Back to Journals » Clinical Interventions in Aging » Volume 13
Psychological effects of exercise on community-dwelling older adults
Authors Tada A
Received 30 September 2017
Accepted for publication 19 December 2017
Published 13 February 2018 Volume 2018:13 Pages 271—276
DOI https://doi.org/10.2147/CIA.S152939
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Walker
Akio Tada
Faculty of Health Science, Hyogo University, Kakogawa, Hyogo, Japan
Background: In recent years, there have been an increasing number of older adults who suffer from mental disorders globally.
Objective: The objective of this study was to examine the effect of an intervention that consisted of an exercise program to improve the mental health of community-dwelling older adults.
Participants and methods: The recruited participants of this study were community-dwelling older adults aged ≥60 years who participated in a comprehensive health promotion program in Kakogawa, Japan. Participants in the intervention group received an exercise program that was developed for older adults using Thera-Band. To measure participants’ mental health status, a Japanese version of the short form of the Profile of Mood States (POMS-SF) was used. Stress markers were measured, such as salivary cortisol, alpha-amylase, and sIgA levels. All participants provided salivary samples and completed psychological questionnaires at baseline and 6-month follow-up.
Results: No significant differences were observed between the intervention and control groups with respect to POMS-SF score and salivary biomarker profile at baseline. After the intervention, the intervention group showed a significant decrease in the POMS-SF “fatigue” score and cortisol level. No significant changes were observed in the control group.
Conclusion: Simultaneous changes in feelings of fatigue and cortisol levels were observed among subjects who had received the intervention of regular exercise. Further research is needed to investigate the effectiveness of exercise intervention in improving mental health among older adults.
Keywords: intervention, exercise, psychological status, stress, cortisol
Introduction
In recent years, the number of older adults who suffer from mental disorders has been increasing globally.1 Common mental disorders in older adults include depression and anxiety, and because of their severe consequences, they should be regarded as an important public health problem. Depression and anxiety in the elderly are associated with medical comorbidities and cognitive decline, in addition to increased risks of dementia, suicide risk, and overall mortality.2–4
The possible association between physical activity and psychological state has been discussed for several years. Many studies have accumulated evidence concerning improvements in the mental health of older adults as a result of exercise/physical activity. An intervention based on exercise has been shown to reduce depression severity in older adults.5 A measurable effect of interventions of mixed aerobic and anaerobic exercise sessions, which are moderate intensity, on depression has already been observed.6 It has been reported that regular physical activity may be effective for improving anxiety symptoms in older adults.7 On the other hand, exercise intervention appeared to have limited effect on reducing the fear of falling.8
Some community-dwelling older adults, although not mentally ill, suffer from stress, although the severity of this differs. There is a possibility that they have some disturbance in their mood state, which may proceed to develop into a mental disorder. It is therefore necessary to prevent people who are in good mental health from developing a mental disorder. Population aging has increased public interest in maintaining a healthy and high-quality life. Exercise is considered as one of the means for the promotion of good mental health and quality of life and lifestyle. Therefore, more studies evaluating the effect of exercise on mood and stress of the general population are necessary. It was reviewed that physical activity have improved mental health of patients with psychological distress.5
In general, community-dwelling older adults show low rates of participation in physical activities due to poor access to facilities that provide exercise programs; in addition, the economic burden of attending these programs can also be a barrier. Moreover, it is difficult to perform physical training with an exercise apparatus at home. Therefore, exercise programs that are properly designed for an individual’s physical conditions and that can be easily performed at home are required. For example, elastic bands are easily used, portable, and inexpensive, and they are widely used to develop muscle strength and power.9 Thera-Band, one such type of elastic band, is known to be a device that makes it possible to do physical training at home, and studies assessing the effect of training using the Thera-Band have been carried out in recent years.10,11 Resistance training using Thera-Band has shown effects on enhancement of muscle strength in older adults.12,13
The primary objective of this study was to investigate the intervention effect of exercise using Thera-Band on mood state and levels of stress-related markers in community-dwelling older adults who participated in a comprehensive health promotion program. In addition, the study aimed to evaluate the association between psychological improvements and biological changes.
