Back to Journals » Journal of Hepatocellular Carcinoma » Volume 6
Prospective Phase II trial of drug-eluting bead chemoembolization for liver transplant candidates with hepatocellular carcinoma and marginal hepatic reserve
Authors Fidelman N , Johanson C , Kohi MP, Kolli KP , Kohlbrenner RM, Lehrman ED, Taylor AG, Kelley RK, Yao FY, Roberts JP, Kerlan RK
Received 27 February 2019
Accepted for publication 7 May 2019
Published 17 June 2019 Volume 2019:6 Pages 93—103
DOI https://doi.org/10.2147/JHC.S206979
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Nicholas Fidelman,1 Curt Johanson,1 Maureen P Kohi,1 K Pallav Kolli,1 Ryan M Kohlbrenner,1 Evan D Lehrman,1 Andrew G Taylor,1 R Kate Kelley,2 Francis Y Yao,3 John P Roberts,4 Robert K Kerlan1
1Department of Radiology and Biomedical Imaging; 2Department of Medicine – Division of Gastrointestinal Oncology; 3Department of Medicine – Division of Hepatology; 4Department of Surgery – Division of Transplant Surgery, University of California San Francisco, San Francisco, CA, USA
Purpose: To determine whether chemoembolization using drug-eluting beads (DEB-TACE) is safe and effective for liver transplantation candidates with liver-limited hepatocellular carcinoma (HCC) without vascular invasion and baseline hepatic dysfunction.
Materials and methods: Seventeen adult liver transplantation candidates (median age 66 years, range 58–73 years; 13 men) with HCC were treated with DEB-TACE as a part of Stage 1 of a prospective single-institution Phase II trial. All patients had marginal hepatic reserve based on at least one of the following criteria: ascites (n=14), bilirubin between 3 and 6 mg/dL (n=5), AST 5–10 times upper normal limit (n=1), INR between 1.6 and 2.5 (n=4), portal vein thrombosis (n=2), and/or portosystemic shunt (n=2). Primary study objectives were safety and best observed radiographic response.
Results: Thirty-seven DEB-TACE procedures were performed. Objective response rate and disease control rate were 63% and 88%, respectively. HCC progression was observed in 12 patients. Median time to progression was 5.6 months (range 0.9–13.6 months). Within 1 month following DEB-TACE, 13 patients (76%) developed grade 3 or 4 AE attributable to the procedure. Four patients (all within Milan Criteria) were transplanted (2.7–6.9 months after DEB-TACE), and 12 patients died (1.8–32 months after DEB-TACE). All deaths were due to liver failure that was either unrelated to HCC (n=5), in the setting of metastatic HCC (n=5), or in the setting of locally advanced HCC (n=2). Mortality rate at 1 month was 0%.
Conclusions: DEB-TACE achieves tumor responses but carries a high risk of hepatotoxicity for liver transplant candidates with HCC and marginal hepatic reserve.
Keywords: hepatocellular carcinoma, chemoembolization, liver transplantation
Introduction
Transarterial chemoembolization (TACE) has been shown to extend survival of patients with intermediate stage unresectable hepatocellular carcinoma (HCC) compared with supportive care,1,2 and in optimal cases may be effective in allowing “bridging” to liver transplantation (LT)3 or “downstaging”4–6 of the tumor to allow transplantation. Moreover, use of TACE in the pre-transplant period has been found to diminish drop-out from the liver transplant wait list.4–6
TACE can be performed by administering chemotherapeutic agents as an emulsion with ethiodized oil (conventional method, cTACE) or by delivering microspheres pre-loaded with doxorubicin (drug-eluting bead TACE, DEB-TACE). Our group has demonstrated that pre-cTACE bilirubin ≥2 mg/dL, aspartate or alanine aminotransferase (AST or ALT) >5 times upper normal limit, international normalized ratio (INR) for prothrombin time >1.5, clinical evidence of ascites, or portal venous thrombus or flow diversion were associated with an increased risk of severe hepatotoxicity after cTACE.7 Multiple recent studies8–10 as well as a meta-analysis11 have demonstrated similar efficacy of cTACE and DEB-TACE for patients with unresectable intermediate-stage HCC. One prospective randomized study8 showed that the overall frequency of treatment-related severe adverse events (AE) per 100 treatments was lower in DEB compared to cTACE group. Furthermore, the number of radiographic objective responses observed for patients with Child-Pugh B and ECOG performance status 1 were significantly higher in DEB group.8 On the basis of this observation, DEB-TACE may be the preferred modality for treating patients with hepatic dysfunction.
