Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 11
Proliferation, odontogenic/osteogenic differentiation, and cytokine production by human stem cells of the apical papilla induced by biomaterials: a comparative study
Authors Saberi E , Farhad-Mollashahi N , Sargolzaei Aval F, Saberi M
Received 16 April 2019
Accepted for publication 24 May 2019
Published 12 July 2019 Volume 2019:11 Pages 181—193
DOI https://doi.org/10.2147/CCIDE.S211893
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Christopher E. Okunseri
Eshaghali Saberi,1 Narges Farhad-Mollashahi,1 Fereydoon Sargolzaei Aval,2,3 Mersad Saberi4
1Oral and Dental Diseases Research Center, Department of Endodontics, Faculty of Dentistry, Zahedan University of Medical Sciences, Zahedan, Iran; 2Cellular and Molecular Research Center, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran; 3Department of Anatomical, School of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran; 4General Dentist, Zahedan, Iran
Introduction: Clinical applications of bioactive materials are increasing in biomedical tissue engineering. This study sought to assess the effect of calcium enriched mixture (CEM) cement, Biodentine, mineral trioxide aggregate (MTA), octacalcium phosphate (OCP), and Atlantik on proliferation, odontogenic/osteogenic differentiation, and pro-inflammatory cytokine production by human stem cells of the apical papilla (SCAPs).
Materials and methods: Proliferation of SCAPs treated with different biomaterials was evaluated using trypan blue exclusion test and flow cytometry. Differentiation of cells was evaluated using ALP activity, alizarin red staining, and RT-PCR. The expression of genes of pro-inflammatory cytokines was also evaluated using RT-PCR.
Results: The SCAPs treated with biomaterials showed significantly higher proliferation, increased ALP activity, higher number of calcified nodules, and up-regulation of genes related to odontogenic/osteogenic markers compared to the control group. The expression of pro-inflammatory cytokines increased in all groups compared to the control group.
Conclusion: The tested biomaterials could induce odontogenic/osteogenic differentiation in SCAPs. MTA had a greater potential for induction of differentiation of SCAPs to odontoblast-like cells while OCP had higher potential to induce differentiation of SCAPs to osteoblast-like cells (MTA↔ BD↔ CEM↔ Atlantik↔ OCP).
Keywords: apical papilla, biomaterials, odontogenic differentiation, stem cells
Introduction
In the past decade, postnatal stem cells have been isolated from many different tissues and are extensively used for regeneration of dental tissue.1 Different postnatal undifferentiated cells from dental mesenchyme (pulp and apical papilla), stem cells of the apical papilla (SCAPs) isolated from immature, permanent human teeth have been extensively studied by Sonoyama et al.2 Although SCAPs show similar characteristics to dental pulp stem cells (DPSC), they have a greater number of population doublings, tissue regeneration capacity, and number of SRTO-1-positive cells when compared with DPSC. In addition, SCAPs express a higher level of survival (antiapoptotic protein) than DPSC.3,4 Because of their anti-inflammatory ability, these stem cells may survive in infectious sites and continue to play a critical role during the development of roots in infected immature permanent teeth with periapical diseases.2,5 These lines of evidence suggest that SCAPs may be a superior cell source for tissue regeneration. Many previous studies have investigated the capacity of SCAPs to differentiate to functional cells when exposed to bioactive materials.2–4
Biomaterials such as calcium and phosphate are either artificially synthesized or derived from natural sources. They play a pivotal role in prevention of demineralization and promotion of remineralization of hard tissues.6 Moreover, biominerals play a fundamental role in bone formation and regeneration and exert their effects on differentiation, proliferation, chemotaxis, and formation of extracellular matrix. Calcium phosphate, hydroxyapatite, calcium silicate, calcium carbonate, and calcium sulfate are among the most commonly used biomaterials as bone cement for biomedical purposes.7
Calcium enriched mixture (CEM) cement is an alkaline cement that releases calcium hydroxide during and after setting. Optimal biocompatibility, low cytotoxicity, and formation of hydroxyapatite are among the advantages of this cement.8 A previous study showed that the number of inflammatory cells significantly decreased at 1, 4, and 8 weeks after implantation of CEM cement and mineral trioxide aggregate (MTA) in the femur of rats, and both materials significantly enhanced osteogenesis.9
MTA is a bioactive compound commonly used in endodontic procedures such as root-end filling, pulpotomy, internal resorption repair, root perforation repair, apexification, and apexogenesis. It has unique properties such as antimicrobial activity, optimal sealing ability, low cytotoxicity, optimal biocompatibility, setting capacity in presence of blood and water, and effective promotion of mineralization in vivo. Moreover, it is non-resorbable, non-mutagenic, osteoinductive, and osteoconductive. It was shown that the ALP activity of bone marrow stem cells isolated from the craniofacial bones significantly increased following their exposure to 0.02 mg/mL MTA. Moreover, they created more mineralized nodules compared to untreated cells. A significant up-regulation was also noted in genes/proteins of odontoblastic/osteoblastic markers in cells treated with MTA, and level of phosphorylated Jun N-terminal kinase and phosphorylated extracellular signal-regulated protein kinase significantly increased over time in these cells; whereas, inhibition of Jun N-terminal kinase and extracellular signal-regulated protein kinase significantly decreased odontoblastic/osteoblastic differentiation and level of ALP.10