Participants and methods
Participants
A total of 61 male and female adults aged ≥60 years who were living independently were enrolled in the study. Participants were recruited from people enrolled in a comprehensive health promotion program conducted by Hyogo University at three community centers in Kakogawa city during 2015–2016. A pragmatic approach was taken to the allocation of participants to the intervention and control groups because community centers were unwilling to be randomized. Participants in the health promotion services were allocated to either group (intervention and control) based on their community center. All participants were Japanese and lived in Kakogawa city. The exclusion criteria were as follows: 1) physically and/or mentally disabled people, and 2) people who could not understand Japanese.
Intervention
Participants in the intervention group received a resistance exercise program devised for older adults using Thera-Band (The Hygenic Corp., Akron, OH, USA). This program included the use of Thera-Band when performing knee flexion to extension while sitting, standing from sitting, elbow flexion to extension while sitting, hip flexion to extension while standing, or hip extension to flexion while standing. The intensity was adjusted with the perceived extension rated at 12–13. Contents of the exercise program were provided through media such as booklets and/or DVDs. Subjects were asked to practice the exercise program at home for 20 minutes twice per week for 6 months. Participants in the control group were asked to keep to their normal daily activities and were requested to avoid starting a new physical activity. A reminder letter was sent to participants in the intervention group at 3 months to remind them to continue with the program.
Measures
All participants provided salivary samples and completed psychological questionnaires (short form of the Profile of Mood States [POMS-SF]) at baseline and 6-month follow-up.
Salivary biomarkers
Saliva samples were collected at the beginning of the comprehensive health promotion program, at 10 am prior to start of exercise, by having participants chew on a cotton roll (Salivettes; Sarstedt AG & Co., Nümbrecht, Germany) for 2 minutes. They were requested to abstain from smoking, food, and strenuous physical activity for 1 hour before each saliva collection. After collection, the specimens were immediately transferred to storage tubes and stored in a freezer. Analysis of specimens was performed at Yanaihara Institute Inc. (Fujinomiya, Shizuoka, Japan) using the Salivary EIA Kit.
POMS-SF
To measure participants’ mental health status, the Japanese version of the POMS-SF14,15 was used. This instrument has achieved wide acceptance as a self-reported measure for assessing psychological distress in a variety of healthy and physically and mentally ill populations in Japan. The POMS-SF consists of 30 items grouped into six subscales: tension–anxiety, depression–dejection, anger–hostility, vigor, fatigue, and confusion. Standardized scores for each item ranged from 20 to 85 using a 5-point Likert scale. The reliability of the POMS-SF and its subscales has been estimated by Cronbach’s alpha values, which ranged from 0.57 to 0.88 (p<0.01) in a study by Yokoyama,15 which indicated that POMS-SF has a fairly high reliability. The internal consistency of the estimates for this study was quite high across all samples and subscales. The value of Cronbach’s alpha was 0.94 for the total mood disturbance score and ranged from 0.84 to 0.95 for each of the six subscales. Thus, the POMS-SF was reasonable to use for this study.
Statistical analysis
Comparisons of participants’ demographics in the intervention and control groups were done using chi-square tests. Baseline data on POMS-SF and salivary biomarkers of both groups were compared using the Mann–Whitney U test. Wilcoxon signed-rank tests were used to compare differences in the groups’ scores at baseline and 6-month follow-up. Correlations between changes in cortisol level and in POMS-SF “fatigue” score were assessed using Pearson correlation coefficients. Statistical analyses were performed using SPSS version 23, and differences with p<0.05 were considered as statistically significant.
Ethical considerations
This study was approved by the Hyogo University Ethics Committee (No 14007). All subjects participated on a voluntary basis and provided written informed consent prior to data collection.
Results
The exercise program was applied over a period of 6 months to 32 older adults. Retention was very high for both the intervention and control groups (6.3% withdrew from the intervention group and 3.4% from the control group) with two participants from the intervention group and one participant from the control group unavailable for follow-up.
