Back to Journals » Clinical Ophthalmology » Volume 14
Prospective, Single-Center, Six-Month Study of Intravitreal Ranibizumab for Macular Edema with Nonproliferative Diabetic Retinopathy: Effects on Microaneurysm Turnover and Non-Perfused Retinal Area
Authors Lee SJ , Shin IC, Jeong IW, Choi CW, Yang YS
Received 6 February 2020
Accepted for publication 28 May 2020
Published 16 June 2020 Volume 2020:14 Pages 1609—1618
DOI https://doi.org/10.2147/OPTH.S248529
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Seung Joon Lee,1,2 In Choel Shin,1,2 Il Won Jeong,1,2 Chang Wook Choi,1,2 Yun Sik Yang1,2
1Department of Ophthalmology, Wonkwang University College of Medicine, Iksan, Korea; 2Institute of Wonkwang Medical Science, Wonkwang University, Iksan, Korea
Correspondence: Yun Sik Yang
Department of Ophthalmology, Wonkwang University Hospital, #895 Muwang-Ro, Iksan, 54538, Korea
Tel +82 63 859 1370
Fax +82 63 855 1801
Email [email protected]
Purpose: To analyze the effects on microaneurysm (MA) and perifoveal perfusion in nonproliferative diabetic retinopathy (NPDR) patients with macular edema (ME) after early intensive treatment using intravitreal ranibizumab (IVR) injections.
Patients and Methods: Prospectively, 25 eyes of 25 type 2 diabetes mellitus patients with ME were included between August 2016 and February 2019. For 6 months, patients were administered 0.5-mg IVR injections monthly. Ocular evaluation, including best-corrected visual acuity (BCVA; using the Early Treatment Diabetic Retinopathy Study chart), central retinal thickness (CRT; using optical coherence tomography), fundus photography, and fluorescein angiography, was performed for all participants. Results obtained at baseline were compared to those observed after 6 months.
Results: Mean BCVA increased significantly from 67.6± 3.29 letters at baseline to 76.36± 1.61 letters after 6 months (P=0.002) of IVR therapy. CRT decreased significantly from 479.12± 16.66 μm at baseline to 369.12± 13.02 μm at 6 months. Similarly, the total number of MAs decreased significantly from 5.68± 3.41 to 1.60± 1.73 (P< 0.0001). MA turnover, calculated by adding the MA formation rate to the MA disappearance rate (both calculated as MA number/month) also decreased significantly from 6.88± 3.83 to 1.92± 1.75 after treatment (P< 0.0001). Perifoveal non-perfused area decreased from 2.517± 0.456 mm2 at baseline to 2.495± 0.293 mm2 at 6 months, but the results were not statistically significant (P=0.954).
Conclusion: Treatment with early intensive IVR therapy in NPDR patients with ME not only improved BCVA and CRT but also decreased MA turnover. However, in the study period of 6 months, IVR therapy did not show significant improvement in perifoveal non-perfused area.
Keywords: nonproliferative diabetic retinopathy, macular edema, ranibizumab, early intensive treatment, microaneurysm
Introduction
Increased prevalence of diabetes mellitus has roused interest in its ophthalmic sequelae such as diabetic retinopathy (DR) and diabetic macular edema (DME).1 DR and DME are major causes of visual impairment in millions of patients worldwide,2 and there are numerous ongoing studies on the efficacy of various treatment modalities for these conditions. While multiple causes of retinopathy have been implicated, vascular endothelial growth factor (VEGF) seems to play an important role in the pathogenesis of these conditions.3,4 DR, in both proliferative and nonproliferative stages, shows an increase in VEGF production,5 which causes structural changes in the retinal vessels, leading to subsequent increase in vessel permeability and angiogenesis, and ultimately, visual impairment.6
Panretinal laser photocoagulation (PRP) and focal laser treatment have played important roles in preventing the progression of DR and in alleviating DME. However, PRP can cause permanent impairment of peripheral vision, night blindness, and deterioration of DME. Further, it cannot be performed in certain patients with concomitant cataract or vitreous hemorrhage.7 Focal laser treatment can also be utilized in selected patients only.8 To address these shortcomings, anti-VEGF therapy has been proposed for the management of both DR and DME.9 Recently, intravitreal, injectable, anti-VEGF antibody has been reported to delay the progression and ameliorate the severity of both DR and DME.10–13
Results of multi-center, randomized-controlled clinical trials such as RIDE, RISE, and RESOLVE studies have shown the efficacy of intravitreal ranibizumab (IVR) injection monotherapy in the treatment of DME, based on markers such as best-corrected visual acuity (BCVA) and central retinal thickness (CRT).14–17 Furthermore, many recent studies have shown that the formation and disappearance of microaneurysms (MAs), as well as the extent of perifoveal non-perfused area, can also be used as markers to predict the progression and prognosis of DME.18–25
In this prospective study, we evaluated the efficacy of IVR therapy by assessing the response to monthly administration of IVR injections for 6 months in nonproliferative diabetic retinopathy (NPDR) patients with DME. Changes in BCVA and CRT, as compared to the baseline, were evaluated monthly, for 6 months. Accompanying markers such as changes in total number of MAs, MA turnover, and extent of perifoveal non-perfused area were also analyzed and compared to their pre-treatment values.
