Back to Journals » Clinical Epidemiology » Volume 9
Psychiatric conditions and general practitioner attendance prior to HPV vaccination and the risk of referral to a specialized hospital setting because of suspected adverse events following HPV vaccination: a register-based, matched case–control study
Authors Lützen TH , Bech BH , Mehlsen J, Høstrup Vestergaard C, Krogsgaard LW, Olsen J, Vestergaard M, Plana-Ripoll O , Rytter D
Received 21 February 2017
Accepted for publication 19 June 2017
Published 12 September 2017 Volume 2017:9 Pages 465—473
DOI https://doi.org/10.2147/CLEP.S135318
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Irene Petersen
Tina Hovgaard Lützen,1 Bodil Hammer Bech,2 Jesper Mehlsen,3 Claus Høstrup Vestergaard,1 Lene Wulff Krogsgaard,1 Jørn Olsen,4 Mogens Vestergaard,1 Oleguer Plana-Ripoll,5 Dorte Rytter2
1Research Unit for General Practice, Department of Public Health, Aarhus University, Aarhus C, Denmark; 2Section for Epidemiology, Department of Public Health, Aarhus University, Aarhus C, Denmark; 3Coordinating Research Centre, Frederiksberg Hospital, Frederiksberg, Denmark, 4Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus N, Denmark; 5National Center for Register-based Research, Aarhus University, Aarhus V, Denmark
Aim: No association between human papilloma virus (HPV) vaccination and numerous diseases has been found. Still, a large number of Danish women are reporting suspected adverse events. Other factors may play a role, and the aim of this study is to examine the association between psychiatric conditions, general practitioner (GP) attendance and indicators of psychological symptoms prior to HPV vaccination and the risk of referral to an HPV center following vaccination.
Study design and setting: Register-based, matched case–control study. Cases were identified from five Danish, regional HPV centers, and health data for cases and controls were obtained from national registries.
Participants: Cases were defined as women referred to an HPV center between January 1, 2015 and December 31, 2015 (n=1,496). Each case was matched with five controls on age, region and time of first vaccine registration. The total study population consisted of 8,976 women.
Results: Overall, women above 18 years who had been referred to an HPV center were more likely to have used psychiatric medication (odds ratio [OR]: 1.88 [95% CI 1.48–2.40]) or to have been hospitalized because of a psychiatric disorder within 5 years prior to the first vaccine registration (OR: 2.13 [95% CI 1.59–2.86]). Specifically, referred women were more likely to have used antipsychotics, antidepressants, attention deficit hyperactivity disorder (ADHD) medication or anxiolytics, and to have been hospitalized for affective disorders or anxiety, but not to have been hospitalized for schizoid, ADHD or eating disorders. In addition, they were more likely to have had talk therapy or psychometric test performed prior to vaccination (OR: 1.72 [95% CI 0.1.35–2.18] and OR: 1.67 [95% CI 1.30–2.13], respectively). Referred women of all ages had higher use of GP before vaccination. Population attributable fraction analyses indicated that psychiatric medication, hospitalization due to a psychiatric disorder and use of talk therapy, or psychometric test “explained” 13%, 10%, 12% and 11% of the referrals, respectively. Results did not change substantially when adjusted for potential confounders.
Conclusion: Women referred to HPV centers because of suspected adverse events after vaccination more often had preexisting psychiatric conditions, psychological symptoms or frequent GP attendance prior to HPV vaccination.
Keywords: papillomavirus vaccines, adverse drug events, psychoactive drugs, general practice
Plain language summary
In Denmark, the human papilloma virus (HPV) vaccination coverage has decreased in recent years probably because of extensive media attention on reports on suspected adverse events. However, several large international studies have not found associations between the HPV vaccines and serious adverse events. It is important to continuously investigate the safety of the HPV vaccines, but in addition, it is also important to look at other factors that may contribute to an explanation to why some women have experienced suspected adverse events after vaccination. Through data retrieved from various national health registries, this study has investigated psychiatric conditions and general practitioner (GP) attendance before vaccination and the risk of being referred to an HPV center because of suspected adverse events after vaccination. The study found that women referred to an HPV center more often had a psychiatric condition, psychological symptoms or higher GP attendance before vaccination. These results indicate that other factors than the vaccination may also play a role when experiencing suspected adverse events after vaccination.
