Back to Journals » Clinical Ophthalmology » Volume 9
Prostaglandin-associated periorbitopathy in latanoprost users
Authors Nakakura S, Yamamoto M, Terao E, Nagatomi N, Matsuo N, Fujisawa Y, Fujio Y, Tabuchi H , Kiuchi Y
Received 10 October 2014
Accepted for publication 5 November 2014
Published 30 December 2014 Volume 2015:9 Pages 51—56
DOI https://doi.org/10.2147/OPTH.S75651
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Shunsuke Nakakura,1 Minamai Yamamoto,1 Etsuko Terao,1 Nozomi Nagatomi,1 Naoko Matsuo,1 Yausko Fujisawa,1 Yuki Fujio,1 Hitoshi Tabuchi,1 Yoshiaki Kiuchi2
1Department of Ophthalmology, Saneikai Tsukazaki Hospital, Himeji, Japan; 2Department of Ophthalmology and Visual Sciences, Graduate School of Biomedical Sciences, Hiroshima University, Hiroshima, Japan
Purpose: We investigated the incidence of prostaglandin-associated periorbitopathy (PAP) in subjects with glaucoma treated with latanoprost ophthalmic solution.
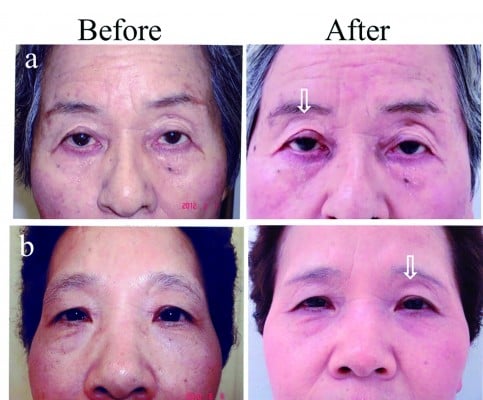
Subjects and methods: One eye and the forehead in 22 subjects were evaluated. All patients had used latanoprost for more than 1 year (range, 12 to 45 months; mean, 26.0 months) and were prostaglandin F2α analogue treatment-naïve. Digital photographs of the subjects obtained before latanoprost therapy and at the last examination were compared retrospectively. Four signs of PAP (deepening of the upper eyelid sulcus (DUES), upper eyelid ptosis, flattening of the lower eyelid bags, and inferior scleral show) and supplemental side effects around the eyelids (eyelash growth, poliosis, and eyelid pigmentation) were judged to be negative or positive by three independent observers. If the observers unanimously rated a sign as positive, the result was defined as positive.
Results: Twelve subjects (54.5%) had no apparent signs. Three subjects were judged to have DUES (13.6%), and two subjects each were judged to have flattening of the lower eyelid bags and eyelid pigmentation (9.0%). The other signs were judged as positive in only one subject each, respectively (4.5%). A univariate logistic regression analysis showed no significant associations between any of the signs and age, sex, or the duration of therapy.
Conclusion: Latanoprost induced DUES, upper eyelid ptosis, flattening of the lower eyelid bags, inferior scleral show, and supplemental side effects around the eyelids; however, the rates of such occurrence might be relatively low.
Keywords: glaucoma, prostaglandin F2α, deepening of the upper eyelid sulcus
Introduction
Peplinski and Smith first reported the side effect of prostaglandin F2α analogues to be “deepening of the upper eyelid sulcus (DUES)” in bimatoprost users in 2004.1 DUES was subsequently reported among users of other prostaglandin F2α analogues, including travoprost,2–6 tafluprost,6,7 latanoprost,5,8,9 and isopropyl unoprostone.7 DUES has since become a frequently recognized cosmetic side effect of prostaglandin F2α analogues. However, the term DUES refers to upper eyelid problems only, and similar side effects in the lower eyelids should be considered. Recently, the new term “prostaglandin-associated periorbitopathy (PAP)” was proposed as a general term for side effects of prostaglandin F2α analogues that occur around the eyelids10 and was later confirmed by Shah et al in 2013.11 PAP includes upper eyelid ptosis, DUES, involution dermatochalasis, orbital fat atrophy, mild enophthalmos, flattening of the lower eyelid bags, inferior scleral show, and a tight orbit.8,10
However, these side effects frequently coexist and do not have their own strict definitions. Previous studies have prospectively reported the incidence of DUES in patients treated with bimatoprost,12,13 travoprost,4 and tafluprost;7 however, there are no prospective data regarding DUES in patients treated with latanoprost. Furthermore, previously reported DUES symptoms in latanoprost users were evaluated in comparison with the unaffected eye,5,8,9 based on the assumption of facial symmetry, not comparison with the natural eye observed before treatment. Moreover, no previous studies have investigated the incidence of other signs of PAP and poliosis in patients treated with prostaglandin F2α analogues. Therefore, the primary purpose of this study (Japan Clinical Trials Register number: UMIN000014127) was to investigate the incidence of signs of PAP in subjects treated with latanoprost for more than 1 year by comparing photographs obtained before and after latanoprost treatment. The second purpose was to investigate the incidence of other side effects around the eyelids, including eyelash growth,14 poliosis,15 and eyelid pigmentation.14
Subjects and methods
This study received approval from the Institutional Review Board of Saneikai Tsukazaki Hospital, Himeji, Japan and was performed according to the tenets of the Declaration of Helsinki. Written informed consent was obtained from each participant prior to enrollment in this cross-sectional retrospective study. Since 2011, all patients who are diagnosed with glaucoma at our clinic have a photograph taken with naturally open eyes, to allow for subsequent evaluation of the ocular side effects associated with glaucoma medication.