Materials and Methods
Study Design
This was an interventional, prospective, single-center study conducted at Wonkwang University Hospital from August 2016 to February 2019.
All patients included in the study provided signed, informed consents prior to enrollment. The consent statement included the purpose and method of the study as well as information on reasonable expected benefits along with potential risks and side effects.
The study protocol was approved by the Institutional Review Board of the Wonkwang University Hospital (Approval no: WKUH 201,602-CTDG-005). The clinical trial has been registered on ClinicalTrials.gov (ID: NCT02834663). This study abided by the principles defined in the Declaration of Helsinki throughout the trial.
Method
All patients underwent the following baseline ophthalmological examinations and investigations: i) Assessment of BCVA using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart, ii) measurement of intraocular pressure, iii) optical coherence tomography (OCT) (Spectralis OCT®: Heidelberg engineering, Heidelberg, Germany), iv) fundus photography (Kowa nonmyd 7®: Kowa American Corporation, Kowa, USA), and v) fluorescein angiography (FA) (SpectralisHRA2®: Heidelberg engineering, Heidelberg, Germany).
Patients were administered 0.5-mg IVR injections monthly for 6 months. At each visit, the above-mentioned ophthalmological tests (except FA) were repeated. FA was performed at baseline and was repeated after 3 and 6 months of treatment.
Enrollment Criteria
NPDR patients aged >40 years, having type 2 diabetes mellitus, with BCVA of the study eye being ≥20/200 (Snellen equivalent using ETDRS testing), and having CRT of ≥300 µm on OCT were included in this study. Patients with proliferative diabetic retinopathy (PDR), previous history of vitreoretinal surgery, post-cataract operation status (≤4 months before participation in this study), uncontrolled hypertension, or glaucoma were excluded from the study. Patients who had received prior treatment with anti-VEGF drugs, intraocular corticosteroids, and/or retinal laser application were also excluded. If both eyes met the study inclusion criteria, only the more severely affected eye was selected for treatment, based on BCVA.
Assessment of Treatment Efficacy
Patients were evaluated for changes in BCVA and CRT of the treated eye, from the start of the study to 6 months, until trial completion.
The MAs and perifoveal non-perfused areas in individual retinas were evaluated at 6 months using fundus photography and FA imaging. The Retmarker (version 1.0.2 by Retmarker Ltd, Coimbra, Portugal) software was used for automatic measurement and analysis of changes in number and extent of MAs on fundus photographs and to calculate the total number and turnover of MAs. It includes a co-registration algorithm that allows comparison within the same retinal location between different visits for the same eye. MA turnover was calculated by adding the MA formation rate (number of new MAs detected/month) to the MA disappearance rate (number of MAs resolved/month) (Figure 1).20–22 Perifoveal non-perfused area was analyzed using the arteriovenous phase in the FA image (from each of the three examinations) when perifoveal capillary network was best visualized, that is, approximately 30 seconds after injection, and was estimated by connecting the blood vessels instead of the branches of superior and inferior temporal venules within 30 degrees. Subsequently, the ImageJ software (version 1.25a 23/04/2018 by ImageJ, USA) was used for scaling each image by 16 pixels, down to 200 µm. Thereafter, the contrast and sensitivity of all photographs were converted to the maximum values to compare the perfused and non-perfused areas. The raw red-green-blue (RGB) images were then converted to 8-bit images, with the threshold set for optimal visualization of the non-perfused area. Thus, FA images that needed analysis were converted to raw RGB images, the program automatically converted the thresholds, and one retina specialist assessed the image for accuracy. The same threshold was applied to all images of the same patient. Subsequently, particle analysis was performed to calculate the total perifoveal non-perfused area (Figure 2).