Biodentine is a calcium silicate cement with mechanical properties resembling those of dentin. The manufacturer first introduced Biodentine as an alternative to dentin and claimed that it can induce the formation of tertiary dentin. Biodentine powder contains high amounts of tricalcium silicate, dicalcium silicate, calcium carbonate, and zirconium oxide as opaquer. Its liquid contains calcium chloride in an aqueous solution combined with polycarboxylate.11 It has been shown that biocompatibility of Biodentine in 1, ½, and ¼ concentrations is significantly less than that of MTA and BioAggregate (which is a bioceramic). Also, mRNA of osteogenic genes experienced a significant increase in presence of MTA and BioAggregate compared to the control group. Although the mRNA of osteogenic genes in Biodentine group also increased compared to the control group, this increase did not reach statistical significance.12
Octacalcium phosphate (OCP) is a precursor for the formation of biological apatite crystals in tooth and bone. Synthetic OCP can induce bone regeneration and is biodegradable. It can also induce differentiation of osteoblasts and formation of osteoclasts from stem cells/progenitor cells. Its application is easy and does not require cell implantation at the site of defect. It induces bone regeneration without requiring exogenous osteogenic cytokines.13 OCP has considerable osteoconductive properties, which have been confirmed both in vitro and following implantation in bone defects.14,15 Several studies have demonstrated osteoblast differentiation and osteoclast formation by OCP.16–18
Atlantik bone powder has applications in medicine as a synthetic bone substitute. It is semi-resorbable and biphasic. As stated by the manufacturer, it is composed of 70% hydroxyapatite and 30% beta-tricalcium phosphate. Raw materials used for the synthesis of this powder are completely similar to bone minerals; therefore, it is highly biocompatible.
The odontogenic/osteogenic differentiation capacity of DPSC, tooth germ, and periodontal ligament tissue have been previously confirmed.1,19 However, there is still controversy regarding the effect of biomaterials on SCAPs. This study sought to assess the effect of CEM cement, Biodentine, MTA, OCP, and Atlantik on proliferation, odontogenic/osteogenic differentiation, and pro-inflammatory cytokine production by SCAPs.
Materials and methods
Sample collection
Five healthy, immature impacted mandibular third molars that were scheduled for extraction in the Oral and Maxillofacial Surgery Department of Tehran University of Medical Sciences were used in this study. The teeth belonged to patients aged between 18 and 24 years and were used after obtaining written informed consent from patients and gaining approval from the ethics committee of the university (no: IR.ZAUMS.REC.1394011). The extracted teeth were immediately rinsed with sterile PBS (Gibco, Thermo Fisher Scientific, Waltham, MA, USA) and stored in this solution until being transferred to a laboratory for isolation of stem cells.
Cell isolation and culture
Stem cells were isolated from the apical papilla by enzymatic digestion using 2 mg/mL of type I collagenase (Worthington Biomedical, Lakewood, NJ, USA), and immersed in DMEM (Gibco, Thermo Fisher Scientific). To obtain higher number of cells, they were re-cultured in a culture medium containing 15% FBS (Gibco, Thermo Fisher Scientific). This was repeated five to eight times to obtain higher number of cells. The cell line was cultured in sterile flasks (SPL Life Science, Gyeonggi-do, South Korea) containing DMEM supplemented with 10% FBS. After 2–3 days, the medium was replaced and after 1 week, the cells were passaged. Adequate confluence was achieved after four passages (Figure 1).
Preparation of biomaterials
ProRoot MTA (Dentsply Maillefer, Switzerland), Biodentine (Septodent, France), CEM cement (NSK, Japan), and Atlantik (Chemin du Catupolan, Vaulx en Velin, France) powders were mixed with their respective liquids. OCP (Iran) was mixed with saline to obtain a paste-like consistency. The biomaterials were dried for 24 hours and ground into powder. The powders were filtered using a 45 µm filter and added to DMEM in 200 mg/mL concentration. The mixture was vortexed and incubated at 37°C for 1 week. The obtained slurry was first filtered with a 2.2 µm filter and then mixed with equal volume of DMEM to obtain the condition medium of biomaterials. The cells were treated with these media, which were freshly prepared daily.
Cell proliferation assay
Stem cells were cultured in a 24-well plate with a primary density of 5×103 cells/plate. The wells contained DMEM supplemented with 10% FBS and were randomly divided into ten groups. They were then serum-starved for 24 hours. The cells were removed in the next 9 consecutive days and counted using a coulter counter (Beckman Coulter, Fullerton, CA, USA). Trypan blue was added to cell suspension to eliminate non-viable cells. The effect of biomaterials on proliferation capacity of SCAPs was evaluated by counting the number of cells in triplicate in each group.
