Back to Journals » Therapeutics and Clinical Risk Management » Volume 13
Protocol for management after thyroidectomy: a retrospective study based on one-center experience
Authors Luo H, Yang HL, Wei T, Gong YP, Su AP, Ma Y, Zou XH, Lei JY, Zhao WJ, Zhu JQ
Received 10 December 2016
Accepted for publication 27 February 2017
Published 15 May 2017 Volume 2017:13 Pages 635—641
DOI https://doi.org/10.2147/TCRM.S129910
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Deyun Wang
Han Luo,1 Hongliu Yang,2,3 Tao Wei,1 Yanping Gong,1 Anping Su,1 Yu Ma,1 Xiuhe Zou,1 Jianyong Lei,1 Wanjun Zhao,1 Jingqiang Zhu3
1Thyroid & Breast Surgery, 2Nephrology, 3Biostatistics Center, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
Background and aim: The optimal approach to detect and treat symptomatic hypocalcemia (SxH) after thyroidectomy is still uncertain. In our retrospective study, we sought to set a standardized postoperative management protocol on the basis of relative change of parathyroid hormone (PTH) and absolute value of postoperative day 1 (POD1) PTH.
Methods: Patients who underwent thyroidectomy were identified retrospectively in our prospective database. Blood was collected 1 day before surgery and on POD1. Extra calcium and calcitriol supplement was prescribed when necessary. Meanwhile, postoperative signs of SxH were treated and recorded in detail. Patients were followed up for 1 month after surgery and then 3 months thereafter.
Results: A total of 744 patients were included in the final analysis. Transient SxH occurred in 86 (11.6%) patients, and persistent SxH occurred in 4 (0.54%) patients in more than half year after surgery. Relative decrease of PTH reached its maximal discriminative effect at 70% (area under the curve [AUC] =0.754), with a sensitivity of 72.1% and a specificity of 75%. In Group 1 (≤70%), 24 (4.67%) patients were interpreted as having SxH, whereas in Group 2, 62 (27.0%) patients had SxH (>70%), P<0.001. Days of symptom relief in Group 1–1 (1, 2) were significantly shorter than those in Group 2–2 (1, 10), P=0.023. In Group 2, 112 (80%) patients with POD1 PTH <1 pmol/L were treated with calcitriol, whereas only 8 (8.89%) patients with POD1 PTH ≥1 pmol/L were treated with calcitriol (P<0.001). According to relief of SxH and recovery of parathyroid function, treating with and without calcitriol showed no difference in patients with POD1 PTH <1 and ≥1 pmol/L.
Conclusion: Relative decrease of PTH >70% is a significant risk factor for SxH in post-thyroidectomy. The decreasing percent of PTH ≤70% ensures discharge on POD1, but longer hospitalization was advocated for patients with decreasing percent of PTH >70%, who needed extra calcitriol supplement when POD1 PTH <1 pmol/L.
Keywords: parathyroid hormone, PTH, relative change, thyroidectomy, calcitriol, discharge
Introduction
Hypocalcemia is the most common complication of thyroidectomy. The incidence of transient hypocalcemia ranges from 10% to 50%,1,2 and permanent hypocalcemia usually occurs in 0%–2% of patients according to different definitions.3,4 It is known that not all patients with hypocalcemia will have associated symptoms such as numbness and spasm. Some surgeons advocated prolonged stays that are not cost-effective nowadays. Meanwhile, when most of the surgeons discharge patients within 24 hours, more emergency room visits or emergency calcium intravenous infusions would also occur.5 With the increasing preference for shorter stays,6–8 identifying patients at high risk is essential for their timely and safe discharge.
In recent years, multiple retrospective studies have approved that the absolute value of postoperative serum parathyroid hormone (PTH) is an accurate predictor of hypocalcemia in postoperative patients. However, we found that the absolute cutoff value is variant.2,9,10 Moreover, as we know, parathyroid insufficiency is the main contributor to hypocalcemia. Consequently, relative change of perioperative PTH is hypothesized as a more reasonable predictor.
To our limited knowledge, no study has been conducted in China considering the relationship between the relative change of PTH and postoperative management. More important is the lack of a standard protocol for the postoperative management after thyroidectomy. Calcium and calcitriol supplement plan is mostly determined by surgeons’ varied experience. Additionally, relatively longer stays – postoperative 4–5 days – are a common issue in China compared with other hospitals abroad. Hence, we anticipate that the results of this study would contribute to the management of post-thyroidectomy patients for future retrospective and prospective studies.