Therefore, between January and June 2014, we recruited primary open-angle glaucoma patients who had been treated with latanoprost (0.005%) for more than 1 year at our hospital. All patients were prostaglandin F2α–naïve at the beginning of the latanoprost therapy.
All subjects had primary open-angle glaucoma, as defined based on 1) the results of a gonioscopic examination that revealed an open angle and 2) the presence of visual field defects, in least one of the eyes, whose location corresponded to glaucomatous disc excavation, namely the presence of a focal or diffuse defect of the optic disc rim, with or without retinal nerve fiber layer defects. None of the subjects had a previous history of glaucoma surgery or neuroophthalmological disease, and no patients underwent ocular surgery during the follow-up period. All subjects underwent photography of the eye and forehead before starting treatment with 0.005% latanoprost therapy, and at the last visit, between January and June 2014, to evaluate the side effects of treatment. Finally, one eye and the forehead in 22 patients were included in this cross-sectional, retrospective study. Complete ophthalmic examinations and intraocular pressure (IOP) measurement with a Goldmann applanation tonometer were performed by SN. All study patients presented in the Figures provided their written informed consent, as outlined in the Clinical Ophthalmology consent form, prior to the publication of their photographs.
Photography
Experienced certified orthoptists photographed the forehead and eyes under the same room conditions, using a digital single-lens camera (the Cyber-shot™ DSCN1 8.1MP [Sony, Tokyo, Japan] until March 2013, when the camera was broken, and then the EXLIM EX-ZR200 [Casio, Tokyo, Japan] after March 2013).
The patient was asked to relax, and a photograph was taken with natural open eyes because the presence of wide-open eyes can cause a DUES-like appearance.16 The images were printed on glossy paper (catalog number KL400PSKR; EPSON, Tokyo, Japan) using a digital printer (Colorio me E-350W; EPSON) and mounted on A4-sized (210×297 mm) paper, in a horizontal line for each patient. If both eyes were under latanoprost therapy, one eye was randomly selected by YF and YF.
Three evaluators (MY, NN, and NM) who were blinded to each other judged two photographs in each case for the four signs of PAP (DUES, upper eyelid ptosis, flattening of the lower eyelid bags, and inferior scleral show) and three supplemental side effects around the eyelids (eyelash growth, poliosis, and eyelid pigmentation). Observations unanimously rated positive by the observers were defined as positive for all seven signs. PAP also included other side effects, such as involution dermatochalasis, orbital fat atrophy, and a tight orbit (as mentioned above). Involution dermatochalasis is similar to DUES, while orbital fat atrophy was excluded from this study because a previous report based on a pathological study showed that this sign is the main cause of DUES.17 Furthermore, judging the presence of orbital fat atrophy or tight orbit syndrome18 using two-dimensional photographs is difficult; therefore, we excluded these side effects in this study.
Statistical analysis
We used the JMP software program, version 10.0.0 (SAS Institute Inc, Cary, NC, USA) to calculate the patient demographics and perform the univariate logistic regression analyses using age, sex, and the duration of therapy as independent predictors of each sign.
The degree of agreement between the three observers regarding the seven signs was evaluated according to Fleiss’ κ factor using the statistical package R, version 2.15.0 software program (R Foundation for Statistical Computing, Vienna, Austria). P-values <0.05 were considered to be statistically significant.
