Back to Journals » Research and Reports in Urology » Volume 11
Prostate cancer “super-active surveillance” era opened by vascular targeted photodynamic therapy
Authors Corradi RB, Travassos TC, Reis LO
Received 26 December 2018
Accepted for publication 26 April 2019
Published 29 May 2019 Volume 2019:11 Pages 157—163
DOI https://doi.org/10.2147/RRU.S178038
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jan Colli
Renato B Corradi,1,2 Thiago C Travassos,1,2 Leonardo O Reis1,2
1UroScience, Pontifical Catholic University of Campinas, Campinas, SP, Brazil; 2University of Campinas, Campinas, SP, Brazil
Abstract: The “super-active surveillance” concept denotes any active surveillance optimization that allows longer surveillance periods, with the main intention of avoiding overtreatment, by safely eliminating or postponing radical treatment. Super-active surveillance might add to the oncological control with minimal functional impact and similar quality of life compared to active surveillance, which has proved to be safe in well-selected patients. Vascular targeted photodynamic therapy has pioneering shown to significantly reduce the upgrade on subsequent biopsies, resulting in fewer cases converted to radical therapy, and any energy source can be applied to the super-active surveillance concept allowing more men to consider a tissue-preserving therapy for prostate cancer.
Keywords: Super-active surveillance, ablation, minimally invasive
The “super-active surveillance” era
In the last decade, we have witnessed a growing interest in ablative techniques to treat solid tumors. Due to the development of prostate imaging with multiparametric magnetic resonance imaging (mp-MRI), physicians are able to visualize, characterize, and target prostate lesions for biopsy and ablation.
This led to the possibility of targeting suspicious lesions and plan partial/focal gland treatment in response to the concern regarding overtreatment of patients with low-risk prostate cancer. Classical treatments such as radiotherapy (RT) and radical prostatectomy (RP) put men at risk of erectile dysfunction (ED), urinary incontinence (UI), and long-term bowel dysfunction. In this regard, different ablative modalities can be used to treat prostate cancer with the intention to decrease the treatment-related adverse effects with similar oncological outcomes compared to established treatment options.1,2
While current guidelines support active surveillance (AS) as the best treatment option for low-risk prostate cancer,3,4 it does not always remain indolent and up to 60% of the patients will undergo radical treatment within 5–10 years.5–9 A primary concern is the underestimation of cancer grade that could compromise long-term cancer control,10 which drives many physicians to recommend more aggressive interventions. Moreover, some of these patients are not comfortable with AS and want to be more active in their surveillance. For these patients, focal ablation is an option to make surveillance “super-active”, and despite adding morbidity to surveillance, it may fill the gap between surveillance and radical definitive treatment, a halfway between hypothetical undertreatment and overtreatment.10,11
Vascular targeted therapy (VTP) is one type of these treatment modalities that are expanding its importance in prostate cancer treatment in the era of the index lesion concept2 and the first ablation therapy that significantly reduced the subsequent finding of higher grade cancer on biopsy in a multicenter, randomized, controlled Phase III trial.12,13 Consequently, fewer cases were converted to radical therapy, a clinically meaningful benefit that lowered treatment-related morbidity, supporting the “super-active surveillance” concept.
Technical aspects
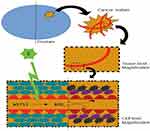
Cytotoxic ROS are well known to cause tissue damage and can be used to destroy tumor cells. Differently of classic photodynamic therapy, in which ROS formation occurs after light activation of a photosensitizer selectively accumulated in the tumor cells, VTP targets tumor vasculature, using the photosensitizing agent WST11 (Figure 1), which stays in the circulation, an effective strategy to efficiently deliver photodynamic therapy to tumor tissue.14,15
In association with a low-power near-infrared laser light in the presence of oxygen, the photosensitizer absorbs light and creates ROS that induces vascular endothelium damage and vascular occlusion, leading to tumor necrosis.16–20
General anesthesia is mandatory since any movement can lead to the need for the complete reinstallation of all transperineal fiber insertion catheters (FICs). After draining the urine, the Foley catheter is clamped to keep the bladder full for a better visualization of prostate limits by transrectal ultrasound. Using dedicated software, ultrasound prostate images are acquired; the pictures are saved and transferred to the software. Then, the urologist has to delineate the prostate limits and to launch the software in order to produce the treatment guidance.21
Transparent FICs are transperineally inserted from right to left and anterior to posterior. Before insertion, fibers have to be calibrated and locked. The optical fibers diffusing lengths (10–50 mm) are chosen according to the sagittal length of the prostate minus 5-mm safety distance from the apex, margin to the urethra, rectal wall, sphincter, and capsule. Each fiber must be positioned in the FIC bottom in order to have the illuminating diffusor perfectly positioned into the prostate.