Introduction
Cervical cancer is the fourth most common cancer among women with 570,000 new cases and 270,000 deaths each year worldwide.1 As a preventive strategy, vaccination against human papilloma virus (HPV) has been implemented in national immunization programs in 58 countries, and in Denmark, the Danish Child Vaccination Programme has offered vaccination to all girls at the age of 12 since 2009.2,3 In addition, two catch-up programs have been available to provide free vaccination, the first one from 2008 to 2010 for women born from 1993 to 1995, and the second in 2012 and 2013 for women born after 1984. The efficacy of both vaccines has been examined extensively and found to be highly protective against HPV infection and thereby cervical dysplasia.4–9 No severe adverse events (SAEs) including several neurological and autoimmune disorders have been reported in the published studies.8,10–18
However, by 2016, the Danish Medicines Agency (DMA) has received >2,300 reports on suspected adverse events among ~600,000 HPV-vaccinated women. From these reports, >1,000 were categorized as serious.19 As a consequence, five specialized hospital settings (hereafter HPV centers) were implemented in 2015, one in each region of Denmark, and by the end of 2015, ~1,600 women had been referred to these centers because of suspected adverse events of the vaccine. The HPV center’s main task is to examine the referred patients from a bio-psycho-social perspective in order to suggest proper treatment and rehabilitation.
There is a clear discrepancy between the scientific research and the clinical experience in Denmark. It is, therefore, important to supplement safety studies with research on specific predictors for experiencing suspected adverse events following HPV vaccination. Although no evidence at present supports a causal link between the HPV vaccines and illness, a Danish case study observed numerous somatic symptoms in referred women.20 Having numerous somatic symptoms has been associated with psychiatric conditions, particularly anxiety and depression, as well as having increased general practitioner (GP) attendance.21–29 This study, therefore, aims to examine the association between psychiatric conditions, GP attendance and indicators for possible psychological symptoms before vaccination and referral to an HPV center because of suspected adverse events after vaccination.
Materials and methods
Study design and source population
The study was conducted as a register-based, matched case–control study.
In Denmark, all citizens are assigned a unique personal identification number (Civil Personal Registration [CPR]) at birth. We used this number to identify the source population consisting of Danish, HPV-vaccinated women born from 1975 to 2008 and to link personal information across national health registers. Criteria for being identified as an HPV-vaccinated woman included having ≥1 service code registration for vaccine injection (Gardasil® [Merck & Co., Whitehouse Station, NJ, USA] or Cervarix® [GSK Biologicals, Rixensart, Belgium]) in the Danish National Health Insurance Service Register (service codes 8328, 8329, 8330 or 8334, 8335 and 8336) or ≥1 registration of redeeming a prescription for the vaccines in the Danish Register of Medicinal Product Statistics (Anatomic Therapeutic Chemical [ATC] code J07BM01, J07BM02 or J07BM03).30,31 The source population consisted of 534,580 HPV-vaccinated women.
Study population
We defined cases as HPV-vaccinated women, born from 1975 to 2008 who were referred to an HPV center from 1.1.2015 to 31.12.2015. We obtained CPR numbers on cases from each HPV center and merged these with the personal data from the Danish Register of Medicinal Product Statistics, the Danish Psychiatric Central Research Register,32 the Danish National Health Insurance Service Register and Statistics Denmark. Among the 1,592 women who were referred, 90 cases were excluded because they were not registered with an HPV vaccination according to our data. This resulted in 1,502 cases. For each case, five HPV-vaccinated women (controls) were randomly selected from the source population, matched on age, region and time of first vaccine registration (±2 months). For six cases, it was not possible to find a full set of controls, and we, therefore, excluded them from the study. Hence, a final sample of 1,496 cases and 7,480 controls were included resulting in a total study population of 8,976 women.