Methods
Patients who underwent thyroidectomy between 2013 and 2015 were identified retrospectively in the prospective clinical database of West China Hospital. Patients were excluded from the final analysis if they 1) had hyperparathyroidism, 2) had chronic kidney disease (CKD), 3) had parathyroid adenoma or carcinoma, 4) took preoperative osteoporotic medications or prophylactic vitamin D and calcium supplementation, 5) underwent lobectomy and 6) had intensive care unit (ICU) history after surgery.
Demographics (sex, age, etc.), history and laboratory data (PTH1, calcium, etc.) were recorded before surgery. Tumor staging was done according to the standard of the Union for International Cancer Control (UICC), sixth edition.
Thyroidectomy and postoperative management were performed by the same thyroid surgical team. In terms of benign disease, such as large goiter, hyperthyroidism, near total thyroidectomy (NTT) or lobectomy was the main choice. NTT refers to the removal of all of thyroid except <1 g of thyroid tissue at the entry into the larynx. With regard to malignant entity, total thyroidectomy (TT) and ipsilateral central nodal dissection (CND) were the main choices. Contralateral CND was performed mainly depending on the intraoperative findings and frozen section of pretracheal lymph node.11
Routine 2-day calcium supplement, 2 and 4 g calcium infused intravenously on the surgery day and on postoperative day 1 (POD1), respectively, was adopted to every postoperative patient. Meanwhile, patients were prescribed with calcium carbonate 600 mg t.i.d. or q.i.d. on POD1. POD1 PTH and calcium were tested and recorded in the next morning after surgery. Extra calcitriol 0.25 μg q.d. or b.i.d. was added into the therapeutic plan on a case-by-case basis. Postoperative patients were monitored cautiously, and hypocalcemic symptoms such as numbness, facial paresthesia, positive Chvostek’s signs and muscular spasm were recorded as soon as they occurred. Usually, the patients were discharged on the third day after surgery. After that, patients were followed up at the outpatient department 1 month later after discharge and then 3 months thereafter focusing on the levels of PTH and calcium and hypocalcemic symptoms.
Relative change of PTH was calculated in all patients according to the formula: [(PTH1 − POD1 PTH)/PTH1] ×100%. Reference range for the normal value of PTH is 1.6–6.9 pmol/L, and that for calcium is 2.1–2.7 mmol/L.
Data analysis was performed using SPSS version 19 (SPSS Inc, Chicago, IL, USA). If normally distributed, continuous variables were presented as mean ± standard deviation and compared using Student’s t-test, and paired t-test was also used when needed; if not, variables were presented as median (range) and compared using Mann–Whitney U-test. Pearson’s chi-square test or Fisher’s exact test was used to compare the frequency (percentage) of categorical variables. Receiver operating characteristic (ROC) curve was used to identify the cutoff value and binary logistic regression for risk factor identification. The P-value <0.05 indicated significant difference.
This study was approved by the ethics committee of West China Hospital, Sichuan University, and was conducted in accordance with the relative guidelines. Written informed consent was obtained from all subjects to use the clinical data in the clinical research.
Results
A total of 806 patients had undergone thyroidectomy between 2013 and 2015. According to the exclusion criteria, 62 patients were excluded from the final analysis (secondary hyperparathyroidism and CKD 13, preoperative calcium or calcitriol supplement 4, parathyroid carcinoma 1, lobectomy 40, ICU history 4). As a result, 744 patients participated in the final analysis, with a mean age of 42.3±11.7 years and a female:male ratio of 538:206. In the light of pathological results, 650 patients had papillary thyroid carcinoma (PTC). Both medullary thyroid carcinoma (MTC) and follicular thyroid carcinoma (FTC) were diagnosed in 8 patients. Thyroid adenoma, hyperthyroidism and thyroiditis were diagnosed in 22, 30 and 96 patients, respectively. Besides, 610 patients underwent TT or completion thyroidectomy and 44 underwent NTT. Auto-transplantation of parathyroid (ATP) and CND were performed in 406 and 656 patients, respectively (Table 1).