Results
The patient demographics are shown in Table 1. The mean follow-up period was 26.0±8.2 (standard deviation [SD]) months, with a range of 12 to 45 months. Eighteen subjects were prescribed 0.005% latanoprost monotherapy from the beginning of the study to the last examination, and three subjects were prescribed latanoprost 0.005%/timolol 0.5% fixed combination therapy from the beginning of the study to the last examination. One subject was prescribed latanoprost for 15 months and switched to latanoprost 0.005%/timolol 0.5% fixed combination therapy (for 9 months). The κ coefficients of the seven signs among the three observers are shown in Table 2. DUES exhibited the best agreement (κ coefficient =0.618), whereas eyelash growth demonstrated the worst agreement (κ coefficient =-0.048), and upper eyelid ptosis displayed relatively poor agreement (κ coefficient =0.287).
  | Table 1 Patient demographics |
  | Table 2 Interobserver agreement for the seven signs |
The other signs showed relatively good agreement (κ coefficient =0.529–0.567).
Table 3 presents the incidence of each side effect. Twelve subjects (54.5%) had no apparent signs. Three subjects were judged to have DUES (13.6%), and two subjects each were judged to have flattening of the lower eyelid bags and eyelid pigmentation (9.0%). The other signs were judged as positive in only one subject each, respectively (4.5%). In addition, only a 76-year-old male who received latanoprost therapy in both eyes for 33 months was judged to have two side effects (DUES and eyelid pigmentation). The other eight patients each had only one sign. The univariate logistic regression analyses showed that the age, sex, and the duration of therapy exhibited no significant associations with seven signs (all P>0.3).
  | Table 3 Incidence of PAP signs and supplemental side effects |
Figure 1 demonstrates the signs of latanoprost-induced DUES.
Discussion
In this study, we assessed the incidence of signs of PAP among patients treated with the prostaglandin F2α analogue latanoprost. In addition, we investigated the frequency of supplemental signs around the eyelids (eyelash growth, poliosis, and eyelid pigmentation) as these side effects may be associated with the original cause of PAP.
The mechanisms underlying the development of PAP have not been precisely demonstrated. Originally, it was believed that prostaglandin F2α analogues reduce the IOP by increasing the uveoscleral outflow, via a reduction in the levels of collagen types I, III, IV in the ciliary smooth muscles and adjacent sclera.19,20 Collagens constitute the stromal extracellular matrix that fills the spaces between ciliary smooth muscle fiber bundles.19,20 Muller muscles and the levator palpebrae superioris function as smooth muscles in the upper eyelids,21 while the posterior layer of the lower eyelid retractor connects to abundant smooth muscles in the lower eyelid.22 The degeneration of smooth muscles fiber due to the reduction of collagens is one hypothesis regarding the mechanism of upper eyelid ptosis and an inferior scleral show. Recently, various reports have demonstrated the possibility that prostaglandin analogues inhibit adipogenesis via FP receptor stimulation,23,24 thus resulting in involution dermatochalasis, orbital fat atrophy, mild enophthalmos, DUES, and flattening of the lower eyelid bags.
Concerning supplemental side effects, the eyelid pigmentation caused by prostaglandin F2α analogues is thought to be due to increased tyrosinase activity, which increases the production of melanin in the eyelids.25 However, this mechanism may be contradicted by the occurrence of poliosis. The cause of poliosis remains unknown; however, Chen et al reported that among seven patients with poliosis, five patients had hypertrichosis, and the authors concluded that prostaglandin affects melanogenesis, which only occurs during the anagen phase of the hair follicle cell cycle.15 Another possible mechanism of poliosis is inflammation. Patients with anterior uveitis caused by latanoprost tend to have poliosis, although in one study, the uveitis completely resolved without topical steroids within 2 weeks and the poliosis improved after 10 months following the discontinuation of latanoprost.26
The incidence of DUES in latanoprost users is relatively low compared with that observed in bimatoprost- and travoprost-users (more than 50%).4,6,10 Meanwhile, the incidence of DUES among tafluprost-users is 14% at 6 months,7 which is similar to our result of 13.6% among patients treated with latanoprost.
No previous studies have investigated the incidence of other signs of PAP in a prospective manner. However, Shah et al reported that bimatoprost, travoprost, and latanoprost were each significantly associated with upper eyelid ptosis, worsening of the levator function, and an inferior scleral show.11 However, with respect to the loss of dermatochalasis and lower lid steatoblepharon (flattening of the lower eyelid), neither have been found to be significantly associated with latanoprost treatment, although they are associated with bimatoprost and travoprost therapy. These results support our findings of a lower incidence of PAP in latanoprost users. Therefore, the incidence of lower lid steatoblepharon (flattening of the lower eyelid) may also be lower than that associated with bimatoprost and travoprost.