Table 1 gives the distribution of sex and age in the intervention and control groups. Participants ranged in age from 60 to 87 years with an average age of 70.9±5.9 years (intervention: 70.5±5.5 years and control: 71.3±6.3 years) and were predominantly female (52.4%). The age and sex distributions did not differ significantly between groups.
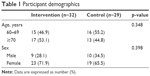  | Table 1 Participant demographics |
Differences in the POMS-SF score between the intervention and control groups at baseline were all nonsignificant, indicating equivalency (Table 2). Overall, the mean values of POMS-SF subscales for both groups were not high.
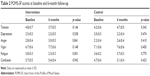  | Table 2 POMS-SF scores at baseline and 6-month follow-up |
In the intervention group, from baseline to 6-month follow-up, the scores for tension–anxiety, depression–dejection, fatigue, and confusion decreased and scores for anger–hostility and vigor increased (Table 2). However, only for “fatigue”, this change reached statistical significance. In the control group, although all scores on subscales had increased at the 6-month follow-up compared to baseline, none were significant.
The salivary cortisol levels significantly decreased from baseline to 6-month follow-up in the intervention group (Table 3). The salivary levels of alpha-amylase and sIgA also decreased, but these changes did not reach statistical significance. In the control group, all measured biomarkers also decreased from baseline to 6-month follow-up, but none of the changes in the salivary levels of biomarkers reached statistical significance.
  | Table 3 Levels of cortisol, alpha-amylase, and sIgA at baseline and 6-month follow-up |
To explore a possible association between changes in biomarker levels and psychological changes, the correlation between changes in cortisol levels and in “fatigue” scores was calculated. No significant correlation was observed.
Discussion
In this investigation of the effectiveness of an exercise program on psychological status and salivary stress biomarkers in the setting of community health service centers, the ease of doing exercise with the use of elastic bands contributed to a lower dropout rate in the intervention group. The ease of using an elastic band may be an advantage for promoting exercise among older adults.
Exercise had a positive impact on the mental health of some older adults in the intervention group, as measured by the “fatigue” dimension of the POMS-SF. Stress caused by daily life can be manifested in different moods, and the subscales of the POMS-SF are considered to represent these. Exercise is expected to positively modify these moods. Participants in the present study were more likely to experience a change in the “fatigue” subscale than in the other expressions of mood in the POMS-SF. It is hypothesized in the present study that exercise contributed to improvement in the participants’ physical functions of daily life and thus prevented feelings of fatigue. Feeling fatigued is a primary, personal feeling that can be concomitant with other cognitive functions such as not wanting to continue or initiate a task.16 Feeling fatigued is common in older adults. The finding that chronic exercise increased feelings of energy and decreased feelings of fatigue has been reported in many epidemiological studies and is not limited to older adults.17 It was concluded in a recent review that exercise can decrease cancer-related fatigue among cancer patients of all ages.18 Several other studies also reported an improvement in the “fatigue” dimension of the POMS-SF as a result of physical activity.19,20 However, these studies differed from the present study in that they included subjects with some type of disorder. It has been reported that levels of habitual physical activity are lower in older adults with greater self-reported fatigue.21 Our results confirm results from another study in which older adults (mean age of 58±5 years) who had an intervention with resistance band exercise similarly showed an improvement in feelings of fatigue.22
No significant changes were observed in the other subscales of the POMS-SF. The scores for “depression” and “anger” at baseline might have been too low to detect any significant reduction after 6 months. There have been studies reporting significant improvements in scores for tension, vigor, and confusion by providing exercise to study subjects.23–27 Further research on this association is needed in older adults who, on average, have higher baseline scores for these subscales.