Statistical Analysis
The SPSS Statistics 18 (IBM, Armonk, NY, USA) software was used for data analysis. The repeated measures analysis of variance with Bonferroni correction was used to analyze continuous outcome measures, including BCVA, CRT, total number of MAs, MA formation rate, MA disappearance rate, MA turnover, and perifoveal non-perfused area, before and after completion of 6 months of IVR therapy. A P-value <0.05 was considered statistically significant.
Results
Baseline Characteristics
Totally, 25 eyes of 25 patients (13 males, 12 females; mean age, 63.80±11.45 years; range, 41–80 years) were included in the study. Baseline characteristics of all study patients are summarized in Table 1.
 |
Table 1 Baseline Characteristics of All Study Patients |
BCVA & CRT Outcomes
The mean BCVA (ETDRS letters) at baseline was 67.6±3.29 letters, which significantly increased to 76.36±1.61 letters after administration of six monthly IVR injections (P<0.0001) (Figure 3).
The associated mean CRT measured at baseline was 479.12±16.66 µm, which significantly decreased to 369.12±13.02 µm after completion of the treatment regimen (P<0.0001) (Figure 4). Considering the definition of improved CRT as a ≥25% reduction in macular edema, 20/25 eyes included in the study demonstrated ≥25% reduction in macular edema. Of the 20 eyes, 14 showed improvement in CRT with administration of ≤3 IVR injections, and 3 eyes demonstrated decrease in CRT after administration of ≥4 IVR doses. The remaining 3 eyes showed fluctuating decreases and increases in CRT during the course of treatment, but finally showed definitive improvement after administration of all 6 IVR injections (Figure 5).
 |
Figure 4 Mean central retinal thickness over the six-month study period. *Statistically significance is P < 0.05 with baseline. Abbreviation: CRT, central retinal thickness. |
Changes in MA Turnover and Perifoveal Non-Perfused Area
The total mean number of MAs before IVR injection was 5.68±3.41, which significantly decreased to 1.60±1.73 after administration of six monthly IVR injections (P<0.0001) (Figure 6). The MA turnover after the first IVR injection was 6.88±3.83, which significantly reduced to 1.92±1.75 after 6 months (P<0.0001) (Figure 7).
 |
Figure 6 Mean total number of microaneurysms over the six-month study period. *Statistically significance is P < 0.05 with baseline. Abbreviation: MA, microaneurysm. |
 |
Figure 7 Mean turnover, rate of formation, and rate of disappearance of microaneurysms over the six-month study period. *Statistically significance is P < 0.05 with baseline. |
The mean perifoveal non-perfused area measured across three FA tests was 2.517±0.456 mm2 at baseline, which after administration of three, monthly IVR injections, decreased to 2.156±0.387 mm2 and after six, monthly IVR injections, increased to 2.495±0.293 mm2. Compared to baseline, after administration of six, monthly IVR injections, perifoveal non-perfused area showed a mild decrease, though the difference was not statistically significant (P=0.221) (Figure 8) (Table 2).
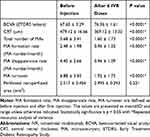 |
Table 2 Comparative Analysis of Prognostic Ophthalmic Markers of Diabetic Retinopathy |
 |
Figure 8 Changes in extent of mean perifoveal non-perfused area over the six-month study period. |
Safety Outcomes
No ocular or systemic adverse events associated with IVR injections were observed in any participant during the study period.
Discussion
In this study, we prospectively analyzed the outcomes following administration of monthly IVR injections for 6 months in NPDR patients with DME. The patients showed visual improvement by 8.76 letters, after completing the study-treatment protocol, as compared to baseline as well as demonstrated a 105.8-µm reduction in CRT. These results were similar to those of other studies that analyzed the use of anti-VEGF drugs for DR.14–17 Further, we found that 80% patients showed improvements in CRT after administration of monthly IVR injections for 6 months. An interesting finding was that among these patients, 70% showed improvement in CRT with administration of ≤3 IVR injections and 30% demonstrated improvements in CRT after administration of ≤6 IVR injections. In other words, we imply that during the treatment of NPDR patients with DME, administration of all 6 injections may not be necessary and in some cases, 3 injections could prove to be quite effective.