Exposures
We defined psychiatric conditions according to redemption of prescriptions for psychiatric medication in the 5 years prior to the first vaccine registration. Redeemed prescriptions on psychiatric medication were identified through ATC codes in the Danish Register of Medicinal Product Statistics. The following ATC codes were used for identification of psychiatric medication: N06A* (antidepressants), N05A* (antipsychotics), N06BA* (attention deficit hyperactivity disorder [ADHD]), N05BA* (anxiolytics), N05BB* (anxiolytics), N03AX16 (anxiolytics) and N05BE01 (anxiolytics). However, N06AA (tricyclic antidepressants) and N06AX12 (bupropion) were excluded because of their frequent use for insomnia, smoking cessation and as pain medication.33,34
We further identified the presence of psychiatric conditions according to hospitalization due to a psychiatric disorder in the 5 years prior to the first vaccine registration. Information on hospitalization for psychiatric disorders was obtained from the Danish Psychiatric Central Research Register using the following International Statistical Classification of Diseases and Related Health Problems 10th Revision codes as main diagnoses: DF20–DF50 and DF90.
Information on GP contacts was obtained from the Danish National Health Insurance Service Register. GP contacts were defined as face-to-face, telephone/email and out-of-office contacts. GP contacts related to pregnancy were excluded. As indicators for possible psychological symptoms, we identified talk therapy and psychometric test in the 5 years prior to the first vaccine registration through services provided by the GP and registered with the following service codes: 6101, 4003, 4106, 4021–4027, 4050, 4247–4249, 4063 and 2149.
Covariates
From Statistics Denmark, we obtained information on household type (six groups) and family socioeconomic group (nine groups), and included these in the study as potential confounders, in addition to the matching variables. All covariates were estimated 1 year prior to the first vaccine registration; however, when data were missing, we obtained data from the year of the first vaccine registration instead in order to minimize the number of missing values.
Statistical analyses
Characteristics for cases and controls on matching variables, covariates and exposures were summarized using numbers and percentages.
We conducted multiple conditional logistic regression analyses in order to describe the association between the use of psychiatric medication, hospitalization, GP attendance and indication for possible psychological symptoms in the 5 years prior to the vaccine registration, respectively, and referral to an HPV center.
Psychiatric medication and hospitalization due to a psychiatric disorder were dichotomized as yes/no, and in subanalyses stratified on type of medication (anxiolytics, ADHD medication, antidepressants and antipsychotics) and type of diagnosis (DF20–29 [schizophrenia, schizotypal and delusional disorders], DF30–39 [affective disorders], DF40–48 [neurotic, stress-related and somatoform disorders], DF50 [eating disorders] and DF90 [ADHDs]).
Frequency of GP contacts in the 5 years prior to the first vaccine registration was defined from the age-specific decile distribution, and the fifth decile was used as reference.35–37 Use of talk therapy or psychometric test during the 5 years prior to the first vaccine registration was dichotomized as yes/no.
The analyses on psychiatric medication, hospitalization and use of talk therapy or psychometric test only included women above 18 years, as few women below 18 years were expected to have experienced psychiatric morbidity.
In addition, we used negative binomial regression analyses in order to examine the overall development of GP attendance within 5 years before the first vaccine registration. This analysis included the entire study population. The results are presented as incidence rate ratios (IRR) with 95% CI for each year, adjusted for matching variables and covariates. A two-sided p value of 0.05 was considered statistically significant.
Finally, we used the estimated adjusted odds ratios (ORs) and the prevalence of exposure among cases (PE) to estimate population attributable fractions (PAF) using the formula (PAF=PE[OR−1]/OR). The PAF can be interpreted as the proportion of cases that can be attributable to the exposures, assuming that the association is causal and all confounders have been accounted for. All statistical analyses were performed using STATA13.1® statistical software (StataCorp LP, TX, USA).