The patient is protected from light exposure by turning off the light, and then WST11 is infused intravenously (4 mg/kg) through an opaque syringe. The activation of VTP is achieved by continuous illumination of the prostate through the optical fibers with 753-nm laser light at a power of 150 mW/cm and light energy of 200 J/cm. The illumination starts immediately after the drug injection for 22 mins and 15 s to coincide with the peak serum concentration. The total duration of the whole procedure is around 2 hrs.17,18
After the procedure, the patient is kept under medical surveillance for at least 6 hrs under dimmed light, avoiding sunlight for 48 hrs. The Foley catheter can be removed 4 hrs after the procedure and alpha-blocker is prescribed for 1 month to avoid/minimize lower urinary tract symptoms. Best results are achieved when used for prostates between 25 and 75 cc, and some absolute contraindications are acute urinary retention in the last 6 months, prior prostate disease and manipulation, and history of urethral stricture disease.21
Histopathological aspects
Histological modifications was observed in 27/56 (48%) of prostate biopsy of patients with localized WHO GG1 (Gleason 3+3 prostate cancer)
adenocarcinoma 6 months after VTP (in the nontreated lobe in 10 cases). When there was cancer in the treated lobe, it was always located outside the scar or the area of necrosis. These areas, replacing prostatic muscle and glands consisted of fibrosis and rare atrophic glands without any atypia.19
Functional aspects
Functional outcomes and adverse effects are important endpoints in prostate cancer treatment evaluation. Since it minimizes the tissue damage in the lesion surroundings, VTP is expected to reduce morbidity compared to radical therapies.
In three Phase II studies that assessed 6-month effects of VTP in patients with localized prostate cancer, there was a small improvement of International Prostate Symptoms Score (IPSS) and on the other hand, a slight deterioration in International Index of Erectile Function (IIEF-5).22
In this regard, a European study that included 68 patients treated with VTP followed for 3.5 years showed a rate of 16.2% of ED 6 months after treatment, which decreased to 15.8%, 5.4%, and 0% after 12, 18, and 36 months, respectively. In a French study with 82 patients followed for a median of 68 months, a total of 18 complaints of ED (22%) with a mean 3-point decrease in IIEF-5 score 6 months after the procedure were reported.23,24
These outcomes were also studied in a Phase III clinical trial comparing VTP to AS. The authors showed that VTP treatment increased the frequency of adverse events from 1 in 10 in men on AS to 1 in 3 men treated with VTP. Most of the events were self-limited and resolved quickly without a sequel. Regarding functional outcomes, the trial showed that patients treated with VTP underwent transient deterioration of urinary and erectile function based on IIEF-5 and IPSS assessments, but after 24 months, the results were similar between groups.12
Oncological aspects
Noweski et al reported the oncological outcomes of low-risk patients treated with VTP, based on the medium-term follow-up of two prospective, multicenter, open-label, nonrandomized Phase II studies. Among 125 patients included in these 2 studies, 68 were treated under optimal conditions and 75% of these remained cancer-free after focal ablation.21,23
In the first open-label Phase III, randomized controlled trial done in 47 European university centers and community hospitals, VTP therapy was compared to AS. The trial showed that the median time to progression from low-to-moderate or high-risk PCa was longer in the VTP group than in AS group (28.3 vs 14.1 months; p<0.0001). As expected, the proportion of patients with a negative biopsy at month 24 was higher in the VTP group, and fewer patients were submitted to radical therapy when compared with AS (6% an 29%, respectively; p<0.0001). Similar outcomes were found in Gill et al study that reported a longer follow-up; the reasons for conversion were an increase in Gleason grade or cancer volume, PSA failure, and patient choice.12,13
A multicentric study including low-volume secondary Gleason pattern 4 published in 2018 explored the proportion of patients with localized prostate cancer that would become safely biopsy negative 12 months after VPT. In the intention-to-treat population (n=81), the proportion of patients with negative biopsies at month 12 was 74% with a slight improvement in urinary function and limited deterioration in sexual function.11
Psychological aspects
Our team recently published a study in which focal cryoablation (FC), brachytherapy (B), and AS were offered to patients diagnosed with very-low-risk prostate cancer (VLRPC) in an equal-access protocol. Along with erectile and voiding symptoms, psychological aspects were evaluated by validated questionnaires. Thirty patients were included with a median follow-up of 18 months. Results showed that patients that have chosen AS were older and presented higher hopelessness and lower healthy perceptions than the patients opting for FC and B, which may indicate that age, hope, and health perceptions may determine the choice of a more active treatment.