Studies that have evaluated safety of DEB-TACE to date have excluded patients with serum bilirubin ≥3 mg/dL, AST or ALT levels greater than 5 times upper normal limit, or clinically apparent ascites,8–10,12–14 owing to risk of confounding by the competing comorbid events of liver dysfunction, which are prevalent in this population. However, patients with significant baseline liver dysfunction are often referred for TACE, particularly under the paradigm of “bridging” or “downstaging” to liver transplantation.5,7,15 The purpose of this study was to evaluate safety and efficacy of DEB-TACE for liver transplantation candidates with HCC and significant baseline hepatic dysfunction.
Materials and methods
Study population
This single-center prospective study was approved by the Institutional Review Board of University of California San Francisco (IRB record #14-13092) and was deemed to be compliant with the Declaration of Helsinki. All patients provided written informed consent. The trial was registered on clinicaltrials.gov (NCT# 02147301). All of the eligible patients were referred for chemoembolization at the treatment center. Incentives for study participation were not provided. The study was designed as a single-arm open-label Phase II two-stage trial, which aimed to enroll 51 adult liver transplant candidates with marginal hepatic reserve and liver-limited HCC without vascular invasion or extracapsular extension diagnosed by imaging criteria in accordance with the Organ Procurement and Transplantation Network (OPTN) guideline.16 The goal of first stage was to enroll 17 patients. Enrollment of the additional 34 patients was subject to documentation of adequate safety and efficacy of DEB-TACE during the first stage. The study was terminated after stage 1 by the study team due to the high rates of severe adverse events (SAE).
Eligibility required imaging16 or histologic diagnosis of HCC with at least one previously untreated HCC tumor nodule (measurable according to modified Response Evaluation Criteria in Solid Tumors, mRECIST) in a previously untreated liver segment. Presence of macrovascular tumor invasion, extracapsular extension, or extrahepatic metastases was not allowed. Patients were required to have impaired hepatic reserve based on the selection criteria delineated by Garwood et al7 and subsequently used by Hansmann J et al.15 Presence of at least one of the following clinical and laboratory abnormalities was required for inclusion: (A) clinical evidence of ascites, (B) serum bilirubin between 3 and 6 mg/dL, (C) aspartate and/or alanine aminotransferase (AST and/or ALT) level between 5 and 10 times upper normal limit, (D) international normalized ratio for prothrombin time (INR) between 1.6 and 2.5 in the absence of ongoing anticoagulation therapy, (E) main portal vein thrombosis, and (F) functioning transjugular intrahepatic portosystemic shunt (TIPS) or surgical shunt. Patients with impaired functional status (Eastern Cooperative Group Performance Status, ECOG, worse than 2), impaired renal function (serum creatinine >1.5 mg/dL), macrovascular tumor invasion or extrahepatic disease, medically refractory hepatic encephalopathy, symptomatic heart disease, previous bile duct sphincterotomy or biliary-enteric anastomosis, and liver transplant recipients were excluded.
DEB-TACE technique
DEB-TACE was performed at a single tertiary transplant center after patients provided written informed consent for the procedure and for study participation. All procedures were performed by fellowship-trained interventional radiologists with 5–25 years of experience with hepatic angiography and embolization. An angiographic survey of the celiac and superior mesenteric arteries was performed via trans-femoral approach using a 5-French catheter. Digital subtraction angiography was also performed after selective catheterization of the proper, right and/or left, hepatic arteries. Cone-beam CT (Philips, Amsterdam, The Netherlands) was used routinely. Subsegmental hepatic artery branches were selected with a coaxially placed microcatheter (Renegade 2.7-French or 2.1-French, Boston Scientific, Natick, MA).