Results
Characteristics for cases and controls are presented in Table 1. The majority of cases came from the Capital Region and were vaccinated in 2009 and 2012 at the ages of 10–15 and 21–25 years. This corresponds to the age where the Danish Child Vaccination Programme is offered and to the years and ages for the catch-up programs. For the potentially confounding covariates, cases and controls were similar regarding both household type and family socioeconomic group (Table 1).
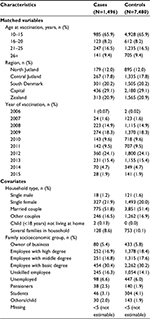  | Table 1 Characteristics of matched variables and covariates for cases and controls at the time of vaccination |
The analyses showed that ~29%, 18% and 28% of cases above 18 years were considered exposed in relation to psychiatric medication, hospitalization and use of talk therapy or psychometric test, respectively (Table 2). The equivalent prevalence among controls was 17%, 9% and 18%, respectively. However, women, who were referred to an HPV center, were 88% more likely to have used psychiatric medication within 5 years prior to the first vaccine registration compared to controls (OR: 1.88 [95% CI 1.48–2.40]). Subanalyses performed on medication type showed that, compared to controls, women, who were referred to an HPV center, were 91%, 96%, 106% and 88% more likely to have used antipsychotics, antidepressants, ADHD medication or anxiolytics 5 years prior to the first vaccine registration, respectively (OR: 1.91 [95% CI 1.22–2.97], OR: 1.96 [95% CI 1.52–2.52], OR: 2.06 [95% CI 1.11–3.81] and OR: 1.88 [95% CI 1.21–2.92]) (Table 2). Referred women were 113% more likely to have had any hospitalization due to a psychiatric disorder within 5 years prior to the first vaccine registration (OR: 2.13 [95% CI 1.59–2.86]), and specifically affective and neurotic, stress-related and somatoform disorders had a higher risk. In addition, eating disorders, schizophrenia, and schizotypal and delusional disorders showed a tendency toward higher risk, which, however, failed to reach significance. Referred women were 72% and 67% more likely to have had talk therapy or a psychometric test performed by their GP, respectively (OR 1.72 [95% CI 1.35–2.18] and OR: 1.67 [95% CI 1.30–2.13]). Adjustment for household type and family socioeconomic group only changed the estimates marginally (Table 2).
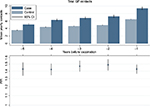
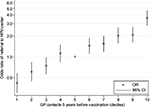
During the 5 years prior to the first vaccine registration, both cases and controls show increasing mean GP attendance up to the time of vaccination. However, the IRR remains steady and consistently higher for cases compared to controls (Figure 1). Descriptive analyses show that 46% of cases and 25% of controls belong to the top three deciles of GP attendance frequency (data not shown). Women belonging to the age-specific 6th–10th decile of frequency of GP contacts have a higher risk of being referred to an HPV center compared to the 5th decile. In contrast, women belonging to the 1st–3rd decile have a lower risk of referral to an HPV center compared to those in the 5th decile (Figure 2).
For the PAFs, assuming an unconfounded, causal association, use of psychiatric medication, hospitalization due to a psychiatric disorder, and use of talk therapy or psychometric test “explained” ~13%, 10%, 12% and 11% of the referrals, respectively. For the specific types of psychiatric medication, the PAF was the highest for the prior use of antidepressants (12%). For GP attendance, belonging to the top quartile “explained” ~25% of the referrals.
Discussion
Principal findings
The study found that women who have been referred to an HPV center because of suspected adverse events after vaccination have 1) more frequent use of psychiatric medication, 2) more psychiatric hospitalizations and 3) a history of more GP contacts, including talk therapy and psychometric test, in the 5 years before vaccination compared with vaccinated women of same age, region and vaccination time. However, this difference in psychiatric morbidity and psychological symptoms could only “explain” a smaller fraction of the referred cases.