As shown by Reis and Carter, the psychological burden in terms of anxiety over the uncertainty, or fear of losing the opportunity for a cure (which are directly related to health perception and care), leads to up to 18% of patients at AS to be overtreated with no evidence of disease progression.10,25
How it compares to other ablation energies
Different types of energies have been utilized in prostate focal ablation in a heterogeneous group of patients, and a large number of studies have assessed the oncological and functional outcomes of different ablative energies in the PCa treatment.
The high focused ultrasound (HIFU) can be guided by magnetic resonance imaging (MRI) or
trans-rectal ultrasound (TRUS). With this technique, a high-intensity beam is created by the generation of focusing ultrasound waves from a high-power spherical transducer. This beam ablates tissue through hyperthermia (temperatures between 60°C and 90°C must be achieved) and cavitation, causing coagulative necrosis.26
A HIFU systematic review carried by Golan et al included 11 studies and showed a large range outcome of ED and UI, due to its variety of definitions and follow-up in each study. Regarding the follow-up biopsies, 8% were reported to have significant cancer.27
A matched pair analysis of 110 men with unilateral (pT2a-b) disease compared robotic RP to HIFU hemi ablation, showing faster return to continence (with comparable rates after 2 years) and better outcomes regarding ED in patients who underwent prostate ablation. The need for secondary treatment was comparable in both groups.28
Cryotherapy is another technique used in cancer treatment that ablates the targeted tissue through denaturation of cellular proteins, intracellular dehydration, and metabolic failure. The delayed vascular injury is the main mechanism of cell death, added to the immediate cellular damage. The target tissue is submitted to two freeze and thaw cycles by an ultrasound-guided system and 17-gauge cryoneedles, thermocouples of argon, and helium inlets. During these cycles, a transurethral warming device is used to prevent urethral damage and the rectal temperature is monitored to avoid damage.29 Published in 2013, a review compared 5 studies that included a varying range of patients, with low-, intermediate-, and high-risk cancer. The authors reported maintained erectile function in 58.1–90% of the patients and UI occurred in 0–3.6% of the patients. Among the studies included in this review, one with data from 1,160 patients from the COLD (Cryo On-Line Database) registry assessed the oncological outcomes in 14% of the patients. The biopsy was made in these patients because of a rise in PSA and showed a 26% of prostate cancer, with a mean WHO GG1 (Gleason 3+3) prostate cancer. Patients presented a pad-free continence rate of 98.4% and spontaneous erections in 58.1% of cases.29,30
Although long-term data on outcomes are still needed about irreversible electroporation, it is a promising treatment. There are no strong data supporting this technique in treating prostate cancer. It is applied under TRUS guidance with up to six needle-like electrode probes placed parallel at a fixed distance using a brachygrip placed to the perineum.31 In the few studies available in the literature, reported functional outcomes are excellent with about the total amount of treated patients without adverse effects such as UI and ED. However, the oncological outcomes are still immature to support this technique as an option in the PCa treatment, and long-term data are still needed.32,33
Table 1 compares ablation techniques based on literature review.12,23,34–38 Comparing these different ablative techniques is not an easy task because of the lack of high-quality studies in the literature. Results must be analyzed carefully since most of them are retrospective with variable follow-up and not standardized for methods of patient’s selection, oncological, and functional outcome assessments.
 | Table 1 Ablation technique comparison based on literature review.12,23,34–38 |
Although regarding functional and oncological outcomes all the ablative techniques seem to perform equally well, cryotherapy and HIFU are the most thoroughly studied, and VTP is, to date, the only one that has been evaluated in a multicenter randomized controlled phase III trial.12
Future perspectives
A few years ago, men with low-risk prostate cancer did not have different treatment options other than RT or RP. This scenario has been widely modified with the improvement of the AS protocols. However, part of these patients will not feel comfortable with surveillance, and despite definitive treatment bringing a low probability of adding years to life in a low-risk scenario, ablative treatment modalities may be an option with low side-effect profile and possibly lower cost in a broad perspective.