After surgery, patients were monitored intensively. Obvious symptomatic hypocalcemia (SxH), in particular, numbness and toe spasm, occurred in 86 (11.6%) patients; only few patients showed positive Chvostek’s signs. Analysis of the relationship between SxH and decreasing percent of PTH was conducted using quintiles of existing data (eg, [0, 0.2], [0.2, 0.4], [0.4, 0.6], [0.6, 0.8], [0.8, 1.0]). This grouping method had a significant discriminative effect (P<0.001). The incidence of SxH was 2.13%, 3.33%, 8.33%, 8.42% and 32.4% in sequence (Figure 1).
  | Figure 1 Incidence of SxH increases with relative decreasing percent of PTH. |
The decreasing percent of PTH impressively had an influence on the incidence of SxH in a continuous manner. Therefore, a cutoff value of decreasing percent should be extracted to guide clinical practice better.
The ROC curve was adopted to identify the cutoff value, which is shown in Figure 2. SxH was set as a state variable, and area under the curve (AUC) was 0.754, which was higher than 0.711 of POD1 PTH. Decreasing percent reached its maximal discriminative effect at 70.3% (sensitivity 72.1%, specificity 75%) and was selected as the cutoff value to predict SxH.
  | Figure 2 AUC in relative decreasing percent was 0.754 and on POD1 was 0.711. |
As a result, 744 patients were divided into 2 groups on the basis of decreasing percent 70% – Group 1 (≤70%) and Group 2 (>70%). A total of 514 patients were in Group 1, with a mean age of 42.6±11.97 years, whereas 230 patients were in Group 2, with a mean age of 42.28±11.22 years. Distribution of the disease and detailed extent of surgery are shown in Table 2. Binary logistic regression analysis showed that the decreasing percent of PTH was an independent risk factor for postoperative SxH (hazard ratio [HR]: 57.6, 95% confidence interval [95% CI]: [15.8, 210.3], P<0.001) (Table 3).
In Group 1, symptoms of 24 (4.67%) patients were interpreted as SxH, especially toe and finger numbness. On the other hand, 62 (27.0%) patients had suffered SxH in Group 2 (P<0.001). Nearly all patients in both the groups had SxH on POD1, which was not significantly different (P=0.452) (Table 4). Only 2 patients in Group 1 had SxH on the fourth postoperative day and relieved on the current day. However, median day of symptom relief was 1 (1, 2) in Group 1, which was significantly shorter than that in Group 2–2 (1, 10), P=0.023. On the other hand, patients with PTH relative decreasing percent >70% needed longer hospitalization. Moreover, absolutely most of the patients in Group 1 could be discharged on POD1.
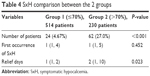  | Table 4 SxH comparison between the 2 groups |
A total of 138 patients were prescribed with calcitriol and 120 (87%) in Group 2. While analyzing the absolute value of POD1 PTH in Group 2, a significant difference was found between patients treated with and without calcitriol (0.63±0.33 vs 1.41±0.62, P<0.001). The absolute value of POD1 PTH seemed to guide the usage of calcitriol. Naturally, a better guiding value should be extracted. POD1 PTH reached its maximal effect at 1 pmol/L (AUC 0.885) with a sensitivity of 74.5% and a specificity of 95%.
Subcomparison was conducted. A total of 140 patients had POD1 PTH <1 pmol/L, of whom 112 (80%) patients were treated with calcitriol, and of 90 patients who had POD1 PTH ≥1 pmol/L, only 8 (8.89%) were treated with calcitriol (P<0.001). Meanwhile, the first occurrence of SxH or relief duration did not show significant difference between patients with POD1 PTH >1 and those with <1 pmol/L (P=0.237 and 0.177, respectively). In patients with POD1 PTH ≥1 pmol/L, 10 SxH patients relieved after 1-month follow-up. And likely, in 52 patients appeared SxH after surgery, only 4 of them still suffered persistent SxH. Paired t-test showed that PTH had a significant increase in all patients, with or without extra calcitriol supplement (both P<0.001) (Table 5). Thus, intensive observation other than calcium supplement was the main choice in terms of patients with POD1 PTH ≥1 pmol/L in Group 2, whereas extra calcitriol supplement was the primary consideration (Figure 3).
  | Table 5 Subcomparison in patients with decreasing percent >70% |
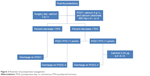  | Figure 3 Flowchart of postoperative management. |
Discussion
In this study, a systematic protocol of identifying high risk of hypocalcemia in patients who underwent thyroidectomy and effective treatment plan for SxH was summarized based on the relative change of PTH with absolute value of POD1 PTH. This protocol could shorten hospital stays and avoid unsafe discharge as much as possible.