Strengths and limitations
As the study is register-based, participation was almost complete. In addition, all cases have been examined by their GPs and subsequently referred to an HPV center. This validates the case definition without making it complete. A total of 1,592 cases were collected from the HPV centers, and from them 96 cases were excluded. For 90 cases, exclusion was due to missing registration of HPV vaccination and six cases did not have a full set of controls. The excluded cases only constituted 6% of the total number of cases. Missing vaccine registration is probably because of register errors and is likely to be independent of a later referral. This is, therefore, not considered to have caused relevant selection bias. The six cases without a full set of controls did not differentiate on either matching variables or covariates, and selection bias from the exclusion of these individuals is, therefore, also considered unlikely.
In relation to obtainment of information on GP attendance and specific services on an individual level from the National Health Insurance Service Register, the accuracy and validity of the information is considered high, as a person’s CPR number is meticulously registered at each GP visit, and GPs are relying on correct registrations in order to receive remuneration.30
For psychiatric medication, the Register of Medicinal Product Statistics provides information on redeemed prescriptions on an individual level. However, it provides no guarantee as to whether the patient took the medication. Still, it is reasonable to assume that no GP would prescribe this type of medication unless indicated, and the patient is, therefore, likely to have psychiatric symptoms even if the redeemed medication is never consumed.
For hospitalization due to a psychiatric disorder, the Danish Psychiatric Central Research Register also provides information on an individual level, and is considered of high validity as research has shown high positive predictive value.32
In addition, all exposure variables were obtained from registries recorded before vaccination and is, therefore, not based on self-reports by patients or relatives.
The analyses have been adjusted for family socioeconomic group and household type, which only changed the estimates slightly. It is, however, possible that other variables, which we did not have information on, may have confounded the associations.
Comparison with other research
To the best of our knowledge, this study is the first to examine the association between psychiatric conditions before HPV vaccination and the risk of being referred to an HPV center because of suspected adverse events to the vaccination. However, the results of this study are in line with the study by Molbak et al38, which found a significant association between use of GP contacts through telephone and email 2 years prior to vaccination and reporting a possible SAE to the DMA after HPV vaccination. In addition, the risk of reporting a possible SAE was also higher among women who had visited a psychologist or psychiatrist prior to vaccination.38 Their results are, however, not directly comparable to the findings of our study because of the difference in case definition. In addition, the study estimated GP contacts 2 years prior to vaccination as yes/no, whereas our study has counted the number of GP contacts. Still, it is likely that there is some overlap between the case populations, as it can be expected that some of the young women with reports to the DMA have been referred to an HPV center later.
Possible explanations for the associations
It is important to underline that only 13% and 10% of referrals can be “explained” by receiving psychiatric medication or being hospitalized before vaccination, respectively, and only 11%–12% can be “explained” by having received either talk therapy or psychometric test. Therefore, being referred to an HPV center cannot be explained by the investigated exposures alone. The results did, however, show that previous psychiatric morbidity was significantly associated with an increased risk of being referred to an HPV center. It is beyond the scope of this study to draw conclusions on the explanations for the associations. However, possible explanations for the estimated associations could be that 1) the symptoms would have occurred irrespective of the vaccination; these symptoms simply correlate over time; 2) people with psychiatric conditions have increased sensitivity toward the vaccine; the mental health problems modify a potential vaccine–adverse events association; or 3) people with psychiatric conditions react differently because of an alternative experience and perception of bodily and physical symptoms.23
Although the PAF on psychiatric conditions may only have a smaller explanatory force as to why the included cases have been referred to an HPV center, the PAF on GP attendance can “explain” 25% of referrals. Having high GP attendance can be related to numerous causes such as having a preexisting somatic or psychiatric disease, injuries and/or frequent infections. It can, however, also reflect differences in illness behavior, which describes the manner in which a person monitors the function of his/her body, interprets symptoms, takes remedial action and makes use of health care facilities.39 It is, however, not possible for this study to distinguish between these possible explanations.