A statistically significant decrease was observed in the salivary levels of cortisol, which is considered to be secreted when human beings experience stress, in the intervention group but not in the control group. It has previously been reported that salivary cortisol levels were higher in subjects who experienced chronically higher stress than in those with lower stress.28–30 Decreases in cortisol levels in the intervention group in the present study may have been a consequence of decreased stress as a result of regular exercise introduced as the intervention. Previous studies also reported that an intervention of exercise lowered cortisol levels, which is consistent with the results of the present study.31–33 However, there have been no studies that reported simultaneous changes in feelings of fatigue and cortisol levels among subjects who had received an intervention of regular exercise. As such, we hypothesize that changes in mood state may have a direct effect on secretion of substances such as cortisol. Data from the present study may help elucidate the involvement of exercise in the reduction of fatigue.
No significant change in levels of alpha-amylase and IgA was seen in either group. There have been studies that reported insignificant effects of long-term exercise intervention on alpha-amylase level, which support the present study.34–36 In contrast, however, Furtado et al37 reported that an intervention of chair yoga exercise reduced alpha-amylase levels in institutionalized older adults. It has also been reported that older adults who received an intervention of exercise experienced an increase in salivary IgA levels.38–41 In another study, there was no significant change in cortisol levels, although there was a significant change in the level of sIgA,38 which contradicts the findings of the present study. This inconsistency may suggest that secretion of stress-related substances could be controlled by several complicated mechanisms.
Effects of physical activity on the improvement of mental health in patients with psychological disease are reviewed.5 However, there are only a few studies42,43 that describe the effect of exercise on the mental health of community-dwelling older adults. Field studies of community-dwelling older adults are useful for elucidating causes of mental diseases and disorders and for developing methods to prevent them. To this end, the present study contributes to the accumulation of knowledge on how to improve the mental health of community-dwelling older adults.
The present study has limitations that must be addressed. First, the study was not a randomized control trial (RCT). People in the government premises such as community centers are difficult to recruit for RCTs because participants in the intervention group, who complete a voluntary exercise program, possibly have higher awareness about exercise and their own health compared to participants in the control group. Second, the present study included a relatively small sample size. Analyses with small sample sizes have the risk of overestimating the impact of associations between items included in the statistical analyses. Third, subjects in the present study were considered to have higher awareness of their health because they participated in physical activities conducted in community centers, and therefore, the results of this study cannot necessarily be applied to other community-dwelling populations.
Conclusion
Simultaneous changes in feelings of fatigue and cortisol levels were observed among subjects who had received an intervention of regular exercise with a Thera-Band. Further research is needed to investigate effectiveness of exercise intervention in improving mental health in older adults.
Acknowledgment
This study was supported by JSPS KAKENHI Grant No 26350857.
Disclosure
The author reports no conflicts of interest in this work.
References
WHO. Mental Health and Older Adults. 2016. Available from: http://www.who.int/mediacentre/factsheets/fs381/en/. Accessed January 19, 2018. | ||
Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58(3):249–265. | ||