Flow cytometry
A total of 1×106 SCAPs were cultured in DMEM containing 2 mg/mL of the biomaterials. After 5 days of culture, cells treated with biomaterials were collected, fixed with 75% ice-cold ethanol, and stored overnight at −20°C. Each sample was rinsed with PBS three times and incubated with propidium iodide (100 mg/mL; Sigma-Aldrich Co., St Louis, MO, USA) on ice for 30 minutes in the dark. The DNA content was determined using a FACScan flow cytometer (BD Biosciences, San Jose, CA, USA). The cell cycle fractions (G0G1/S/G2M phases) were determined using flow cytometry. This was repeated three times.
ALP activity and alizarin red staining
A total of 20,000 cells were added to each well of a 12-well plate containing DMEM supplemented with 10% FBS. After 24 hours, 0, 0.002, 0.02, 0.2, 2, and 20 mg of CEM, Biodentine, MTA, OCP, and Atlantik per 1 mL of DMEM supplemented with FBS were added to the cells. After 72 hours, the cells were lysed using 0.2 mol/L Triton X-100 for 2 hours and were then centrifuged at 5,000 rpm for 10 minutes. The concentration of ALP and the protein content were read at 405 nm wavelength using an auto-analyzer (902; Hitachi Ltd., Tokyo, Japan). For alizarin red staining, cells treated with the biomaterials were cultured in mineralization-inducing medium in 6-well plates for 21 days. The cells were then treated with 95% ethanol for 30 minutes and incubated with 40 mmol/L alizarin red (pH of 5.5) for 5 minutes at room temperature. The cells were rinsed with distilled water three times, scanned with a high-resolution scanner, and their calcium content was quantitatively analyzed using 10% cetylpyridinium chloride. Other 6-well plates were used with the same density of SCAPs to determine the protein content in different groups. The final concentration of calcium was normalized with the total protein content and expressed as nanograms per milligram protein. It was analyzed six times in each group.
Real-time RT-PCR
For molecular assessments with regard to gene expression, RNA was extracted from the cells exposed to adequate concentration of biomaterials for 3 and 7 days using RNX Plus according to the manufacturer’s instructions (Sinagen, Iran). The concentration and purity of extracted RNA were assessed using spectrophotometry (Nanodrop). The amount of impurity due to the presence of protein or DNA in RNA solution was determined by calculating the 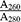 ratio. This ratio must be 2±0.15 for pure RNA sample and 1.8±0.15 for pure DNA sample. Calculated ratios lower than the standard value indicate higher contamination with protein.
ratio. This ratio must be 2±0.15 for pure RNA sample and 1.8±0.15 for pure DNA sample. Calculated ratios lower than the standard value indicate higher contamination with protein.
cDNA synthesis from the extracted RNA
After the extraction of RNA with high purity and adequate concentration from all samples, cDNA was synthesized according to the protocol recommended by the manufacturer (Fermentas, USA). The synthesized cDNA was then used for reverse-transcription.
Quantitative assessment of gene expression using real-time PCR
Preparation of primers
Primers were obtained in lyophilized form (Table 1). For their preparation, a certain volume of sterile distilled water was added to each tube containing lyophilized primer (according to the information provided for each primer). This stock solution was stored at −20°C. The samples were placed on ice until being transferred to the machine. RT-PCR was used to confirm expression of the respective genes. Each PCR reaction was performed using PCR master mix (Applied Biosystems, Thermo Fisher Scientific) and SYBR Green in ABI Step One (Sequence Detection System, Applied Biosystems, Thermo Fisher Scientific) according to the manufacturer’s protocol. Forty cycles were considered for each real-time PCR reaction at the following temperatures: 94°C for 20 seconds, 58°C–60°C for 30 seconds, and 72°C for 30 seconds. The ratio of expression of genes in this study was assessed using the threshold cycle (Ct) method. The expression of target genes was normalized with the reference genes using ΔΔCt and 2–ΔΔCt formula. At each step, gene expression in the previous step was considered as the calibrator.
 |
Table 1 Sense and anti-sense primers for real-time RT-PCR |
Data were analyzed using one-way ANOVA followed by Tukey’s test. P≤0.05 was considered statistically significant.
Results
Determining optimal biomaterial concentration
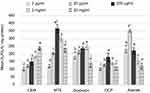
First, the potential of SCAPs for proliferation and differentiation following exposure to the biomaterials was evaluated using ALP activity. CEM cement, MTA, Biodentine, OCP, and Atlantik in 2 µg/mL to 20 mg/mL concentrations increased ALP activity of SCAPs compared to the control group. CEM cement in 20 mg/mL concentration caused the highest ALP activity and its effect was dose-dependent. Biodentine at 2 mg/mL concentration, OCP and MTA at 200 µg/mL, and Atlantik at 20 µg/mL concentration caused the highest ALP activity. Since CEM cement, Biodentine, OCP/MTA, and Atlantik caused the highest ALP activity at 20 mg/mL, 2 mg/mL, 200 µg/mL, and 20 µg/mL concentrations, respectively, these concentrations were considered as optimal concentrations of these biomaterials for use in the next steps of the experiment (Figure 2).