MAs develop in the early stages of DR, and the total number as well as turnover rate are known to play an important role in identifying and defining the disease cycle. Furthermore, MAs can be a predictive factor for the occurrence of complications that may cause visual impairment by worsening retinopathy or ME.18,21,26 Formation and disappearance of MAs is a dynamic activity, of which MA disappearance occurs mostly due to platelet and fibrin thrombi, is an irreversible process, and can indicate the occurrence of retinal capillary occlusion and vascular damage. However, it could result in the restructuring of retinal vasculature and capillary-flow bypass.27,28 Therefore, not only the formation of MAs but also their disappearance should be considered important during the evaluation of MA turnover.
MA counts and MA turnover are good markers for DR progression, and high MA turnover is a risk factor for the development of clinically significant macular edema (CSME) requiring photocoagulation. Further, MA turnover has been shown to be more reliable than MA counts.18,20 Leicht et al,29 reported that high MA turnover can result in a higher risk of developing CSME and worsening DR without DME. They also suggested that anti-VEGF therapy using IVR could delay the progression to diabetic fundus and reverse existing fundus changes to restore normality. Limited research exists regarding the effects of VEGF inhibitors such as ranibizumab on MA turnover in NPDR patients with DME, and no prospective study has evaluated the efficacy of intensive treatment using ranibizumab, which has been performed in our study. This is the strength of our study. In this study, we prospectively observed that the total number, formation rate, disappearance rate, and overall turnover of MAs, each reduced significantly after administration of six monthly IVR injections. Our results showed that these MA-related parameters could be used as appropriate markers for evaluating the progression of retinopathy and that IVR injections could delay the progression of DR.
Hyperglycemia in diabetes induces high glucose concentration within the retina, eventually damaging the endothelium and pericytes of the retinal vessels. These structural damages lead to retinal ischemia and destruction of the blood-retinal barrier, causing the development of MAs, further decreasing retinal perfusion. Increased VEGF level is known to play an important role in mediating this process.30 However, physiologic levels of VEGF are important in maintaining retinal microvascular circulation during ischemic states.24 Thus, although the mechanism of action of an anti-VEGF antibody such as ranibizumab on retinal vessels in DME is not yet elucidated, it may have a detrimental effect on retinal circulation.
Capillary loss is known to be a risk factor for DR progression. In a subanalysis of the RESTORE (extension) study, all treatment arms (ranibizumab monotherapy, ranibizumab plus laser combination therapy, laser monotherapy) showed increased capillary loss, but no difference in capillary loss around fovea was reported. This shows that IVR injections do not increase the risk of aggravating central capillary perfusion.24 Findings of the RISE and RIDE trials conducted by Ip et al,12,13 showed that administration of IVR injections can reduce the risk of progression and severity of DR and that capillary loss within the macula is an important factor for the progression to PDR. However, IVR injections for DME lead to improved vision on their own accord, regardless of the absence of macular capillary perfusion. This suggests that the presence of retinal non-perfusion may not significantly correlate with improving vision, despite being an important risk factor for predicting the severity and progression of DR, and early intervention may be indicated for achieving maximal therapeutic effects. Additionally, Bonnin et al,25 showed that 3 monthly injections of anti-VEGF agents improved BCVA and CRT and reduced grades of DR severity, such as regression of red dots (hemorrhages or MAs) were observed on color photographs, but no improvement in vessel perfusion in capillary non-perfusion area was reported. Similarly, in this study, despite a decrease in perifoveal non-perfused area after 3 months of treatment and an increase after 6 months, a significant improvement in vision was observed after early intensive treatment.
A retrospective analysis of FA images from the RISE and RIDE trials performed by Campochiaro et al,30 over a period of 3 years revealed that the area of retinal non-perfusion increased from baseline over time in groups of patients who were administered IVR in concentrations of 0.3 mg, 0.5 mg, and sham (including patients crossed over to treatment with ranibizumab). However, slower changes were evident in the IVR injection group, implying that IVR injections retarded the progression of non-perfusion faster, compared with sham. Furthermore, in patients treated using IVR injections, retinal non-perfusion area decreased paradoxically until 12 months. Karst et al,24 analyzed data from the RESTORE study for 3 years and showed that capillary loss decreased at 6 months and increased after 6 months on FA with IVR monotherapy. Although our result was not statistically significant and the study was conducted in a short period, we observed a decrease in perifoveal non-perfused area after six monthly IVR injections, similar to the results obtained by Campochiaro et al and Karst et al.