Brailean A, Comijs HC, Aartsen MJ, et al. Late-life depression symptom dimensions and cognitive functioning in the Longitudinal Aging Study Amsterdam (LASA). J Affect Disord. 2016;201:171–178. | ||
Mirza SS, Wolters FJ, Swanson SA, et al. 10-year trajectories of depressive symptoms and risk of dementia: a population-based study. Lancet Psychiatry. 2016;3(7):628–635. | ||
Bridle C, Spanjers K, Patel S, Atherton NM, Lamb SE. Effect of exercise on depression severity in older people: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry. 2012;201(3):180–185. | ||
Schuch FB, Vancampfort D, Rosenbaum S, et al. Exercise for depression in older adults: a meta-analysis of randomized controlled trials adjusting for publication bias. Rev Bras Psiquiatr. 2016;38(3):247–254. | ||
Mochcovitch MD, Deslandes AC, Freire RC, Garcia RF, Nardi AE. The effects of regular physical activity on anxiety symptoms in healthy older adults: a systematic review. Rev Bras Psiquiatr. 2016;38(3):255–261. | ||
Kendrick D, Kumar A, Carpenter H, et al. Exercise for reducing fear of falling in older people living in the community. Cochrane Database Syst Rev. 2014;(11):CD009848. | ||
McMaster D, Cronin J, McGuigan M. Forms of variable resistance training. J Strength Cond Res. 2009;31:50–64. | ||
Uchida MC, Nishida MM, Sampaio RA, Moritani T, Arai H. Thera-band(®) elastic band tension: reference values for physical activity. J Phys Ther Sci. 2016;28(4):1266–1271. | ||
Colado JC, Garcia-Masso X, Triplett NT, et al. Construct and concurrent validation of a new resistance intensity scale for exercise with thera-band® elastic bands. J Sports Sci Med. 2014;13(4):758–766. | ||
Lin SF, Sung HC, Li TL, et al. The effects of Tai-Chi in conjunction with thera-band resistance exercise on functional fitness and muscle strength among community-based older people. J Clin Nurs. 2015;24(9–10):1357–1366. | ||
Delshad M, Ghanbarian A, Mehrabi Y, Sarvghadi F, Ebrahim K. Effect of strength training and short-term detraining on muscle mass in women aged over 50 years old. Int J Prev Med. 2013;4(12):1386–1394. | ||
Curran SL, Andrykowski MA, Studts JL. Short form of the profile of mood states psychometric information. Psychol Assess. 1995;7:80–83. | ||
Yokoyama K. Profile of Mood States, Short Form in Japanese Version, Guideline and Case Studies. Tokyo, Japan: Kaneko Publishers; 2005:1–105. | ||
Berrios GE. Feelings of fatigue and psychopathology: a conceptual history. Compr Psychiatry. 1990;31(2):140–151. | ||
Puetz TW, O’Connor PJ, Dishman RK. Effects of chronic exercise on feelings of energy and fatigue: a quantitative synthesis. Psychol Bull. 2006;132(6):866–876. | ||
Scott K, Posmontier B. Exercise interventions to reduce cancer-related fatigue and improve health-related quality of life in cancer patients. Holist Nurs Pract. 2017;31(2):66–79. | ||
Hsu CY, Moyle W, Cooke M, Jones C. Seated T’ai Chi in older Taiwanese people using wheelchairs: a randomized controlled trial investigating mood states and self-efficacy. J Altern Complement Med. 2016;22(12):990–996. | ||
Oka T, Tanahashi T, Chijiwa T, Lkhagvasuren B, Sudo N, Oka K. Isometric yoga improves the fatigue and pain of patients with chronic fatigue syndrome who are resistant to conventional therapy: a randomized, controlled trial. Biopsychosoc Med. 2014;8(1):27. | ||
Nicklas BJ, Beavers DP, Mihalko SL, Miller GD, Loeser RF, Messier SP. Relationship of objectively-measured habitual physical activity to chronic inflammation and fatigue in middle-aged and older adults. J Gerontol A Biol Sci Med Sci. 2016;71(11):1437–1443. | ||
Smith MF, Ellmore M, Middleton G, Murgatroyd PM, Gee TI. Effects of resistance band exercise on vascular activity and fitness in older adults. Int J Sports Med. 2017;38(3):184–192. | ||
Halpern J, Cohen M, Kennedy G, Reece J, Cahan C, Baharav A. Yoga for improving sleep quality and quality of life for older adults. Altern Ther Health Med. 2014;20(3):37–46. | ||
Katsura Y, Yoshikawa T, Ueda SY, et al. Effects of aquatic exercise training using water-resistance equipment in elderly. Eur J Appl Physiol. 2010;108(5):957–964. | ||
