to 95% of reported cases.1–6 As mentioned before, it is a primary intramuscular infection, not seeded by penetrating trauma or adjacent soft tissue or bone infections. A plethora of pathogens have been described in prior literature, including both gram positives, negatives and even parasites and viruses.5 Gram positives are more frequently isolated, while E. coli is the most common gram negative.8 In our case, pyomyositis was caused by simultaneous E. coli and VRE infections, consistent with bowel flora as the initial source from perforated diverticulitis. In non-tropical areas, pyomyositis is uncommon, primarily affecting immunosuppressed, elderly patients. This disease is commonly associated with trauma and the immunodeficient state, which may include HIV, steroid use, diabetes mellitus, and neutropenia of any cause.1–2 Large muscles of the lower extremity are more commonly involved: the quadriceps, gluteal and iliopsoas muscles being the most common sites.2,4 Muscles remote to this location have been affected, including the sternocleidomastoid, erector spinae, and even muscles of the head and neck.4
Pyomyositis is divided into three stages. The first stage (invasive) typically lasts 2 weeks, with general symptoms of swelling, erythema, with or without pain or signs on radiologic imaging. The second stage (purulent) is when the disease is usually diagnosed, as in the case presented. Patients may have high fever, chills, sepsis with local tenderness, swelling, fluctuance over affected muscles, myalgia and inflammatory skin changes. In the third stage (late), the disease spreads systemically with worsening of local symptoms. Complications can occur at this stage, including rhabdomyolysis, hematologic spread of abscesses, arthritis and toxic shock syndrome from S. aureus.1,2,4
A high clinical suspicion must be maintained because imaging may not show discrete abscesses early in the course of the disease. Initial CT imaging in our patient showed gas in the gluteal muscles, which should have prompted further investigation. CT imaging in the first stage of the disease may only show swelling of the involved muscles. When abscesses are present, they appear as fluid collections with rim enhancement. MRI has been proven to be the most sensitive imaging modality in the diagnosis of this disease. It shows hyperintense signals on T2-weighted images and a hyperintense rim on enhanced T1-weighted images and peripheral enhancement after gadolinium administration. CT imaging and ultrasound can aid with diagnosis and treatment.4,7
Treatment of pyomyositis includes antimicrobial medical therapy and surgical or image-guided percutaneous drainage. If discovered in the invasive stage, antibiotic therapy alone is sufficient for treatment.6 Once the disease has progressed to the second or third stages, surgical or percutaneous drainage is necessary in conjunction with antibiotic therapy. Antibiotic therapy should be tailored to the speciation of cultures obtained from the abscesses.4,7
Conclusion
In this case, pyomyositis was secondary to bacteremia caused by purulent peritonitis from perforated diverticulitis evidenced by the isolation of bowel flora found intraoperatively in each intramuscular abscess. Several diagnostic challenges were present during this case. First, the diagnosis of diverticulitis was delayed, and the duration of perforation was unknown. The patient’s immunosuppressed state obscured the usual clinical findings of diverticulitis, and the patient had a minimum of 5 days of purulent peritonitis before the disease was recognized and treated. Second, pyomyositis was not recognized, nor expected in the management of this disease. Several other diagnoses for extremity swelling and pain were explored before a CT scan was performed, revealing intramuscular abscesses. Once the abscesses were definitively treated with percutaneous drainage, the patient rapidly improved. Pyomyositis has not previously been described as a complication of complicated diverticulitis, and our experience shows that if unrecognized, can significantly prolong hospitalization and potentially lead to further complications.
Acknowledgments
The authors acknowledge and thank Richmond University Medical Center and the State University of New York Downstate Medical Center for their support in writing this report.
Disclosure
The authors report no conflicts of interest in this work.
References
Crum NF. Bacterial Pyomyositis in the United States. Am J Med. 2004;117(6):420–428. | ||
Crum-Cianflone NF. Bacterial, fungal, parasitic and viral myositis. Clin Microbiol Rev. 2008;21(3):473–494. | ||
Scriba J. Beitrag zur aetiologie der myositis acuta. Dtsch Z Chir. 1885;22:497–502. | ||
Comegna L, Guidone PI, Prezioso G, et al. Pyomyositis is not only a tropical pathology: a case series. J Med Case Rep. 2006;10(1):372. | ||
Levin MJ, Gardner P, Waldvogel FA. Pyomyositis: an unusual infection due to Staphylococcus aureus. N Engl J Med. 1971;284:196–198. | ||
Ea HK, Zeller V, Chicheportiche V, Desplaces N, Ziza JM. Polybacterial pyomyositis following laparoscopic colectomy for complicated diverticulosis. Joint Bone Spine. 2007;74(6):653–655. | ||
Drosos G. Pyomyositis. A literature review. Acta Orthop Belg. 2005;71(1):9–16. | ||
Khan S, Khan MA, Iqbal J, Saleem S. Pyomyositis, frequency and its common bacteria with their antibiotic sensitivity among children with highly suspected clinical features. Professional Med J. 2017;24(1):188–194. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.