DEB-TACE was performed with up to two 2-mL vials of spherical polyvinyl alcohol microspheres (LC Bead®; BTG PLC, London, UK) impregnated per standard manufacturer-supplied protocol with a total of 100 mg of reconstituted doxorubicin hydrochloride powder (25 mg per milliliter of beads; Mylan, Canonsburg, PA). Investigational Device Exemption (IDE number G13-0023) was obtained from the United States Food and Drug Administration (FDA) per the requirement of the IRB. Microspheres in the size range of 100–300 microns were used. Prior to administration, each 2-mL vial of drug-eluting beads was resuspended in 10 mL of Omnipaque 350 contrast (Amersham Health, Princeton NJ). Procedures were stopped when arterial flow approached stasis and/or when the entire DEB dose had been administered. If stasis had not been achieved after administration of two vials of DEB, additional embolic was not administered. Patients with multiple liver lesions, lesions >3 cm, or lesions with more than one feeding hepatic artery branch received up to 3 additional DEB-TACE at approximately 1-month intervals. Additional DEB-TACE was allowed until liver transplantation or untreatable progression upon demonstration of residual and/or recurrent liver-limited HCC as long as patients continued to meet study inclusion and had lesions that were amenable to treatment with DEB-TACE.
Safety assessments
Adverse events (AE) were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.03. Clinical and laboratory evaluations were performed approximately 1, 7, and 30 days after each DEB-TACE procedure, and subsequently every 3 months for up to 1 year after last DEB-TACE procedure. Laboratory investigations included complete blood count, serum electrolytes, creatinine, liver function tests, and prothrombin time. All AE that resulted in at least one category increase under the CTCAE v. 4.03 definitions were followed until resolution or until completion of follow-up. Cardiac function assessment monitoring using transthoracic echocardiogram was performed prior to first DEB-TACE and approximately 1 month following the last planned (1st, 2nd, 3rd, or 4th) DEB-TACE procedure.
Efficacy assessments
Efficacy was assessed based on multi-phase dynamic contrast-enhanced CT or MRI (CECT or CEMRI) according to modified Response Evaluation Criteria in Solid Tumors (mRECIST). The same imaging modality was used for pre- and post-treatment evaluations. Imaging was performed one month following last planned DEB-TACE procedure. Subsequently, CECT or CEMRI exams were repeated at three-month intervals until HCC recurrence, liver transplantation, or death. Serum alpha-fetoprotein (AFP) levels were checked at the time of the imaging tests.
Study objectives
Primary study objectives were to determine the safety of DEB-TACE in accordance to NCI CTCAE and to determine the best observed overall radiographic response (ORR) rate to DEB-TACE according to mRECIST.
Secondary objectives were to determine the proportion of patients who remained active on the wait list for a liver transplant and were eventually transplanted; to determine the proportion of patients who were inactivated or dropped out from the wait list for any reason but transplantation; to measure ORR according to mRECIST 6 months after the first planned DEB-TACE procedure; time to progression (TTP); time to hepatic progression (TTHP); time to progression beyond Milan Criteria; time to untreatable progression (TTUP); overall progression-free survival (PFS); hepatic progression-free survival (HPFS) rates at 1, 3, 6, 12, and 24 months; overall survival (OS); to determine whether cardiac function changes occurred as a result of DEB-TACE; and to obtain pharmacokinetic data for doxorubicin administered as a part of DEB-TACE procedures. The date of the first on-study DEB-TACE procedure was used as the starting point for calculations of TTP, TTHP, TTUP, PFS, HPFS, and OS. The study protocol as well as deidentified participant data regarding TTP, TTHP, TTUP, PFS, HPFS, and OS will be available at
Pharmacokinetics
Pharmacokinetics (PK) data for doxorubicin were collected per the request of the USA FDA since PK data for 100–300 micron drug-eluting microspheres has not been previously reported. Peripheral venous blood samples were obtained from all patients during the first planned DEB-TACE procedure prior to chemotherapy administration, at the time of completion of DEB administration, and subsequently 5±1 mins, 30±5 mins, 60±5 mins, 120±10 mins, 6±0.5 hrs, 20±2 hrs, and 8±2 days following DEB administration. The data were used to determine maximum doxorubicin concentration (Cmax) and total systemic doxorubicin dose (area under the curve, AUC).