Cassilhas RC, Viana VA, Grassmann V, et al. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc. 2007;39(8):1401–1407. | ||
Galantino ML, Shepard K, Krafft L, et al. The effect of group aerobic exercise and t’ai chi on functional outcomes and quality of life for persons living with acquired immunodeficiency syndrome. J Altern Complement Med. 2005;11(6):1085–1092. | ||
O’Connor PJ, Puetz TW. Chronic physical activity and feelings of energy and fatigue. Med Sci Sports Exerc. 2005;37(2):299–305. | ||
Steptoe A, Cropley M, Griffith J, Kirschbaum C. Job strain and anger expression predict early morning elevations in salivary cortisol. Psychosom Med. 2000;62(2):286–292. | ||
Ockenfels MC, Porter L, Smyth J, Kirschbaum C, Hellhammer DH, Stone AA. Effect of chronic stress associated with unemployment on salivary cortisol: overall cortisol levels, diurnal rhythm, and acute stress reactivity. Psychosom Med. 1995;57(5):460–467. | ||
Powell LH, Lovallo WR, Matthews KA, et al. Physiologic markers of chronic stress in premenopausal, middle-aged women. Psychosom Med. 2002;64(3):502–509. | ||
Najafi P, Moghadasi M. The effect of yoga training on enhancement of adrenocorticotropic hormone (ACTH) and cortisol levels in female patients with multiple sclerosis. Complement Ther Clin Pract. 2017;26:21–25. | ||
Chaturvedi A, Nayak G, Nayak AG, Rao A. Comparative assessment of the effects of hatha yoga and physical exercise on biochemical functions in perimenopausal women. J Clin Diagn Res. 2016;10(8):KC01–KC04. | ||
Ida M, Ida I, Wada N, Sohmiya M, Tazawa M, Shirakura K. A clinical study of the efficacy of a single session of individual exercise for depressive patients, assessed by the change in saliva free cortisol level. Biopsychosoc Med. 2013;7(1):18. | ||
Kusaka M, Matsuzaki M, Shiraishi M, Haruna M. Immediate stress reduction effects of yoga during pregnancy: one group pre-post test. Women Birth. 2016;29(5):e82–e88. | ||
Edmonds R, Burkett B, Leicht A, McKean M. Effect of chronic training on heart rate variability, salivary IgA and salivary alpha-amylase in elite swimmers with a disability. PLoS One. 2015;10(6):e0127749. | ||
Kageta T, Tsuchiya Y, Morishima T, Hasegawa Y, Sasaki H, Goto K. Influences of increased training volume on exercise performance, physiological and psychological parameters. J Sports Med Phys Fitness. 2016;56(7–8):913–921. | ||
Furtado GE, Uba-Chupel M, Carvalho HM, Souza NR, Ferreira JP, Teixeira AM. Effects of a chair-yoga exercises on stress hormone levels, daily life activities, falls and physical fitness in institutionalized older adults. Complement Ther Clin Pract. 2016;24:123–129. | ||
Fornieles G, Rosety MA, Elosegui S, et al. Salivary testosterone and immunoglobulin A were increased by resistance training in adults with Down syndrome. Braz J Med Biol Res. 2014;47(4):345–348. | ||
Martins RA, Cunha MR, Neves AP, Martins M, Teixeira-Veríssimo M, Teixeira AM. Effects of aerobic conditioning on salivary IgA and plasma IgA, IgG and IgM in older men and women. Int J Sports Med. 2009;30(12):906–912. | ||
Shimizu K, Kimura F, Akimoto T, et al. Effects of exercise, age and gender on salivary secretory immunoglobulin A in elderly individuals. Exerc Immunol Rev. 2007;13:55–66. | ||
Sloan CA, Engels HJ, Fahlman MM, Yarandi HE, Davis JE. Effects of exercise on S-IGA and URS in postmenopausal women. Int J Sports Med. 2013;34(1):81–86. | ||
Wang CY, Yeh CJ, Wang CW, Wang CF, Lin YL. The health benefits following regular ongoing exercise lifestyle in independent community-dwelling older Taiwanese adults. Australas J Ageing. 2011;30(1):22–26. Epub 2010 Aug 12. | ||
Tarazona-Santabalbina FJ, Gómez-Cabrera MC, Pérez-Ros P, Martínez-Arnau FM, Cabo H, Tsaparas K, et al. A multicomponent exercise intervention that reverses frailty and improves cognition, emotion, and social networking in the community-dwelling frail elderly: a randomized clinical trial. J Am Med Dir Assoc. 2016;17(5):426–433. Epub 2016 Mar 3. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