Proliferation of biomaterial-treated SCAPs
Cell proliferation was assessed by cell counting (using the trypan blue technique). At baseline, MTA, Atlantik, and Biodentine-treated SCAPs showed greater proliferation than the control group (P<0.05). However, OCP/CEM cement-treated SCAPs showed no significant difference compared with the control group. On days 3 and 5, the proliferation capacity of SCAPs in MTA, Biodentine, and Atlantik groups was similar to that of the control group. But, SCAPs in CEM cement and OCP groups, had a significantly lower proliferation rate than the control group. At 9 days, no significant difference existed in proliferation capacity of the cells (MTA-treated SCAPs > Atlantik-treated SCAPs > control/Biodentine-treated SCAPs > CEM cement-treated SCAPs > OCP-treated SCAPs (Figure 3).
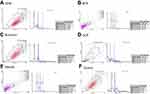
Flow cytometry revealed that there was a significant difference in proliferation index (PI) of OCP-treated SCAPs (PI=S%+G2M%) compared with the control group (34.8 vs 25.07, P<0.001, Figure 4D). No significant difference was noted in this respect between Atlantik (Figure 4E, 24.8), MTA (Figure 4B, 23.16), CEM cement (Figure 4A, 24.8), and Biodentine (Figure 4C, 27.67) compared with the control group (Figure 4F, 25.07).
Odontogenic/osteogenic differentiation of biomaterial-treated SCAPs
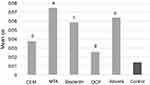
Alizarin red staining and cetylpyridinium chloride assay indicated that SCAPs treated with the tested biomaterials created a significantly higher number of mineralized nodules (Figure 5) and had significantly higher calcium content (Figure 6) at 21 days compared to the control group. SCAPs treated with MTA formed the highest and those treated with OCP formed the lowest number of calcified nodules.
The expression of genes related to odontogenic/osteogenic markers was evaluated using real-time RT-PCR. The genes related to odontogenic/osteogenic markers (including BSP, OCN, OSX, RUNX2, and ALP) and odontoblastic marker (DSPP) were significantly up-regulated at 3 and 7 days in the biomaterial groups. The SCAPs treated with Atlantik, OCP, and CEM cement, showed significant up-regulation of ALP and RUNX2 genes compared to the other two groups. OSX and OCN showed the highest expression in MTA-treated SCAPs. The highest expression of odontoblast specific marker (DSPP) was noted in OCP-treated SCAPs on day 3 and MTA-treated SCAPs on day 7 (Figure 7).
Cytokine production by biomaterial-treated SCAPs
The expression of pro-inflammatory cytokines, namely IL-1α, IL-1β, and IL-6 significantly increased in a time-dependent manner in all groups except for the control group and the OCP group. The Biodentine group showed the highest expression of IL-1α and IL-1β on day 3 and this expression increased over time but on day 7, the MTA group showed the highest expression of these cytokines. The SCAPs treated with OCP showed the lowest expression of IL-1α, IL-1β, and IL-6 (Figure 8). The highest expression of IL-6 was noted in Atlantik group on day 3 and the CEM cement group on day 7.
Discussion
Biomaterials used in endodontics are in direct contact with the pulp and periapical tissues. Therefore, they should be biocompatible and have no adverse effect on differentiation and proliferation of stem cells present in the area. SCAPs are among the stem cells present in this area, and are ideal for dental tissue engineering. Several studies have indicated that SCAPs and human DPSC treated with MTA can differentiate to osteoblasts/odontoblasts and form bone-like or dentin-like tissues under suitable in vitro conditions.5,20 However, information regarding the effect of other biomaterials on SCAPs is limited. Therefore, this study assessed the effect of OCP, Biodentine, MTA, CEM cement, and Atlantik on proliferation and odontogenic/osteogenic differentiation of SCAPs.
In this study, 2, 20, and 200 µg/mL and 2 and 20 mg/mL concentrations of biomaterials were first tested to find the optimal concentration of each biomaterial for induction of cell differentiation. The concentration of biomaterial resulting in the highest ALP activity was used for induction of proliferation and differentiation of SCAPs in the next steps of the experiment. Atlantik at 20 µg/mL, MTA and OCP at 200 µg/mL, Biodentine at 2 mg/mL, and CEM cement at 20 mg/mL concentration showed the highest ALP activity. In our study, MTA at 200 µg/mL concentration caused the highest ALP activity and by increasing its concentration, the ALP activity decreased. Zhao et al reported that concentrations higher than 2 mg/mL were toxic.21 In our study, the ALP activity increased in cells treated with different concentrations of CEM cement in a dose-dependent manner.