Limitations of the current study include the small sample size (n=25 eyes), short study-duration of 6 months, and the lack of a control group. However, this study is unique, as it prospectively analyzed not only the improvement in BCVA and reduction in CRT but also changes in both MA turnover and perifoveal non-perfused area in NPDR patients with DME, following IVR therapy. Furthermore, this study employed RetmakerDR and ImageJ software programs to assess the occurrence of changes within the same retinal location, despite differing timings of examinations ensuring consistency and continuity of findings and enabling a more accurate analysis of the examination results.
Conclusion
After administration of early, intensive IVR injections, BCVA improved and CRT decreased in NPDR patients with ME. Further, they decreased the MA turnover as compared to baseline. However, the extent of perifoveal non-perfused area showed a mild overall decrease as compared to baseline, after 6 months. Thus, our data suggest that early intensive IVR injections can delay DR progression, but over a 6-month period, IVR therapy did not show a significant improvement in perifoveal non-perfused area. Hence, further randomized-controlled studies of longer duration are indicated to understand the impact of newer treatment strategies on the progression and prognosis of DR.
Data Sharing Statement
Available from the corresponding author on reasonable request.
Ethics and Approval
All procedures performed in this study were approved by the Institutional Review Board of the Wonkwang University Hospital and conducted according to the principles of the Declaration of Helsinki. Prior to the procedure, written informed consent was obtained from all patients. Also, potential risks and side effects were explained.
Acknowledgments
This study was presented at the 122nd Annual Meeting of the Korean Ophthalmological Society (Seoul, Korea) on November 2, 2019. Financial support for the study was received from Novartis, Korea.
Disclosure
The authors report no conflicts of interest with respect to this work.
References
1. De Barros Garcia JMB, Isaac DLC, Avila M. Diabetic retinopathy and OCT angiography: clinical findings and future perspectives. Int J Retina Vitreous. 2017;3:14. doi:10.1186/s40942-017-0062-2
2. Reddy RK, Pieramici DJ, Gune S, et al. Efficacy of ranibizumab in eyes with diabetic macular edema and macular nonperfusion in RIDE and RISE. Ophthalmology. 2018;125:1568–1574. doi:10.1016/j.ophtha.2018.04.002
3. Simunovic MP, Maberley DA. Anti-vascular endothelial growth factor therapy for proliferative diabetic retinopathy: A systematic review and meta-analysis. Retina. 2015;35:1931–1942. doi:10.1097/IAE.0000000000000723
4. Figueira J, Silva R, Henriques J, et al. Ranibizumab for high-risk proliferative diabetic retinopathy: an exploratory randomized controlled trial. Ophthalmologica. 2016;235:34–41. doi:10.1159/000442026
5. Ishida S, Usui T, Yamashiro K, et al. VEFG164 is proinflammatory in the diabetic retina. Invest Ophthalmol Vis Sci. 2003;44:2155–2162. doi:10.1167/iovs.02-0807