The degree of eyelash growth in patients treated with latanoprost was found to be relatively low in previous reports: 0% and 4.4% at 3 months, as reported by Parrish et al27 and Gandolfi et al28 respectively, which are similar to our results. In contrast, Chiba et al reported an incidence of eyelash growth of 46.2% at 1 year using slit lamp photography.29 The cause of this difference in incidence is likely due to differences in the magnification of the photographs, and this same phenomenon may have also caused the worse κ coefficients in eyelash growth that were observed in the current study. Tight orbit syndrome was recognized as a narrow palpebral fissure, difficulty in measuring the IOP with the Goldmann applanation tonometer due to tight eyelids, and difficulty in controlling the IOP.18 All subjects were under good IOP control with topical antiglaucoma medications, and tight eyelids cannot be judged on photographs. Therefore, we excluded this side effect in this study.
One limitation of this study is that the tone of the color photographs was different in some patients because the camera used in the first part of the study broke and had to be replaced, which may have affected the judgment of the observers. The second limitation is the strict definition of a positive judgment, which has resulted in the low incidence of side effects. However, according to previous work using comparisons between subjective evaluations based on photographs and objective evaluations using questionnaires, the presence of DUES is less frequently reported in subjective evaluations than in objective evaluations.4,6,7,12,13 Furthermore, DUES was rated as positive by at least two of the three observers6 or by consensus after consultations among the three observers.4,7,12,13
Therefore, the data obtained using the method involving unanimous findings by three independent observers may be more accurate. Meanwhile, the patients’ perception of these side effects might be a crucial counterpoint to the clinician’s view because if patients have noticed the side effect, that side effect will affect their adherence.
The third limitation is that our subjects constituted a relatively small group; we did not obtain a sample size estimation prior to sampling because providing follow-up for more than 1 year using the same eye drops is relatively difficult in real clinical practice. Kashiwagi and Furuya reported rates of continuation of glaucoma eye drops at 3, 6, and 12 months, and at 3 years after the initiation of therapy, of 73.2%, 68.1%, 60.9% and 52.5%, respectively, in newly diagnosed glaucoma patients.30 This surprising data support our hypothesis that it is challenging to gather a large group of patients.
If a patient was using latanoprost for long term, he or she may have multiple PAP signs; however, we herein demonstrated a relatively low incidence for latanoprost-associated PAP and side effects around the eyelids. Clinicians and patients should both be aware of the potential for DUES as well as other new side effects of PAP, in order to increase adherence with therapy.
Acknowledgments
The authors thank Hajime Yamakage for his advice on the statistical analyses performed in this study. All facial photographs and patient data are available for review by interested readers. Please contact the corresponding author with any questions.
Disclosure
The authors report no conflicts of interest in this work.
References
Peplinski LS, Albiani Smith K. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81(8):574–577. | ||
Yang HK, Park KH, Kim TW, Kim DM. Deepening of eyelid superior sulcus during topical travoprost treatment. Jpn J Ophthalmol. 2009;53(2):176–179. | ||
Nakakura S, Tabuchi H, Kiuchi Y. Latanoprost therapy after sunken eyes caused by travoprost or bimatoprost. Optom Vis Sci. 2011;88(9):1140–1144. | ||
Maruyama K, Shirato S, Tsuchisaka A. Incidence of deepening of the upper eyelid sulcus after topical use of travoprost ophthalmic solution in Japanese. J Glaucoma. 2014;23(3):160–163. | ||
Kucukevcilioglu M, Bayer A, Uysal Y, Altinsoy HI. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Experiment Ophthalmol. 2014;42(2):126–131. | ||
Inoue K, Shiokawa M, Wakakura M, Tomita G. Deepening of the upper eyelid sulcus caused by 5 types of prostaglandin analogs. J Glaucoma. 2013;22(8):626–631. | ||
Sakata R, Shirato S, Miyata K, Aihara M. Incidence of deepening of the upper eyelid sulcus on treatment with a tafluprost ophthalmic solution. Jpn J Ophthalmol. 2014;58(2):212–217. | ||