Statistical analysis
For sample size calculations, the best observed ORR was assumed to be at least 40% in treated lesions using mRECIST criteria. The best observed ORR of less than 20% was considered unacceptable for the study treatment. Under binomial approximation with 5% type I error and 85% power, a total of 51 patients were required for the study. For the purpose of safety analysis, the study was divided into two stages. After 17 patients have received at least 1 DEB-TACE and had potential for at least 4 weeks of follow-up, the safety profile was analyzed. Because of the high incidence of severe AE (SAE), the study was terminated after completion of the first stage. The study DEB-TACE procedures were performed between 01/12/2015 and 06/24/2016. Data were censored on February 1, 2019. The correlation between maximum doxorubicin concentration (Cmax), AUC, and delivered doxorubicin dose, serum bilirubin level, MELD-Na, and CPT, and ALBI scores were evaluated using linear regression analysis (SAS version 9.4, SAS Institute Inc., Cary NC).
Results
Patient population
A total of 155 adult patients with liver-limited HCC without vascular invasion or extracapsular tumor extension who were referred for TACE were screened for study participation between 11/01/2014 and 08/01/2015 (Figure 1). The majority of patients (n=124) were not deemed to be study candidates due to absence of significant underlying liver disease (n=117), and/or poor candidacy for liver transplantation (n=48), and/or lack of previously untreated liver lesions (n=14). An additional four patients were excluded due to impaired renal function. Of the remaining 27 patients who met inclusion criteria and who were approached for study participation, 4 declined to participate, 5 did not undergo DEB-TACE due to clinical liver function decompensation between screening and the scheduled procedure date, while 1 patient underwent diagnostic angiography, which did not reveal any targetable liver lesions. The latter six patients were enrolled in the study and were replaced, as allowed per protocol.
 | Figure 1 Flow diagram that demonstrates criteria for patient inclusion and exclusion. |
Clinical characteristics of the 17 patients treated on study are summarized in Table 1. A total of 17 patients (median age 66 years, range 58–73 years; 13 men) were treated with at least 1 DEB-TACE procedure during the first stage of the study. Hepatitis C virus infection was the most common cause for cirrhosis (8 patients, 47%). The majority of the patients qualified for inclusion based on presence of symptomatic ascites (14 patients, 82%), serum bilirubin between 3 and 6 mg/dL (5 patients, 29%), and/or coagulopathy (4 patients, 24%). Three patients qualified for inclusion based on presence of main portal vein thrombosis, patent transjugular intrahepatic portosystemic shunt (TIPS), or a patent surgical shunt. Median CTP score was 10 (range 6–12), and median MELD score was 12 (range 7–26). Most study participants (12 patients, 71%) had HCC within Milan Criteria at the time of inclusion. Median lesion size was 3 cm (range 1.1–6.5 cm). Solitary lesions were present for 14 patients, while 2 patients had 2 lesions each, and 1 patient had 4 lesions. Prior to inclusion in the study, seven patients (41%) had received between one and seven (median 3) liver-directed therapy procedures, which included conventional or DEB-TACE (5 and 7 patients, respectively) or radiofrequency ablation (3 patients).
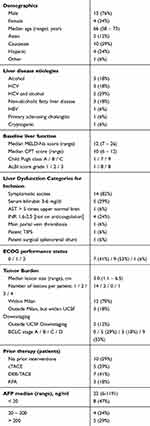 | Table 1 Population demographics and baseline characteristics |
Procedure details
A total of 37 DEB-TACE procedures were performed for the 17 enrolled patients. Nine patients (53%) were treated once, three patients twice, one patient three times, three patients four times, and one patient seven times. Median time interval between DEB-TACE was 2 months (range 1–7 months). Median doxorubicin dose administered during each procedure was 49 mg (range 4.9–98 mg). No major protocol deviations were encountered.
Safety assessments
All patients experienced at least one AE as a result of the DEB-TACE procedures (Table 2). Most common clinical AEs were self-limited grade 1 or 2 fatigue, anorexia, nausea, abdominal pain, and insomnia, all of which resolved within 1 month of DEB-TACE. New Grade 3 or 4 AE attributed to DEB-TACE occurred in 13 patients (76%). Seven patients (41%) developed symptoms of fluid retention. Three patients (18%) had worsening ascites, two of whom started requiring periodic large-volume paracenteses, while one was successfully treated with diuretics. New or worsening lower extremity edema developed in six patients (35%) within one month of DEB-TACE. Symptoms were grade 1 or 2 in severity for four patients and resolved after supportive treatment and/or diuretics within one month of onset. Two patients developed severe (grade 3) leg edema, which was life-long and refractory to diuretics. A decline in ECOG performance status within 1 month of DEB-TACE was experienced by 8 patients (47%), of whom 2 were able to regain pre-treatment level of function within 3 months. The remaining six patients experienced irreversible decline in performance status.