On the basis of our findings in the retrospective study, routine 2-day intravenous infusion of calcium with or without oral supplement on POD1 plus extra supplement of calcitriol according to the relative decreasing percent and absolute value of POD1 PTH was an effective protocol, which meanwhile could identify patients needing longer hospitalization. Decreasing percent of PTH, POD1 PTH that was obtained in the morning after surgery compared with preoperative PTH, had an impressive discriminative effect between high- and low-risk SxH. When the decreasing percent is ≤70%, only <5% of patients were interpreted as having SxH, and nearly all SxH patients appeared and relieved on POD1, which ensures safe discharge on POD1. On the contrary, it is significantly different that 27% of patients suffered SxH when the decreasing percent is >70% (P<0.001). Moreover, relief days was significantly longer than in patients with deceasing percent ≤70% (P=0.023). On the other hand, when PTH decreased >70% relatively, patients needed longer stays.
Another issue was the timing of postoperative measurement of PTH. Some studies advocated 4 hours after surgery or even later, while others suggested 1 hour or before.9,10,12,13 In the review of Grodski and Serpell,14 it was concluded that PTH measurement varies from 10 minutes postoperatively to several hours, which would provide equally accurate results. Lee et al15 concluded in a meta-analysis that intraoperative PTH has no significant advantage over early postoperative PTH when used as a clinical guide for discharge after thyroidectomy. In our study, PTH, measured in the next morning after surgery, presented an excellent predicting and discriminative ability. In patients of PTH decreasing percent >70%, POD1 PTH 1 pmol/L (sensitivity 74.5% and specificity 95%) could identify accurately patients who need calcitriol supplement other than calcium supplement and longer stays.
Puzziello et al16 stated that >62% decreasing percent of PTH in 2 hours after surgery, though normocalcemia in POD1, suggested a longer hospitalization and additional therapy after discharge, however, <62% did not. Chapman et al17 reported that patients with a >44% PTH decrease from preoperative to 6 hours postoperative are more likely to develop hypocalcemia. Our study showed that >70% decrease of PTH on POD1 could accurately predict the incidence of SxH. Rather than simply focusing on the calcium levels, we paid more attention to symptoms associated with hypocalcemia. Regarding hypocalcemic symptoms, both Lecerf and Schlottmann et al18 concluded that patients with <80% drop in PTH levels can be safely discharged on the day of the surgery.19 Despite various setting points, a consensus that relative decrease of PTH, not the absolute value, does have a determined influence on the outcome after surgery was reached.
In terms of retrospective study, limitations are inevitable. First, it is a retrospective study from one institution, and hence selection bias is inevitable. Moreover, recording of symptoms may be missed on occasion. Besides, the number of patients in some parts was still relatively insufficient. In addition, the follow-up information was not complete, and whether discharged asymptomatic patients visited emergency room was unknown. This may lead to some bias. Furthermore, postoperative calcium supplement would affect the calcium level. However, the trend is much clear that PTH dramatic change (>70% in our study and >80% in other studies) may lead to SxH.18,19 Because of calcium supplement, the cutoff value in our study is less than that in other studies. In addition, in China and other areas, very few surgeons would supply calcium to patients after thyroidectomy. Hence, we can provide training to surgeons to stratify the risk of SxH after surgery. Meanwhile, we have conducted a randomized controlled trial (RCT) of calcium supplement to confirm the efficiency and safety of postoperative management without intravenous calcium supplement. We believe that we can find evidence about it. Moreover, discharging patients within 24 hours is the main choice for surgeons abroad; however, 3–5 postoperative stays are adopted in most of the Chinese hospitals.
Despite the limitations in our studies, we still have confidence in our protocol about postoperative management. On the basis of our protocol, shorter hospital stays are the first step to standardize postoperative management. Meanwhile, the rational usage of calcitriol is also considered in our study. A prospective research based on this retrospective study has to be conducted to guide the use of supplement and the dose of calcium.
Conclusion
Routine 2-day intravenous and oral calcium supplement is a basic therapeutic plan for post-thyroidectomy patients. When the relative decreasing percent is ≤70%, patients could be discharged on the first postoperative day, and when the relative decreasing percent is >70%, longer hospitalization is needed other than calcium supplement. In addition, when POD1 PTH <1 pmol/L, extra calcitriol could relieve hypocalcemic symptoms and recover parathyroid function.