Implications for clinicians and future research
The results of this study are probably applicable in young women elsewhere, who have also received the vaccination at age 12 or older; however, the results need not be applicable in study populations where the vaccine is provided at a younger age.
The study may provide knowledge to GPs for identification of especially vulnerable girls and women, as well as supporting a better understanding and thereby possibly a more optimal treatment of women already referred to an HPV center. Further research, which includes epidemiological research on predictors and risk factors in order to more fully comprehend the mechanisms behind the current situation, is needed, as well as biological studies in order to fully investigate possible safety issues with the vaccines.
As the study only includes vaccinated girls it is not possible from this study to draw conclusions on the possible causal link between the vaccine and potential adverse events. Hence, the results do not rule out that referral to an HPV center was related to vaccine-induced adverse events. It merely states that the women’s medical history should be taken into consideration when planning a treatment strategy.
Conclusion
Being referred to an HPV center because of suspected adverse events after vaccination was associated with use of psychiatric medication and hospitalization due to a psychiatric disorder in the 5 years prior to HPV vaccination. Referrals were also associated with higher frequency of GP attendance and use of talk therapy or psychometric test in the 5 years prior to HPV vaccination. Although the study has identified these associations, other designs are needed to shed light on the causal mechanisms and directions behind the associations.
Acknowledgments
Collected data: Dr. Med. Erik Østergaard, Department of Woman-Child and Urology, Aalborg University Hospital, Dr. Med. Michael Nielsen, Department of Neurology, Aalborg University Hospital, Dr. Med. Svend Stenvang Pedersen, Department of Infectious Diseases, Odense University Hospital, Dr. Med. Niels Fisker, H.C. Andersen Children’s Hospital, Odense University Hospital, Dr. Med. Martin Faber Boxill, Department of Pediatrics, Regional Hospital of Viborg, Dr. Med. Dan Pradsgaard, Department of Pediatrics, Regional Hospital of Viborg, Dr. Med. Vibeke Neergaard Sørensen, Diagnostics Centre, Regional Hospital of Silkeborg, Dr. Med. Reinar Bue Falck Juvik, Diagnostics Centre, Sjælland University Hospital Roskilde, Dr. Med. Lise Heilmann Jensen, Department of Pediatrics, Sjælland University Hospital Roskilde and Dr. Med. Jesper Mehlsen, Frederiksberg Syncope Centre, Frederiksberg Hospital.
Ethics coordinator: Hanne Birgitte Hede Jørgensen, Center for Integrated Register-based Research at Aarhus University (CIRRAU). This work was supported by the Program for Clinical Research Infrastructure (PROCRIN) established by the Lundbeck Foundation and the Novo Nordisk Foundation and administered by the Danish Regions, as well by an unrestricted grant from the Lundbeck Foundation (grant number: R155–2012-11280). The funding sources had no role in the design and conduct of the study; the collection, analysis and interpretation of data; or the preparation, review or approval of the manuscript. The Danish Data Protection Agency (journal number 2015–57-0002) and the Danish Patient Safety Authority approved the study. According to Danish legislation, ethical approval of registry studies is not required. The abstract of this paper was presented at the Nordic Epi Congress in September 2017 as a poster presentation with final findings. The abstract was published in the “Abstract book,” in the congress app and online (https://mkon.nu/nordicepi).
Disclosure
The authors report no conflicts of interest in this work.