Part of the prostate can be spared from the treatment-related adverse effects with focal or hemi-gland ablation. Thus, these techniques rely on imaging exam ability to properly stage cancer. Prostate MRI has shown high sensitivity and negative predictive value to clinically significant prostate cancer diagnoses.2,10,39
Advances in focal or hemi-gland ablative therapies walk together with the improvements of imaging modalities. The adoption of MRI into clinics made it possible to treat men with WHO GG1 (Gleason 3+3) by ablative treatment, and it is likely that men with well-characterized low volume secondary Gleason 4 will be also candidates in the near future.11
Despite the promising results, there are still concerns whether current biopsy and imaging techniques can adequately localize the index cancer.2 Furthermore, more data should be generated in terms of cancer treatment since VTP was not completely effective in eliminating cancer in the targeted lobe in a previous study.2,23
Also, as the mechanism of VTP involves tumor tissue ablation by ROS generation, it is not expected to potentially lead to an increased incidence in genome-wide mutations in cancer cells and there is no rationale to support resistance to any targeted therapy after treatment. However, technical challenges and limitations of VTP therapy are mainly related to light penetration, sunshine toxicity, and light exposure during and soon after the procedure. While its applicability is well described in localized tumors, there is substantial potential to metastatic tumors treatment, though still underexplored. As more data accumulate to confirm the efficacy and safety of prostate ablation, the challenge shifts to proper patient selection.
Future trials should focus on the treatment of patients with Gleason 4 patterns. We then might have answers about if this therapy is only an option for patients who are uncomfortable with AS or if it is a way to postpone radical treatment and their adverse effects or even a successful therapy to eradicate low- and intermediate-risk PCa by “precise multifocal partial gland ablation”.40
Another important point to be assessed in future research is the treatment-related effects of the therapy in the tissues in terms of fibrosis and how it would impact future treatments. Does it harm the feasibility of future radical treatment? Is it possible to make nerve bundle preservation after ablation? The previous gland ablation can impact negatively in the functional outcomes of possible future therapies, and it is one of the main points to be discussed with patients.
Also, despite there is evidence that an index lesion dictates the tumor metastatic potential, one strong argument against focal therapy is PCa multifocality.41 There are concerns that nonindex lesions may harbor significant lesions and potentially originate metastasis.2 Future studies should also report on oncological and immunological outcomes on the untreated prostate lobe. Our team has pioneering explored the vaccine potential of PCa ablation, awarding 2018 American Urological Association best poster. The ablation immunology understanding is underway, and there is evidence that supports an immune system boost by partially ablating42 that warrants future investigations.
Take home message
Supporting the "super-active surveillance" concept, prostate cancer focal ablation therapy has recently shown to significantly reduce the upgrading on subsequent biopsy, resulting in fewer cases converted to radical therapy, a clinically meaningful benefit that lowered treatment-related morbidity.
Acknowledgments
Thanks goes to the involved institution(s), the patients and those that provided and cared for study patients and to CAPES, BEX 14679/13-2, and CNPq Research Productivity, 304747/2018-1 (Reis LO).
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ficarra V, Novara G, Rosen RC, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2012;62(3):405–417. doi:10.1016/j.eururo.2012.05.045
2. Reis LO, Billis A, Zequi SC, et al. Supporting prostate cancer focal therapy: a multidisciplinary International consensus of experts (“ICE”). Aging Male. 2014;17(2):66–71. doi:10.3109/13685538.2014.895319
3. Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part I: risk stratification, shared decision making, and care options. J Urol. 2018;199(3):683–690. doi:10.1016/j.juro.2017.11.095
4. Sanda MG, Cadeddu JA, Kirkby E, et al. Clinically localized prostate cancer: AUA/ASTRO/SUO guideline. Part II: recommended approaches and details of specific care options. J Urol. 2018;199(4):990–997. doi:10.1016/j.juro.2018.01.002
5. Musunuru HB, Yamamoto T, Klotz L, et al. Active surveillance for intermediate risk prostate cancer: survival outcomes in the sunnybrook experience. J Urol. 2016;196(6):1651–1658. doi:10.1016/j.juro.2016.06.102
6. Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and longer-term outcomes from a prospective active-surveillance program for favorable-risk prostate cancer. J Clin Oncol. 2015;33(30):3379–3385. doi:10.1200/JCO.2015.62.5764
7. Godtman RA, Holmberg E, Khatami A, Pihl C-G, Stranne J, Hugosson J. Long-term results of active surveillance in the Göteborg randomized, population-based prostate cancer screening trial. Eur Urol. 2016;70(5):760–766. doi:10.1016/j.eururo.2016.03.048
8. Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28(1):126–131. doi:10.1200/JCO.2009.24.2180
9. Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol. 2013;63(4):597–603. doi:10.1016/j.eururo.2012.11.005
10. Reis LO, Carter HB. The mind. Int Braz J Urol. 2015;41(1):10–14. doi:10.1590/S1677-5538.IBJU.2015.01.03
11. Rodriguez-Rivera JA, Rodriguez-Lay R, Zegarra-Montes L, et al. Extensión de la indicación de terapia fotodinámica dirigida vascular con padeliporfina (WST11): resultados de un estudio multicéntrico latinoamericano del cáncer de próstata. Actas Urológicas Españolas. 2018;42(10):632–638. doi:10.1016/j.acuro.2018.02.009
12. Azzouzi A-R, Vincendeau S, Barret E, et al. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): an open-label, phase 3, randomised controlled trial. Lancet Oncol. 2017;18(2):181–191. doi:10.1016/S1470-2045(16)30661-1
13. Gill IS, Azzouzi A-R, Emberton M, et al. Randomized trial of partial gland ablation with vascular targeted phototherapy versus active surveillance for low risk prostate cancer: extended followup and analyses of effectiveness. J Urol. 2018;200(4):786–793. doi:10.1016/j.juro.2018.05.121
14. Azzouzi A-R, Barret E, Moore CM, et al. TOOKAD ® soluble vascular-targeted photodynamic (VTP) therapy: determination of optimal treatment conditions and assessment of effects in patients with localised prostate cancer. BJU Int. 2013;112(6):766–774. doi:10.1111/bju.12265
15. Moore CM, Azzouzi A-R, Barret E, et al. Determination of optimal drug dose and light dose index to achieve minimally invasive focal ablation of localised prostate cancer using WST11-vascular-targeted photodynamic (VTP) therapy. BJU Int. 2015;116(6):888–896. doi:10.1111/bju.12816
16. Tempel-Brami C, Pinkas I, Scherz A, Salomon Y. Detection of light images by simple tissues as visualized by photosensitized magnetic resonance imaging. Secomb T, ed. PLoS One. 2007;2(11):e1191. doi:10.1371/journal.pone.0001191
17. Mazor O, Brandis A, Plaks V, et al. WST11, A novel water-soluble bacteriochlorophyll derivative; cellular uptake, pharmacokinetics, biodistribution, and vascular targeted photodynamic activity against melanoma tumors. Photochem Photobiol. 2004. doi:10.1562/2004-06-14-RA-199
18. Vakrat-Haglili Y, Weiner L, Brumfeld V, et al. The microenvironment effect on the generation of reactive oxygen species by Pd−bacteriopheophorbide. J Am Chem Soc. 2005;127(17):6487–6497. doi:10.1021/ja046210j
19. Eymerit-Morin C, Zidane M, Lebdai S, Triau S, Azzouzi AR, Rousselet M-C. Histopathology of prostate tissue after vascular-targeted photodynamic therapy for localized prostate cancer. Virchows Arch. 2013;463(4):547–552. doi:10.1007/s00428-013-1454-9
20. Koudinova NV, Pinthus JH, Brandis A, et al. Photodynamic therapy with Pd-bacteriopheophorbide (TOOKAD): successful in vivo treatment of human prostatic small cell carcinoma xenografts. Int J Cancer. 2003;104(6):782–789. doi:10.1002/ijc.11002
21. Azzouzi A-R, Lebdai S, Benzaghou F, Stief C. Vascular-targeted photodynamic therapy with TOOKAD® soluble in localized prostate cancer: standardization of the procedure. World J Urol. 2015;33(7):937–944. doi:10.1007/s00345-015-1535-2