Our results revealed that all biomaterials tested induced the proliferation of SCAPs (were mitogen). This mitogenic effect was the highest in MTA, Atlantik, and Biodentine groups and the lowest in OCP group, indicating higher biocompatibility of MTA, Atlantik, and Biodentine compared to OCP. The proliferation rate of SCAPs treated with CEM cement was significantly higher than that of OCP group, which indicates higher cytotoxicity of OCP compared to other biomaterials. It appears that the similarity in PI of SCAPs in the control group and CEM cement, MTA, Biodentine, and Atlantik groups is due to the biocompatibility of these biomaterials and the fact that they all promote cell survival.22 Also, increased PI of SCAPs treated with OCP was probably due to the significantly increased expression of TNF-α by this biomaterial, especially on day 7. It has been demonstrated that CEM cement, MTA, Biodentine, and OCP have acceptable biocompatibility in the presence of SCAPs; however, among these biomaterials, CEM cement had the lowest cytotoxicity.23
The current study revealed that SCAPs treated with CEM cement, MTA, Biodentine, OCP, and Atlantik had significantly higher ALP activity and resulted in significantly higher calcium content; they also produced significantly higher number of calcified nodules compared to the control group. The ALP activity is pivotal for deposition of calcium on collagen plates.24 SCAPs treated with MTA showed the highest ALP activity and calcium nodule formation (in contrast to those treated with OCP). The mechanism of effect of MTA on mineralization of stem cells is induction of expression of BMP-2 gene, the alkaline pH of MTA, release of calcium hydroxide, and deposition of hydroxyapatite.25,26
In our study, the expression of odontoblast (DSPP) and odontoblast/osteoblast markers including BSP, OSX, OCN, RUNX2, and ALP was up-regulated in all study groups compared to the control group. DSPP is a specific marker for differentiation of odontoblasts, which is often detected in secretory odontoblasts.27,28 This gene produces DSP and DPP, which are both non-collagenous dentin matrix proteins. DSP is secreted into predentin, while DPP is released into the mineralized part of dentin and remains there. Increased expression of DSPP indicates higher potential to induce dentinogenesis.29–31
ALP is a well-recognized marker for detection of early osteogenic differentiation of cells. Also, it is a biologic marker for turn-over and a protein marker in primary development of odontoblasts. It indicates severe secretory activity of cells while OCN indicates cell entry into quiescent phase.21,32–34 OCN (Gla protein) is a non-collagenous protein released by bone cells in final stages of osteogenesis and is a specific marker for osteogenic differentiation.20 In our study, MTA and OCP resulted in the highest expression of OCN, while CEM cement resulted in the lowest expression of this gene, which probably indicates lower potential of CEM cement for osteoblast differentiation. Evidence shows that calcium phosphate scaffolds are suitable for hard tissue regeneration and are comparable to natural mineralized tissue in terms of biocompatibility and osteoconductivity.28
RUNX2 is the first transcription factor expressed by the osteoblastic cell line. OSX is extensively expressed by functional odontoblasts/osteoblasts.35 RUNX2 and OSX transcription factors are necessary for osteoblast and odontoblast differentiation, while DSPP is important for odontoblast differentiation. The relationship between the level of RUNX2, OSX, and DSPP during craniofacial bone and dental development has yet to be understood. However, a hypothesis suggests that RUNX2 and OSX play independent roles in differentiation of odontoblasts and osteoblasts. However, it has been shown that the expression of RUNX2 by mesenchymal odontogenic/osteogenic cells overlaps with the expression of OSX, while in final phases of differentiation, RUNX2 and OSX are intensely expressed by osteoblasts of the alveolar bone. In contrast, decreased expression of RUNX2 in odontoblasts is associated with increased expression of OSX. In the next phases, transcription of DSPP significantly decreases in osteoblasts while it significantly increases in odontoblasts, and is associated with increased expression of OSX. A previous study on pre-odontoblasts in rats demonstrated that increased expression of OSX increased the transcription of DSPP, which highlights the different biological functions of RUNX2, OSX, and DSPP in the process of osteogenesis/odontogenesis.35 Evidence shows that the expression of RUNX2 in osteochondroprogenitor cells is a determining factor in primary phases, while OSX is mainly expressed during maturation of osteoblasts.36 Differentiation of osteoblasts/odontoblasts and bone and dentin formation are regulated by complex and multi-step molecular pathways, but OSX is the only specific osteoblast transcription factor that is necessary for proliferation and differentiation of osteoblasts and bone formation.37
The current results indicate that expression of RUNX2 increased in all biomaterial groups in our study in a time-dependent manner, such that the highest expression of RUNX2 was noted in OCP, Atlantik, and CEM cement groups, while its lowest expression was seen in MTA group. The lowest expression of DSPP was noted in OCP group while its highest expression was recorded in MTA group. Therefore, it appears that SCAPs treated with OCP, Atlantik, and CEM cement mainly underwent osteoblastic differentiation, while SCAPs treated with MTA mostly underwent odontoblastic differentiation.