6. Funatsu H, Yamashita H, Noma H, et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol. 2005;243:3–8.
7. Chandra S, Sheth J, Anantharaman G, Gopalakrishnan M. Ranibizumab-induced retinal reperfusion and regression of neovascularization in diabetic retinopathy: an angiographic illustration. Am J Ophthalmol Case Rep. 2018;4(9):41–44. doi:10.1016/j.ajoc.2018.01.006
8. Lee SN, Chhablani J, Chan CK, et al. Characterization of microaneurysm closure after focal laser photocoagulation in diabetic macular edema. Am J Ophthalmol. 2013;155:905–912. doi:10.1016/j.ajo.2012.12.005
9. Writing committee for the Diabetic Retinopathy Clinical Research Network. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: A randomized clinical trial. J Am Med Assoc. 2015;314:2137–2146. doi:10.1001/jama.2015.15217
10. The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–1203. doi:10.1056/NEJMoa1414264
11. Stewart MW. A review of ranibizumab for the treatment of diabetic retinopathy. Ophthalmol Ther. 2017;6:33–47. doi:10.1007/s40123-017-0083-9
12. Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012;130:1145–1152. doi:10.1001/archophthalmol.2012.1043
13. Ip MS, Domalpally A, Sun JK, Ehrlich JS. Long-term effects of therapy with ranibizumab on diabetic retinopathy severity and baseline risk factors for worsening retinopathy. Ophthalmology. 2015;122:367–374. doi:10.1016/j.ophtha.2014.08.048
14. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 Phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi:10.1016/j.ophtha.2011.12.039
15. Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013–2022. doi:10.1016/j.ophtha.2013.02.034
16. Boyer DS, Nguyen QD, Brown DM, Basu K, Ehrlich JS. Outcomes with as-needed ranibizumab after initial monthly therapy: long-term outcomes of the phase III RIDE and RISE trials. Ophthalmology. 2015;122:2504–2513. doi:10.1016/j.ophtha.2015.08.006
17. Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter Phase II study. Diabetes Care. 2010;33:2399–2405. doi:10.2337/dc10-0493
18. Nunes S, Pires I, Rosa A, Duarte L, Bernardes R, Cunha-Vaz J. Microaneurysm turnover is a biomarker for diabetic retinopathy progression to clinically significant macular edema: findings for type 2 diabetics with nonproliferative retinopathy. Ophthalmologica. 2009;223:292–297. doi:10.1159/000213639
19. Sjølie AK, Klein R, Porta M, et al. Retinal microaneurysm count predicts progression and regression of diabetic retinopathy. Post-hoc results from the DIRECT Programme. Diabet Med. 2011;28:345–351. doi:10.1111/j.1464-5491.2010.03210.x
20. Ribeiro ML, Nunes SG, Cunha-Vaz JG. Microaneurysm turnover at the macula predicts risk of development of clinically significant macular edema in persons with mild nonproliferative diabetic retinopathy. Diabetes Care. 2013;36:1254–1259. doi:10.2337/dc12-1491
21. Pappuru RKR, Ribeiro L, Lobo C, Alves D, Cunha-Vaz J. Microaneurysm turnover is a predictor of diabetic retinopathy progression. Br J Ophthalmol. 2019;103:222–226. doi:10.1136/bjophthalmol-2018-311887
22. Haritoglou C, Kernt M, Neubauer A, et al. Microaneurysm formation rate as a predictive marker for progression to clinically significant macular edema in nonproliferative diabetic retinopathy. Retina. 2014;34:157–164. doi:10.1097/IAE.0b013e318295f6de
23. Krawitz BD, Phillips E, Bavier RD, et al. Parafoveal nonperfusion analysis in diabetic retinopathy using optical coherence tomography angiography. Transl Vis Sci Technol. 2018;7:4. doi:10.1167/tvst.7.4.4
24. Karst SG, Deak GG, Gerendas BS, et al. Association of changes in macular perfusion with ranibizumab treatment for diabetic macular edema: a subanalysis of the RESTORE (Extension) study. JAMA Ophthalmol. 2018;136:315–321. doi:10.1001/jamaophthalmol.2017.6135
25. Bonnin S, Dupas B, Lavia C, et al. Anti-vascular endothelial growth factor therapy can improve diabetic retinopathy score without change in retinal perfusion. Retina. 2019;39:426–434. doi:10.1097/IAE.0000000000002422
26. Horii T, Murakami T, Nishijima K, Sakamoto A, Ota M, Yoshimura N. Optical coherence tomographic characteristics of microaneurysms in diabetic retinopathy. Am J Ophthalmol. 2010;150:840–848. doi:10.1016/j.ajo.2010.06.015
27. Boeri D, Maiello M, Lorenzi M. Increased prevalence of microthromboses in retinal capillaries of diabetic individuals. Diabetes. 2001;50:1432–1439. doi:10.2337/diabetes.50.6.1432
28. Ribeiro L, Nunes S, Cunha-Vaz J. Microaneurysm turnover in the macula is a biomarker development of clinically significant macular edema in type 2 diabetes. Current Biomarker Findings. 2013;3:11–15.
29. Leicht SF, Kernt M, Neubauer A, et al. Microaneurysm turnover in diabetic retinopathy assessed by automated RetmarkerDR image analysis - potential role as biomarker of response to ranibizumab treatment. Ophthalmologica. 2014;231:198–203. doi:10.1159/000357505
30. Campochiaro PA, Wykoff CC, Shapiro H, Rubio RG, Ehrlich JS. Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology. 2014;121:1783–1789. doi:10.1016/j.ophtha.2014.03.021
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.