 | Table 2 All-cause adverse events observed within 30 days of DEB-TACE procedure |
A total of 16 patients (94%) experienced new or worsening laboratory AE within 1 month of DEB-TACE. Trends of mean AST, ALT, and bilirubin levels following the last DEB-TACE are shown in Figure 2. Twelve patients (71%) developed new-onset grade 3 laboratory AE, which included hyponatremia (n=5), hyperbilirubinemia (n=3), AST elevation (n=3), thrombocytopenia (n=3), anemia (n=2), and creatinine elevation (n=1). All grade 3 AE returned to baseline (majority within 30 days of DEB-TACE), except for 1 patient with hyperbilirubinemia that was attributed to an unplanned total hip arthroplasty performed 10 days following DEB-TACE and 1 patient with thrombocytopenia as well as grade 4 hyponatremia.
 | Figure 2 Trends of mean serum bilirubin (A), AST and ALT (B) levels following the last DEB-TACE procedure. |
Hepatic failure grade 3 (defined in NCI CTCAE v 4.03 as asterixis, mild encephalopathy, and limited ability to perform activities of daily living) was observed for 4 patients and was deemed unrelated to DEB-TACE for 2 patients (1 patient with previously prescribed diuretic medication non-compliance and 1 patient who underwent an unplanned total hip arthroplasty 10 days following DEB-TACE). Grade 3 hepatic failure resolved with supportive measures for three of the patients less than 1 month after the preceding DEB-TACE procedure. The fourth patient was found to have metastatic HCC to the brain and died 3 months after DEB-TACE. One patient died from liver failure 1.8 months following the first DEB-TACE, which may have been related to DEB-TACE. Procedure-related mortality was 1 out of 17 (6%).
Echocardiograms demonstrated no significant change in myocardial function. Median left ventricular ejection fraction was 70% at baseline (range 66–81%) and 68% at 1 month following last pre-planned DEB-TACE (range 60–74%).
Efficacy
There were 16 patients who were evaluable for radiographic response. One patient did not have follow-up cross-sectional imaging of the abdomen due to inter-current illness unrelated to TACE.
Efficacy data are summarized in Table 3. Best observed radiographic tumor responses according to mRECIST were complete, partial, and stable disease response rates of 25%, 38%, and 25%, respectively. Best observed ORR and disease control rate (DCR) were 63% and 88%, respectively. Median time from the first DEB-TACE to best observed ORR was 1.2 months (range 0.9–9.6 months). ORR and DCR at 6 months were 38% and 46%, respectively. Median number of DEB-TACE procedures required to achieve maximum observed response was 1 (range 1–7). HCC progression was observed in 12 patients. Median time to progression was 5.6 months (range 0.9–13.6 months). HCC progression to beyond Milan Criteria was observed for 8 patients (5 patients with extrahepatic metastases, 2 with multifocal HCC outside Milan Criteria, and 1 with portal vein tumor thrombus) after a median time of 5.6 months (range 1.4–30.4 months). Median time to untreatable progression was 9.7 months (range 1.4–30.4 months). Nine patients (53%) had baseline AFP greater than 20 ng/mL at the time of first on-study DEB-TACE procedure (median 214 ng/mL, range 22–1191 ng/mL). Five of these patients (56%) demonstrated at least 50% reduction in AFP level from the median of 248 ng/mL to median of 16 ng/mL at the time of best observed radiographic response.
 | Table 3 Efficacy data |
Clinical follow-up
Of the 17 patients treated, 4 patients (24%) received orthotopic liver transplants a median of 3.1 months (range 2.7–6.9 months) after the first DEB-TACE. These patients had HCC within Milan Criteria at the time of the first DEB-TACE. All of the patients who had received a liver transplant were alive at the time of the study censor date of 02/01/2019 and were without HCC recurrences following a median 44.1 months (range 39.7–45.9 months) of follow-up.