BSP is an important component of the extracellular matrix of bone. It is mainly released by osteoblasts, which are responsible for deposition and mineralization of matrix.33,38 Expression of BSP is exclusive to mineralized tissues such as bone, mineralized cartilages, dentin, and cementum.34 Although found in dentin, BSP is considered to be an osteoblastic phenotype marker. The highest expression of BSP was noted in regenerated and newly formed bone matrix. In our study, SCAPs treated with Biodentine and Atlantik showed the highest expression of this gene on day 7, which highlights the stronger osteogenic differentiation potential of this biomaterial compared to its odontogenic differentiation potential.39,40 Expression of BSP in pre-osteoblasts is low and hard to detect but its expression increases in osteoblasts and osteocytes and is therefore, well detectable.41 This finding confirms the results obtained in all groups in our study over time (especially Biodentine and Atlantik).
Our current findings indicate that CEM cement, MTA, Biodentine, OCP, and Atlantik significantly enhanced the release of pro-inflammatory cytokines namely IL-1α, IL-1β, IL-6, and TNF-α compared to the control group. The level of IL-1α and IL-1β significantly increased in all groups in a time-dependent manner such that their level in MTA group at 7 days increased to more than six times the value at 3 days. However, they did not undergo a significant change in OCP group. The MTA group at 3 days and the Atlantik and OCP groups at 7 days expressed the highest level of TNF-α. It has been confirmed that MTA enhances the expression of pro-inflammatory cytokines such as IL-1α, IL-1β, IL-6, and TNF-α and subsequently activates the NFKB pathway. Also, it has been confirmed that TNF-α is the canonical activator of NFKB.42 TNF-α leads to fast phosphorylation, ubiquitination, and proteolytic degradation of IкB, which mediates the transfer of NFKB into the nucleus and regulates gene transcription. The NFKB pathway triggers the biological events such as regulatory induction of odontogenesis/osteogenesis by BMP2, OSX, DSP, and RUNX2 and consequent differentiation of stem cells.34,42–49
Conclusion
All biomaterials evaluated in this study caused proliferation and differentiation of SCAPs to odontoblasts/osteoblasts and significantly induced the formation of calcified nodules. All biomaterials up-regulated the genes related to odontogenesis/osteogenesis compared to the control group. However, higher expression of ALP, RUNX2, and OSX genes by Atlantik and OCP suggests greater potential of these two biomaterials for induction of differentiation of SCAPs to osteoblasts; whereas, MTA and Biodentine had greater potential for induction of differentiation of SCAPs to odontoblasts.
Acknowledgment
The authors thank the Vice-Chancellery of Zahedan University of Medical Sciences for supporting this research (no: IR.ZAUMS.REC.1394011).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tziafas D, Kodonas K. Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J Endod. 2010;36(5):781–789. doi:10.1016/j.joen.2010.02.006
2. Sonoyama W, Liu Y, Yamaza T, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34(2):166–171. doi:10.1016/j.joen.2007.11.021
3. Sonoyama W, Liu Y, Fang D, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1(1):e79. doi:10.1371/journal.pone.0000079
4. Huang GT-J, Sonoyama W, Liu Y, Liu H, Wang S, Shi S. The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod. 2008;34(6):645–651. doi:10.1016/j.joen.2008.03.001
5. Yan M, Wu J, Yu Y, et al. Mineral trioxide aggregate promotes the odonto/osteogenic differentiation and dentinogenesis of stem cells from apical papilla via nuclear factor kappa B signaling pathway. J Endod. 2014;40(5):640–647. doi:10.1016/j.joen.2014.01.042
6. He G, Dahl T, Veis A, George A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat Mater. 2003;2(8):552. doi:10.1038/nmat945
7. Murphy W, Simmons C, Kaigler D, Mooney D. Bone regeneration via a mineral substrate and induced angiogenesis. J Dent Res. 2004;83(3):204–210. doi:10.1177/154405910408300304
8. Asgary S, Shahabi S, Jafarzadeh T, Amini S, Kheirieh S. The properties of a new endodontic material. J Endod. 2008;34(8):990–993. doi:10.1016/j.joen.2008.05.006
9. Rahimi S, Mokhtari H, Shahi S, et al. Osseous reaction to implantation of two endodontic cements: mineral trioxide aggregate (MTA) and calcium enriched mixture (CEM). Med Oral Patol Oral Cir Bucal. 2012;17(5):e907. doi:10.4317/medoral.18136
10. Wang Y, Yan M, Fan Z, Ma L, Yu Y, Yu J. Mineral trioxide aggregate enhances the odonto/osteogenic capacity of stem cells from inflammatory dental pulps via NF‐κB pathway. Oral Dis. 2014;20(7):650–658. doi:10.1111/odi.12183
11. Nowicka A, Lipski M, Parafiniuk M, et al. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J Endod. 2013;39(6):743–747. doi:10.1016/j.joen.2013.01.005
12. Lee B-N, Lee K-N, Koh J-T, et al. Effects of 3 endodontic bioactive cements on osteogenic differentiation in mesenchymal stem cells. J Endod. 2014;40(8):1217–1222. doi:10.1016/j.joen.2014.01.036
13. Tanuma Y, Matsui K, Kawai T, et al. Comparison of bone regeneration between octacalcium phosphate/collagen composite and β-tricalcium phosphate in canine calvarial defect. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115(1):9–17. doi:10.1016/j.oooo.2011.12.029
14. Suzuki O. Octacalcium phosphate: osteoconductivity and crystal chemistry. Acta Biomater. 2010;6(9):3379–3387. doi:10.1016/j.actbio.2010.04.002
15. Saberi EA, Aval FS, Arab MR, Sargolzaei N, Shahraki H, Aval FS. Bone regeneration by octacalcium phosphate (OCP)-gelatin composites in rat mandibular bone defects-a qualitative study. Adv Biores. 2017;8:1.