Acknowledgments
The authors acknowledge Dr Qianqian Han and Dr Bin Wang for their kind guidance over the use of statistics software. This study was funded by the 1-3-5 project of West China Hospital, Sichuan University.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Pattou F, Combemale F, Fabre S, et al. Hypocalcemia following thyroid surgery: incidence and prediction of outcome. World J Surg. 1998;22(7):718–724. | ||
Noordzij JP, Lee SL, Bernet VJ, et al. Early prediction of hypocalcemia after thyroidectomy using parathyroid hormone: an analysis of pooled individual patient data from nine observational studies. J Am Coll Surg. 2007;205(6):748–754. | ||
Reeve T, Thompson NW. Complications of thyroid surgery: how to avoid them, how to manage them, and observations on their possible effect on the whole patient. World J Surg. 2000;24(8):971–975. | ||
La Quaglia MP, Black T, Holcomb GW 3rd, et al. Differentiated thyroid cancer: clinical characteristics, treatment, and outcome in patients under 21 years of age who present with distant metastases. A report from the Surgical Discipline Committee of the Children’s Cancer Group. J Pediatr Surg. 2000;35(6):955–959. [discussion 960]. | ||
Youngwirth L, Benavidez J, Sippel R, Chen H. Postoperative parathyroid hormone testing decreases symptomatic hypocalcemia and associated emergency room visits after total thyroidectomy. Surgery. 2010;148(4):841–844. [discussion 844–846]. | ||
McHenry CR. “Same-day” thyroid surgery: an analysis of safety, cost savings, and outcome. Am Surg. 1997;63(7):586–589. [discussion 589–590]. | ||
Mowschenson PM, Hodin RA. Outpatient thyroid and parathyroid surgery: a prospective study of feasibility, safety, and costs. Surgery. 1995;118(6):1051–1053. [discussion 1053–1054]. | ||
Schwartz AE, Clark OH, Ituarte P, Lo Gerfo P. Therapeutic controversy: thyroid surgery – the choice. J Clin Endocrinol Metab. 1998;83(4):1097–1105. | ||
Vescan A, Witterick I, Freeman J. Parathyroid hormone as a predictor of hypocalcemia after thyroidectomy. Laryngoscope. 2005;115(12):2105–2108. | ||
Payne RJ, Tewfik MA, Hier MP, et al. Benefits resulting from 1- and 6-hour parathyroid hormone and calcium levels after thyroidectomy. Otolaryngol Head Neck Surg. 2005;133(3):386–390. | ||
Wei T, Li Z, Jin J, et al. Autotransplantation of inferior parathyroid glands during central neck dissection for papillary thyroid carcinoma: a retrospective cohort study. Int J Surg. 2014;12(12):1286–1290. | ||
Lombardi CP, Raffaelli M, Princi P, et al. Early prediction of postthyroidectomy hypocalcemia by one single iPTH measurement. Surgery. 2004;136(6):1236–1241. | ||
Sywak MS, Palazzo FF, Yeh M, et al. Parathyroid hormone assay predicts hypocalcaemia after total thyroidectomy. ANZ J Surg. 2007;77(8):667–670. | ||
Grodski S, Serpell J. Evidence for the role of perioperative PTH measurement after total thyroidectomy as a predictor of hypocalcemia. World J Surg. 2008;32(7):1367–1373. | ||
Lee DR, Hinson AM, Siegel ER, Steelman SC, Bodenner DL, Stack BC Jr. Comparison of intraoperative versus postoperative parathyroid hormone levels to predict hypocalcemia earlier after total thyroidectomy. Otolaryngol Head Neck Surg. 2015;153(3):343–349. | ||
Puzziello A, Gervasi R, Orlando G, Innaro N, Vitale M, Sacco R. Hypocalcaemia after total thyroidectomy: could intact parathyroid hormone be a predictive factor for transient postoperative hypocalcemia? Surgery. 2015;157(2):344–348. | ||
Chapman DB, French CC, Leng X, Browne JD, Waltonen JD, Sullivan CA. Parathyroid hormone early percent change: an individualized approach to predict postthyroidectomy hypocalcemia. Am J Otolaryngol. 2012;33(2):216–220. | ||
Schlottmann F, Arbulu AL, Sadava EE, et al. Algorithm for early discharge after total thyroidectomy using PTH to predict hypocalcemia: prospective study. Langenbecks Arch Surg. 2015;400(7):831–836. | ||
Lecerf P, Orry D, Perrodeau E, et al. Parathyroid hormone decline 4 hours after total thyroidectomy accurately predicts hypocalcemia. Surgery. 2012;152(5):863–868. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