After discontinuation of DEB-TACE, additional anti-cancer therapy was administered to nine patients (53%), one of whom ultimately received a liver transplant. These patients received a median of 2 (range 1–5) courses of treatment a median of 2.7 months (range 1.2–4.8 months) following the last DEB-TACE procedure. Modalities included stereotactic body radiotherapy (4 patients), percutaneous ethanol injection (3 patients), conventional TACE with doxorubicin, mitomycin C, ethiodized oil, and gelatin sponge slurry (2 patients), and sorafenib (1 patient). Conventional TACE was used following discontinuation of DEB-TACE in selected patients due to partial obliteration of the arterial supply, which resulted from previous hepatic artery embolization. It was expected that a liquid embolic (emulsion of chemotherapy and ethiodized oil) would pass through the small caliber collateral vessels supplying liver tumors more readily than microspheres.
A total of 13 patients were removed from the transplant list due to detection of extrahepatic HCC metastases (5 patients), locally advanced untreatable HCC (2 patients), frailty (4 patients), severe coronary artery disease (1 patient), and lack of interest in transplantation (1 patient). Twelve patients died 1.8–32 months after the first on-study DEB-TACE (median overall survival 11.4 months). Mortality rates at 1, 3, 6, 9, and 12 months were 0%, 12%, 18%, 24%, and 29%, respectively. All deaths were due to hepatic failure that was either unrelated to HCC (n=5), in the setting of metastatic HCC (n=5), or in the setting of locally advanced HCC (n=2). One patient was not transplanted and was lost to follow-up 21 months following first DEB-TACE.
Pharmacokinetic profile
The pharmacokinetic analysis for doxorubicin demonstrated that peak drug concentration (Cmax) was reached within 5 mins after drug-eluting microsphere administration (Figure 3). Mean and median Cmax were 71.2 and 42.2 ng/mL. Cmax range was 8.4–444.4 ng/mL. Standard deviation was 103.1 ng/mL. Cmax values for 15 of the patients were less than 100 ng/mL. Mean and median AUC were 592 and 593 ng/mL-hr. AUC range was 175–2030 ng/mL-hr. Standard deviation was 310 ng/mL-hr. No significant correlation between either Cmax or AUC and delivered doxorubicin dose, serum bilirubin level, MELD-Na, and CPT, and ALBI scores could be demonstrated.
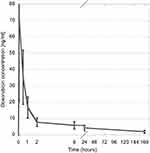 | Figure 3 Serum doxorubicin concentration over time. Error bars represent 95% confidence intervals. |
Discussion
This study provided prospectively acquired data on the outcomes of DEB-TACE for patients with HCC and marginal hepatic reserve. In current clinical practice, bridging and downstaging liver-directed therapy is offered to patients with HCC who have varying levels of liver function impairment. Of 606 patients with HCC who underwent liver-directed therapy for bridging or downstaging to liver transplantation5 at a US transplant center with long expected waiting time on the transplant list, 232 patients (38%) had Child-Pugh class B cirrhosis, while 67 patients (11%) had Child-Pugh class C cirrhosis at the time of listing.
The majority of the published studies on TACE have focused on patients with preserved liver function (CPT score 5–7), no clinical evidence of ascites, and patent portal vein.8–10,12–14 Limited data on the value of TACE for patients with hepatic dysfunction and decompensation are available. A retrospective study based on a population of 1,344 patients with HCC and Child-Pugh C cirrhosis demonstrated that TACE was associated with a significant survival prolongation (10 months versus 7 months) compared to best supportive care.17 Similarly, another retrospective multi-center study of 236 patients with Child-Pugh C cirrhosis and HCC was able to demonstrate an improvement in overall survival from 13.8 to 22.2 months for patients who received liver-directed therapy, either TACE or thermal ablation.18
In this study, the majority of patients (76%) developed severe (Grade 3 or 4) AE attributed to DEB-TACE, which included four patients with clinical hepatic failure. There was 1 death that occurred 1.8 months following DEB-TACE in the setting of decompensated hepatic failure that may have been in part due to the procedure. The rate of severe treatment-related AE was higher than previously reported in other studies describing high-risk patients.7,19 For instance, a large retrospective study by Garwood et al7 has reported on 251 patients with hyperbilirubinemia, coagulopathy and/or clinically evident ascites who underwent 443 conventional TACE procedures. Grade 3 or 4 hepatotoxicity developed after 90 TACE procedures (20%) in 78 patients (31%). Liver function abnormalities became irreversible after 41 procedures (9%) in 37 patients. Six patients (2%) received urgent liver transplants, and 5 (2%) patients died within 30 days of TACE in the setting of irreversible severe hepatotoxicity. Another study by Kothary et al19 reported complications outcomes for 52 patients with hyperbilirubinemia, hypoalbuminemia, CTP score >9, multifocal HCC, a TIPS, and/or biliary obstruction who underwent 65 TACE procedures. The procedure-related morbidity rate was 10.8%, and the 30-day mortality rate was 7.7%. Similar to the study By Garwood et al,7 the majority of adverse events observed in this clinical trial were self-limited.