References
World Health Organization. Human Papillomavirus (HPV) and cervical cancer. 2016; Available from: http://www.who.int/mediacentre/factsheets/fs380/en/. Accessed February 17, 2017. | ||
World Health Organization. Human papillomavirus vaccines: WHO position paper, October 2014. Wkly Epidemiol Rec. 2014;89(43):465–492. | ||
Statens Serum Institut. HPV-vaccine. 2016; Available from: http://www.ssi.dk/Vaccination/Boernevaccination/Vaccination%20mod%20livmoderhalskraeft.aspx. Accessed August 30, 2017. | ||
Drolet M, Benard E, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2015;15(5):565–580. | ||
Sando N, Kofoed K, Zachariae C, Fouchard J. A reduced national incidence of anogenital warts in young Danish men and women after introduction of a national quadrivalent human papillomavirus vaccination programme for young women an ecological study. Acta Derm Venereol. 2014;94(3):288–292. | ||
Baldur-Felskov B, Dehlendorff C, Junge J, Munk C, Kjaer SK. Incidence of cervical lesions in Danish women before and after implementation of a national HPV vaccination program. Cancer Causes Control. 2014;25(7):915–922. | ||
Delere Y, Wichmann O, Klug SJ, et al. The efficacy and duration of vaccine protection against human papillomavirus: a systematic review and meta-analysis. Dtsch Arztebl Int. 2014;111(35–36):584–591. | ||
Lu B, Kumar A, Castellsague X, Giuliano AR. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review & meta-analysis. BMC Infect Dis. 2011;11:13. | ||
Naud PS, Roteli-Martins CM, De Carvalho NS, et al. Sustained efficacy, immunogenicity, and safety of the HPV-16/18 AS04-adjuvanted vaccine: final analysis of a long-term follow-up study up to 9.4 years post-vaccination. Hum Vaccin Immunother. 2014;10(8):2147–2162. | ||
Angelo MG, David MP, Zima J, et al. Pooled analysis of large and long-term safety data from the human papillomavirus-16/18-AS04-adjuvanted vaccine clinical trial programme. Pharmacoepidemiol Drug Saf. 2014;23(5):466–479. | ||
Stillo M, Carrillo Santisteve P, Lopalco PL. Safety of human papillomavirus vaccines: a review. Expert Opin Drug Saf. 2015;14(5):697–712. | ||
Scheller NM, Pasternak B, Svanstrom H, Hviid A. Quadrivalent human papillomavirus vaccine and the risk of venous thromboembolism. JAMA. 2014;312(2):187–188. | ||
Scheller NM, Svanstrom H, Pasternak B, et al. Quadrivalent HPV vaccination and risk of multiple sclerosis and other demyelinating diseases of the central nervous system. JAMA. 2015;313(1):54–61. | ||
Arnheim-Dahlstrom L, Pasternak B, Svanstrom H, Sparen P, Hviid A. Autoimmune, neurological, and venous thromboembolic adverse events after immunisation of adolescent girls with quadrivalent human papillomavirus vaccine in Denmark and Sweden: cohort study. BMJ. 2013;347:f5906. | ||
Angelo MG, Zima J, Tavares Da Silva F, Baril L, Arellano F. Post-licensure safety surveillance for human papillomavirus-16/18-AS04-adjuvanted vaccine: more than 4 years of experience. Pharmacoepidemiol Drug Saf. 2014;23(5):456–465. | ||
Chao C, Klein NP, Velicer CM, et al. Surveillance of autoimmune conditions following routine use of quadrivalent human papillomavirus vaccine. J Intern Med. 2012;271(2):193–203. | ||
Donegan K, Beau-Lejdstrom R, King B, Seabroke S, Thomson A, Bryan P. Bivalent human papillomavirus vaccine and the risk of fatigue syndromes in girls in the UK. Vaccine. 2013;31(43):4961–4967. | ||
Cameron RL, Ahmed S, Pollock KG. Adverse event monitoring of the human papillomavirus vaccines in Scotland. Intern Med J. 2016;46(4):452–457. | ||