16. Sena M, Yamashita Y, Nakano Y, et al. Octacalcium phosphate–based cement as a pulp-capping agent in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontics. 2004;97(6):749–755. doi:10.1016/j.tripleo.2003.10.029
17. Steuer P, Voegel J-C, Cuisinier F. First experimental evidence for human dentine crystal formation involving conversion of octacalcium phosphate to hydroxyapatite. Acta Crystallogr Sect D: Biol Crystallogr. 1998;54(6):1377–1381. doi:10.1107/S0907444998005769
18. Takagi S, Frukhtbeyn S, Chow LC, et al. In vitro and in vivo characteristics of fluorapatite-forming calcium phosphate cements. J Res Natl Inst Stand Technol. 2010;115(4):267. doi:10.6028/jres.115.020
19. Yun H-M, Chang S-W, Park K-R, Herr L, Kim E-C. Combined effects of growth hormone and mineral trioxide aggregate on growth, differentiation, and angiogenesis in human dental pulp cells. J Endod. 2016;42(2):269–275. doi:10.1016/j.joen.2015.08.020
20. Li J, Yan M, Wang Z, et al. Effects of canonical NF-κB signaling pathway on the proliferation and odonto/osteogenic differentiation of human stem cells from apical papilla. Biomed Res Int. 2014;2014.
21. Zhao X, He W, Song Z, Tong Z, Li S, Ni L. Mineral trioxide aggregate promotes odontoblastic differentiation via mitogen-activated protein kinase pathway in human dental pulp stem cells. Mol Biol Rep. 2012;39(1):215–220. doi:10.1007/s11033-011-0728-z
22. Ruparel NB, Teixeira FB, Ferraz CC, Diogenes A. Direct effect of intracanal medicaments on survival of stem cells of the apical papilla. J Endod. 2012;38(10):1372–1375. doi:10.1016/j.joen.2012.06.018
23. Saberi EA, Karkehabadi H, Mollashahi NF. Cytotoxicity of various endodontic materials on stem cells of human apical papilla. Iran Endod J. 2016;11(1):17.
24. Beertsen W, Van Den Bos T. Alkaline phosphatase induces the mineralization of sheets of collagen implanted subcutaneously in the rat. J Clin Invest. 1992;89(6):1974–1980. doi:10.1172/JCI115805
25. Yasuda Y, Ogawa M, Arakawa T, Kadowaki T, Saito T. The effect of mineral trioxide aggregate on the mineralization ability of rat dental pulp cells: an in vitro study. J Endod. 2008;34(9):1057–1060. doi:10.1016/j.joen.2008.06.007
26. Okiji T, Yoshiba K. Reparative dentinogenesis induced by mineral trioxide aggregate: a review from the biological and physicochemical points of view. Int J Dent. 2009;2009.