The higher rate of AE reported in this study is likely related to the differences in the patient populations and to more complete AE reporting in a prospective trial. Patients included in this study were at risk for developing liver decompensation even without liver-directed therapy, as evidenced by the observation that five patients were not able to undergo DEB-TACE after enrollment (Figure 1).
DEB-TACE proved to be an effective means of local tumor control, with reported best observed ORR and DCR of 63% and 88%, respectively. ORR and DCR at 6 months were 38% and 46%, respectively. Lammer et al8 reported somewhat higher ORR and DCR at 6 months (51.6% and 63.4%) in a cohort of patients with significantly better liver function.
DEB-TACE was an effective bridging strategy for four of 12 patients (33%) with HCC within Milan Criteria who ultimately received liver transplants. The other eight patients dropped out from the transplant list due to a variety of causes including development of extrahepatic metastases, locally advanced untreatable HCC, and frailty. All five patients with HCC tumor burden outside Milan Criteria dropped out from the transplant list due to a similar set of causes. The 33% rate of survival to transplantation observed in this study for patients within Milan is considerably lower than the 68% rate reported for a large population of patients with HCC with predominantly Child-Pugh A cirrhosis who were within Milan Criteria at the time of listing.5 This finding suggests that poor liver function may be an independent factor for drop-out from liver transplant list. The observed lack of patients outside Milan who survived until transplantation suggests that DEB-TACE may not be a suitable downstaging strategy for patients who present with significant hepatic dysfunction, especially at transplant centers with long expected waiting times.
The pharmacokinetic profile of doxorubicin release from drug-eluting microspheres has been studied for microspheres in 500–700 micron size.13 However, PK data for doxorubicin release from smaller microspheres have not been previously published. Our study demonstrated that the pharmacokinetic pattern of doxorubicin release from 100 to 300 micron microspheres mirrors the pattern observed by Varela et al13 for 500–700 micron particles. Maximum doxorubicin concentration in plasma (Cmax) occurred at 5 mins following completion of particle deposition, and the observed AUC range of 175–2030 ng/mL-hr was similar to the values observed by Varela et al and was independent of the delivered doxorubicin dose.
This study is limited by small sample size and heterogeneity of the high-risk population. Patients with a number of high-risk factors were allowed for participation, including ascites, hyperbilirubinemia, transaminitis, coagulopathy, main portal vein thrombosis, and a portosystemic shunt. Seven of the patients have had liver-directed therapy (including TACE) prior to study enrollment, which could have an effect on the underlying liver function. It is possible that previous liver-directed therapy has contributed to the underlying liver dysfunction at the time of study enrollment. Patients with liver dysfunction that develops in the course of HCC therapy are routinely encountered at transplant centers with long waiting times. Thus, the cross-section of patients included in this study depicts a representative population of liver transplant candidates with HCC and liver dysfunction who may undergo TACE for bridging to transplantation. It is unlikely that prior trans-arterial therapy had an effect on technical ability to perform on-study DEB-TACE, as patients were required to have previously untreated lesions in liver segment(s) that have not been previously targeted for embolization.
The investigators elected to terminate the study after completion of Stage I due to one death that was possibly related to study treatment. This study lacked a control arm. Conventional TACE or transarterial embolization control arm would be reasonable to include in a future study multi-center study, which could focus on transplant candidates with HCC within Milan Criteria and CPT score 9–12 with the goal of increasing the odds of survival to liver transplantation and limiting participation to patients with marginal liver reserve.
In conclusion, this small prospective study suggests that DEB-TACE achieves tumor responses but carries a high risk of severe toxicity and, therefore, may not be a safe bridging or down-staging strategy for liver transplant candidates with HCC and marginal liver reserve.