Danish Medicines Agency. Bivirkninger ved HPV-vaccinen. 2016; Available from: https://laegemiddelstyrelsen.dk/da/bivirkninger/bivirkninger-ved-medicin/~/media/4DCEFB530117489AB2AE35A871DE3805.ashx. Accessed August 30, 2017. | ||
Brinth L, Theibel AC, Pors K, Mehlsen J. Suspected side effects to the quadrivalent human papilloma vaccine. Dan Med J. 2015;62(4):A5064. | ||
Haftgoli N, Favrat B, Verdon F, et al. Patients presenting with somatic complaints in general practice: depression, anxiety and somatoform disorders are frequent and associated with psychosocial stressors. BMC Fam Pract. 2010;11:67. | ||
Campo JV. Annual research review: functional somatic symptoms and associated anxiety and depression--developmental psychopathology in pediatric practice. J Child Psychol Psychiatry. 2012;53(5):575–592. | ||
Bekhuis E, Boschloo L, Rosmalen JG, Schoevers RA. Differential associations of specific depressive and anxiety disorders with somatic symptoms. J Psychosom Res. 2015;78(2):116–122. | ||
Zhu C, Ou L, Geng Q, et al. Association of somatic symptoms with depression and anxiety in clinical patients of general hospitals in Guangzhou, China. Gen Hosp Psychiatry. 2012;34(2):113–120. | ||
Henningsen P, Zimmermann T, Sattel H. Medically unexplained physical symptoms, anxiety, and depression: a meta-analytic review. Psychosom Med. 2003;65(4):528–533. | ||
Lipsitz JD, Hsu DT, Apfel HD, et al. Psychiatric disorders in youth with medically unexplained chest pain versus innocent heart murmur. J Pediatr. 2012;160(2):320–324. | ||
Tomenson B, McBeth J, Chew-Graham CA, et al. Somatization and health anxiety as predictors of health care use. Psychosom Med. 2012;74(6):656–664. | ||
Barsky AJ, Orav EJ, Bates DW. Somatization increases medical utilization and costs independent of psychiatric and medical comorbidity. Arch Gen Psychiatry. 2005;62(8):903–910. | ||
Andersen NL, Eplov LF, Andersen JT, Hjorthoj CR, Birket-Smith M. Health care use by patients with somatoform disorders: a register-based follow-up study. Psychosomatics. 2013;54(2):132–141. | ||
Andersen JS, Olivarius Nde F, Krasnik A. The Danish National Health Service Register. Scand J Public Health. 2011;39(7 Suppl):34–37. | ||
Kildemoes HW, Sorensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 Suppl):38–41. | ||
Mors O, Perto GP, Mortensen PB. The Danish Psychiatric Central Research Register. Scand J Public Health. 2011;39(7 Suppl):54–57. | ||
Katon W, Pedersen HS, Ribe AR, et al. Effect of depression and diabetes mellitus on the risk for dementia: a national population-based cohort study. JAMA Psychiatry. 2015;72(6):612–619. | ||
Hartmann-Boyce J, Aveyard P. Drugs for smoking cessation. BMJ. 2016;352:i571. | ||
Vedsted P, Sorensen HT, Mortensen JT. Drug prescription for adult frequent attenders in Danish general practice: a population-based study. Pharmacoepidemiol Drug Saf. 2004;13(10):717–724. | ||
Vedsted P, Christensen MB. Frequent attenders in general practice care: a literature review with special reference to methodological considerations. Public Health. 2005;119(2):118–137. | ||
Vedsted P, Fink P, Sorensen HT, Olesen F. Physical, mental and social factors associated with frequent attendance in Danish general practice. A population-based cross-sectional study. Soc Sci Med. 2004;59(4):813–823. | ||
Molbak K, Hansen ND, Valentiner-Branth P. Pre-vaccination care-seeking in females reporting severe adverse reactions to HPV vaccine. A Registry Based Case-Control Study. PLoS One. 2016;11(9):e0162520. | ||
Mechanic D. The concept of illness behaviour: culture, situation and personal predisposition. Psychol Med. 1986;16(1):1–7. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.