27. Wang Y, Yan M, Yu Y, Wu J, Yu J, Fan Z. Estrogen deficiency inhibits the odonto/osteogenic differentiation of dental pulp stem cells via activation of the NF-κB pathway. Cell Tissue Res. 2013;352(3):551–559. doi:10.1007/s00441-013-1604-z
28. Brar GS, Toor RSS. Dental stem cells: dentinogenic, osteogenic, and neurogenic differentiation and its clinical cell based therapies. Indian J Dent Res. 2012;23(3):393. doi:10.4103/0970-9290.102239
29. Prasad M, Butler WT, Qin C. Dentin sialophosphoprotein in biomineralization. Connect Tissue Res. 2010;51(5):404–417. doi:10.3109/03008200903329789
30. Iohara K, Zheng L, Ito M, Tomokiyo A, Matsushita K, Nakashima M. Side population cells isolated from porcine dental pulp tissue with self‐renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells. 2006;24(11):2493–2503. doi:10.1634/stemcells.2006-0161
31. Zheng L, Papagerakis S, Schnell SD, Hoogerwerf WA, Papagerakis P. Expression of clock proteins in developing tooth. Gene Expression Patterns. 2011;11(3):202–206. doi:10.1016/j.gep.2010.12.002
32. Shui C, Scutt A. Mild heat shock induces proliferation, alkaline phosphatase activity, and mineralization in human bone marrow stromal cells and Mg‐63 cells in vitro. J Bone Mineral Res. 2001;16(4):731–741. doi:10.1359/jbmr.2001.16.4.731
33. Matsumoto S, Hayashi M, Suzuki Y, Suzuki N, Maeno M, Ogiso B. Calcium ions released from mineral trioxide aggregate convert the differentiation pathway of C2C12 cells into osteoblast lineage. J Endod. 2013;39(1):68–75. doi:10.1016/j.joen.2012.10.006
34. Rathinam E, Rajasekharan S, Chitturi RT, Martens L, De Coster P. Gene expression profiling and molecular signaling of dental pulp cells in response to tricalcium silicate cements: a systematic review. J Endod. 2015;41(11):1805–1817. doi:10.1016/j.joen.2015.07.015
35. Chen S, Gluhak-Heinrich J, Wang Y, et al. Runx2, osx, and dspp in tooth development. J Dent Res. 2009;88(10):904–909. doi:10.1177/0022034509342873
36. Artigas N, Ureña C, Rodríguez-Carballo E, Rosa JL, Ventura F. Mitogen activated protein kinase (MAPK)-regulated Interactions between osterix and Runx2 are critical for the transcriptional osteogenic program. J Biol Chem. 2014;289:27105–27117. doi:10.1074/jbc.M114.576793
37. Zhang C. Transcriptional regulation of bone formation by the osteoblast-specific transcription factor Osx. J Orthop Surg Res. 2010;5(1):37. doi:10.1186/1749-799X-5-37
38. Seo M-S, Hwang K-G, Lee J, Kim H, Baek S-H. The effect of mineral trioxide aggregate on odontogenic differentiation in dental pulp stem cells. J Endod. 2013;39(2):242–248. doi:10.1016/j.joen.2012.11.004
39. Wei X, Ling J, Wu L, Liu L, Xiao Y. Expression of mineralization markers in dental pulp cells. J Endod. 2007;33(6):703–708. doi:10.1016/j.joen.2007.02.009
40. Khan SZ, Kokubu E, Matsuzaka K, Inoue T. Behaviour of rat‐cultured dental pulp cells in three‐dimensional collagen type‐1 gel in vitro and in vivo. Aust Endodontic J. 2013;39(3):137–145. doi:10.1111/j.1747-4477.2012.00351.x
41. Arai N, Ohya K, Kasugai S, et al. Expression of bone sialoprotein mRNA during bone formation and resorption induced by colchicine in rat tibial bone marrow cavity. J Bone Mineral Res. 1995;10(8):1209–1217. doi:10.1002/jbmr.5650100811
42. Chang J, Liu F, Lee M, et al. NF-κB inhibits osteogenic differentiation of mesenchymal stem cells by promoting β-catenin degradation. Proc National Acad Sci. 2013;110(23):9469–9474. doi:10.1073/pnas.1300532110
43. Minamikawa H, Deyama Y, Nakamura K, et al. Effect of mineral trioxide aggregate on rat clonal dental pulp cells: expression of cyclooxygenase-2 mRNA and inflammation-related protein via nuclear factor kappa B signaling system. J Endod. 2009;35(6):843–846. doi:10.1016/j.joen.2009.03.008
44. Silva MJB, Vieira LQ, Sobrinho APR. The effects of mineral trioxide aggregates on cytokine production by mouse pulp tissue. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontics. 2008;105(5):e70–e76. doi:10.1016/j.tripleo.2008.01.025
45. Smithson G, Couse JF, Lubahn DB, Korach KS, Kincade PW. The role of estrogen receptors and androgen receptors in sex steroid regulation of B lymphopoiesis. J Immunol. 1998;161(1):27–34.
46. Ferreira DCD, Brito DG, Cavalcanti BN. Cytokine production from human primary teeth pulp fibroblasts stimulated by different pulpotomy agents. J Dent Child. 2009;76(3):194–198.
47. Paula-Silva F, Ghosh A, Silva L, Kapila Y. TNF-α promotes an odontoblastic phenotype in dental pulp cells. J Dent Res. 2009;88(4):339–344. doi:10.1177/0022034509334070
48. Nanes MS. Tumor necrosis factor-α: molecular and cellular mechanisms in skeletal pathology. Gene. 2003;321:1–15.
49. Hess K, Ushmorov A, Fiedler J, Brenner RE, Wirth T. TNFα promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-κB signaling pathway. Bone. 2009;45(2):367–376. doi:10.1016/j.bone.2009.04.252
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.