22. Azzouzi AR, Barret E, Bennet J, et al. TOOKAD® soluble focal therapy: pooled analysis of three phase II studies assessing the minimally invasive ablation of localized prostate cancer. World J Urol. 2015;33(7):945–953. doi:10.1007/s00345-015-1505-8
23. Noweski A, Roosen A, Lebdai S, et al. Medium-term follow-up of vascular-targeted photodynamic therapy of localized prostate cancer using TOOKAD soluble WST-11 (phase II trials). Eur Urol Focus. 2018. doi:10.1016/j.euf.2018.04.003
24. Lebdai S, Bigot P, Leroux P-A, Berthelot L-P, Maulaz P, Azzouzi A-R. Vascular targeted photodynamic therapy with padeliporfin for low risk prostate cancer treatment: midterm oncologic outcomes. J Urol. 2017;198(2):335–344. doi:10.1016/j.juro.2017.03.119
25. de Cerqueira MA, Laranja WW, Sanches BCF, Monti CR, Reis LO. Burden of focal cryoablation versus brachytherapy versus active surveillance in the treatment of very low-risk prostate cancer: a preliminary head-to-head comprehensive assessment. Eur J Cancer Care (Engl). 2015;24(6):929–937. doi:10.1111/ecc.12307
26. de Senneville BD, Mougenot C, Moonen CTW. Real-time adaptive methods for treatment of mobile organs by MRI-controlled high-intensity focused ultrasound. Magn Reson Med. 2007;57(2):319–330. doi:10.1002/mrm.21124
27. Golan R, Bernstein AN, McClure TD, et al. Partial gland treatment of prostate cancer using high-intensity focused ultrasound in the primary and salvage settings: a systematic review. J Urol. 2017;198(5):1000–1009. doi:10.1016/j.juro.2017.03.137
28. Nguyen HD, Allen BJ, Pow-Sang JM. Focal cryotherapy in the treatment of localized prostate cancer. Cancer Control. 2013;20(3):177–180. doi:10.1177/107327481302000305
29. Gangi A, Tsoumakidou G, Abdelli O, et al. Percutaneous MR-guided cryoablation of prostate cancer: initial experience. Eur Radiol. 2012;22(8):1829–1835. doi:10.1007/s00330-012-2411-8
30. Ward JF, Jones JS. Focal cryotherapy for localized prostate cancer: a report from the national Cryo on-line database (COLD) registry. BJU Int. 2012;109(11):1648–1654. doi:10.1111/j.1464-410X.2011.10578.x
31. Lodeizen O, de Bruin M, Eggener S, et al. Ablation energies for focal treatment of prostate cancer. World J Urol. 2019;37(3):409–418. doi:10.1007/s00345-018-2364-x
32. Ting F, Tran M, Böhm M, et al. Focal irreversible electroporation for prostate cancer: functional outcomes and short-term oncological control. Prostate Cancer Prostatic Dis. 2016;19(1):46–52. doi:10.1038/pcan.2015.47
33. Valerio M, Stricker PD, Ahmed HU, et al. Initial assessment of safety and clinical feasibility of irreversible electroporation in the focal treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2014;17(4):343–347. doi:10.1038/pcan.2014.33
34. Taneja SS, Bennett J, Coleman J, et al. Final results of a phase I/II multicenter trial of WST11 vascular targeted photodynamic therapy for hemi-ablation of the prostate in men with unilateral low risk prostate cancer performed in the United States. J Urol. 2016;196(4):1096–1104. doi:10.1016/j.juro.2016.05.113
35. Valerio M, Ahmed HU, Emberton M, et al. The role of focal therapy in the management of localised prostate cancer: a systematic review. Eur Urol. 2014;66(4):732–751. doi:10.1016/j.eururo.2013.05.048
36. Wagstaff P, Buijs M, van Den Bos W, et al. Irreversible electroporation: state of the art. Onco Targets Ther. 2016:2437. doi:10.2147/OTT.S88086.
37. Aoun F, Limani K, Peltier A, et al. High intensity focused ultrasound versus brachytherapy for the treatment of localized prostate cancer: a matched-pair analysis. Adv Urol. 2015;2015:1–9. doi:10.1155/2015/350324
38. Veereman G, Jonckheer P, Desomer A, et al. Systematic review of the efficacy and safety of high-intensity focussed ultrasound for localised prostate cancer. Eur Urol Focus. 2015;1(2):158–170. doi:10.1016/j.euf.2015.04.006
39. Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389(10071):815–822. doi:10.1016/S0140-6736(16)32401-1
40. Reis LO, Andrade DL, Bianco FJ
41. Liu W, Laitinen S, Khan S, et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med. 2009;15(5):559–565. doi:10.1038/nm.1944
42. Cerqueira MA, Ferrari KL, de Mattos A, Reis LO. Local immune modulation by decreasing CD4+/CD8+ t cells ratio after prostate cancer hemi-cryoablation. J Urol. 2018;199(4S). doi:10.1016/j.juro.2018.02.957
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.