Acknowledgments
This work was supported by a research grant from BTG, Inc. The abstract of this paper was presented at the Society of Interventional Radiology 2018 Annual Scientific Meeting in March 2018 as a conference talk.
Disclosure
Dr Nicholas Fidelman report grants from BTG, Inc, outside the submitted work. Dr Robin Kate Kelley reports grants from Bayer, BMS, AstraZeneca, Merck and Exelixis, grants and personal fees from Target Pharma Solutions and personal fees from Genentech/Roche, during the conduct of the study; and grants from Agios, Taiho, and Novartis, outside the submitted work. All authors report no other conflicts of interest in this work.
References
1. Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi:10.1016/S0140-6736(02)08649-X
2. Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–1171. doi:10.1053/jhep.2002.33156
3. Rubinstein MM, Kaubisch A, Kinkhabwala M, Reinus J, Liu Q, Chuy JW. Bridging therapy effectiveness in the treatment of hepatocellular carcinoma prior to orthotopic liver transplantation. J Gastrointest Oncol. 2017;8(6):1051–1055. doi:10.21037/jgo.2017.08.11
4. Yao FY, Kerlan RK, Hirose R, et al. Excellent outcome following downstaging of hepatocellular carcinoma prior to liver. transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819–827. doi:10.1002/hep.22412
5. Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61(6):1968–1977. doi:10.1002/hep.27752
6. Yao FY, Fidelman N. Reassessing the boundaries of liver transplantation for hepatocellular carcinoma: where do we stand with tumor downstaging? Hepatology. 2016;63(3):1014–1025. doi:10.1002/hep.28139
7. Garwood E, Fidelman N, Hoch SE, Kerlan RK. Morbidity and mortality following transarterial liver chemoembolization in patients with hepatocellular carcinoma and hepatic dysfunction. Liver Transpl. 2013;19:164–173. doi:10.1002/lt.23552
8. Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(1):41–52. doi:10.1007/s00270-009-9711-7
9. Sacco R, Bargellini I, Bertini M, et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011;22:1545–1552. doi:10.1016/j.jvir.2011.07.002
10. Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolization for hepatocellular carcinoma. Br J Cancer. 2014;111:255–264. doi:10.1038/bjc.2014.199
11. Chen P, Yuan P, Chen B, Sun J, Shen H, Qian Y. Evaluation of drug-eluting beads versus conventional transcatheter arterial chemoembolization in patients with unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2017;41:75–85. doi:10.1016/j.clinre.2016.05.013
12. Poon RT, Tso WK, Pang RW, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007;5(9):1100–1108. doi:10.1016/j.cgh.2007.04.021
13. Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46(3):474–481. doi:10.1016/j.jhep.2006.10.020
14. Gomes AS, Monteleone PA, Sayre JW, et al. Comparison of triple-drug transcatheter arterial chemoembolization (TACE) with single-drug TACE using doxorubicin-eluting beads: long-term survival in 313 patients. AJR Am J Roentgenol. 2017;209(4):722–732. doi:10.2214/AJR.17.18219
15. Hansmann J, Evers MJ, Bui JT, et al. Albumin-bilirubin and platelet-albumin-bilirubin grades accurately predict overall survival in high-risk patients undergoing conventional transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2017;28:1224–1231. doi:10.1016/j.jvir.2017.05.020
16. Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology. 2013;266:376–382. doi:10.1148/radiol.12121698
17. Kitai S, Kudo M, Nishida N, et al. Survival benefit of locoregional treatment for hepatocellular carcinoma with advanced liver cirrhosis. Liver Cancer. 2016;5:175–189. doi:10.1159/000367765
18. Hiraoka A, Kumada T, Michitaka K, et al. Is there a survival benefit in interventional radiology for hepatocellular carcinoma in patients with Child-Pugh C liver cirrhosis?: A multicenter study. Hepatol Res. 2016;46(6):521–528. doi:10.1111/hepr.12583
19. Kothary N, Weintraub JL, Susman J, Rundback JH. Transarterial chemoembolization for primary hepatocellular carcinoma in patients at high risk. J Vasc Interv Radiol. 2007;18:1517–1526. doi:10.1016/j.jvir.2007.07.035
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