Tan J, Berke S. Latanoprost-induced prostaglandin-associated periorbitopathy. Optom Vis Sci. 2013;90(9):e245–e247; discussion 1029. | ||
Yoshino T, Fukuchi T, Togano T, Seki M, Ikegaki H, Abe H. Eyelid and eyelash changes due to prostaglandin analog therapy in unilateral treatment cases. Jpn J Ophthalmol. 2013;57(2):172–178. | ||
Pasquale LR. Prostaglandin-associated periorbitopathy: a postmarketing surveillance observation. Glaucoma Today [serial on the Internet]. 2011 Summer [cited 2014 Jun 10];(9):51–52; 58. Available from: http://bmctoday.net/glaucomatoday/pdfs/gt0611_thera_pasquale.pdf. Accessed November 6, 2014. | ||
Shah M, Lee G, Lefebvre DR, et al. A cross-sectional survey of the association between bilateral topical prostaglandin analogue use and ocular adnexal features. PLoS One. 2013;8(5):e61638. | ||
Aihara M, Shirato S, Sakata R. Incidence of deepening of the upper eyelid sulcus after switching from latanoprost to bimatoprost. Jpn J Ophthalmol. 2011;55(6):600–604. | ||
Sakata R, Shirato S, Miyata K, Aihara M. Recovery from deepening of the upper eyelid sulcus after switching from bimatoprost to latanoprost. Jpn J Ophthalmol. 2013;57(2):179–184. | ||
Lee AJ, McCluskey P. Clinical utility and differential effects of prostaglandin analogs in the management of raised intraocular pressure and ocular hypertension. Clin Ophthalmol. 2010;4:741–764. | ||
Chen CS, Wells J, Craig JE. Topical prostaglandin F(2alpha) analog induced poliosis. Am J Ophthalmol. 2004;137(5):965–966. | ||
Nakakura S, Terao E, Nagatomi N, et al. Cross-sectional study of the association between a deepening of the upper eyelid sulcus-like appearance and wide-open eyes. PLoS One. 2014;9(4):e96249. | ||
Park J, Cho HK, Moon JI. Changes to upper eyelid orbital fat from use of topical bimatoprost, travoprost, and latanoprost. Jpn J Ophthalmol. 2011;55(1):22–27. | ||
Lee GA, Ritch R, Liang SY, et al. Tight orbit syndrome: a previously unrecognized cause of open-angle glaucoma. Acta Ophthalmol. 2010;88(1):120–124. | ||
Sagara T, Gaton DD, Lindsey JD, Gabelt BT, Kaufman PL, Weinreb RN. Topical prostaglandin F2alpha treatment reduces collagen types I, III, and IV in the monkey uveoscleral outflow pathway. Arch Ophthalmol. 1999;117(6):794–801. | ||
Lindsey JD, Kashiwagi K, Kashiwagi F, Weinreb RN. Prostaglandins alter extracellular matrix adjacent to human ciliary muscle cells in vitro. Invest Ophthalmol Vis Sci. 1997;38(11):2214–2223. | ||
Kakizaki H, Zako M, Nakano T, Asamoto K, Miyaishi O, Iwaki M. The levator aponeurosis consists of two layers that include smooth muscle. Ophthal Plast Reconstr Surg. 2005;21(5):379–382. | ||
Kakizaki H, Zhao J, Nakano T, et al. The lower eyelid retractor consists of definite double layers. Ophthalmology. 2006;113(12):2346–2350. | ||
Choi HY, Lee JE, Lee JW, Park HJ, Lee JE, Jung JH. In vitro study of antiadipogenic profile of latanoprost, travoprost, bimatoprost, and tafluprost in human orbital preadiopocytes. J Ocul Pharmacol Ther. 2012;28(2):146–152. | ||
Taketani Y, Yamagishi R, Fujishiro T, Igarashi M, Sakata R, Aihara M. Activation of the prostanoid FP receptor inhibits adipogenesis leading to deepening of the upper eyelid sulcus in prostaglandin-associated periorbitopathy. Invest Ophthalmol Vis Sci. 2014;55(3):1269–1276. | ||
Dutkiewicz R, Albert DM, Levin LA. Effects of latanoprost on tyrosinase activity and mitotic index of cultured melanoma lines. Exp Eye Res. 2000;70(5):563–569. | ||
Waheed K, Laganowski H. Bilateral poliosis and granulomatous anterior uveitis associated with latanoprost use and apparent hypotrichosis on its withdrawal. Eye (Lond). 2001;15(Pt 3):347–349. | ||
Parrish RK, Palmberg P, Sheu WP; XLT Study Group. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol. 2003;135(5):688–703. | ||
Gandolfi S, Simmons ST, Sturm R, Chen K, VanDenburgh AM; Bimatoprost Study Group 3. Three-month comparison of bimatoprost and latanoprost in patients with glaucoma and ocular hypertension. Adv Ther. 2001;18(3):110–121. | ||
Chiba T, Kashiwagi K, Ishijima K, et al. A prospective study of iridial pigmentation and eyelash changes due to ophthalmic treatment with latanoprost. Jpn J Ophthalmol. 2004;48(2):141–147. | ||
Kashiwagi K, Furuya T. Persistence with topical glaucoma therapy among newly diagnosed Japanese patients. Jpn J Ophthalmol. 2014;58(1):68–74. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.