Back to Journals » OncoTargets and Therapy » Volume 7
Prior EGFR tyrosine-kinase inhibitor therapy did not influence the efficacy of subsequent pemetrexed plus platinum in advanced chemonaïve patients with EGFR-mutant lung adenocarcinoma
Authors Tseng J , Yang T, Chen K, Hsu K , Yu C , Liao W, Tsai C, Tsai M, Yu S, Su K, Chen J, Chen H, Chang G
Received 18 February 2014
Accepted for publication 19 March 2014
Published 23 May 2014 Volume 2014:7 Pages 799—805
DOI https://doi.org/10.2147/OTT.S62639
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Jeng-Sen Tseng,1,2 Tsung-Ying Yang,2 Kun-Chieh Chen,1,2 Kuo-Hsuan Hsu,1,3 Chong-Jen Yu,4 Wei-Yu Liao,4 Chi-Ren Tsai,5,6 Meen-Hsin Tsai,2,7 Sung-Liang Yu,8–11 Kang-Yi Su,8,12 Jeremy JW Chen,1 Hsuan-Yu Chen,7 Gee-Chen Chang1,2,13–15
1Institute of Biomedical Sciences, National Chung-Hsing University, 2Division of Chest Medicine, Department of Internal Medicine, Taichung Veterans General Hospital, 3Division of Critical Care and Respiratory Therapy, Department of Internal Medicine, Taichung Veterans General Hospital, Taichung, 4Department of Internal Medicine, National Taiwan University Hospital and National Taiwan University College of Medicine, Taipei, 5Department of Pediatrics, Taichung Veterans General Hospital, 6Institute of Molecular Biology, National Chung-Hsing University, Taichung, 7Institute of Statistical Science, Academia Sinica, 8Department of Clinical Laboratory Sciences and Medical Biotechnology, College of Medicine, National Taiwan University, 9Center for Optoelectronic Biomedicine, College of Medicine, National Taiwan University, 10Graduate Institute of Pathology, College of Medicine, National Taiwan University, 11Department of Laboratory Medicine, National Taiwan University Hospital, 12Center of Genomic Medicine, National Taiwan University, Taipei, 13School of Medicine, China Medical University, 14Comprehensive Cancer Center, Taichung Veterans General Hospital, Taichung, 15Faculty of Medicine, School of Medicine, National Yang-Ming University, Taipei, Taiwan
Background: Tumor cells before and after epidermal growth-factor receptor (EGFR) tyrosine-kinase inhibitor (TKI) therapy might display different characteristics. The aim of this study was to evaluate the influence of prior EGFR TKI therapy on the efficacy of subsequent pemetrexed plus platinum (PP) in advanced chemonaïve patients with EGFR-mutant lung adenocarcinoma.
Materials and methods: Advanced chemonaïve patients with EGFR-mutant lung adenocarcinoma receiving PP as first-line chemotherapy were enrolled retrospectively in two medical centers of Taiwan. The objective of this study was to compare objective response rate (ORR), disease-control rates (DCR), progression-free survival (PFS), and overall survival (OS) of PP in patients with and without prior EGFR TKI therapy.
Results: In total, 105 patients were analyzed. Sixty-one patients (58.1%) had prior EGFR TKI therapy and used PP as second-line treatment. The other 44 patients (41.9%) received PP as first-line therapy. ORRs of PP in patients with and without prior EGFR TKI therapy were 24.6% and 38.6%, respectively (P=0.138). DCRs of the two groups were 62.3% and 65.9%, respectively (P=0.837). The median PFS (6.1 versus 6.1 months, P=0.639) and OS (34.4 versus 32.3 months, P=0.394) were comparable between the groups with and without prior EGFR TKI therapy. In a subgroup analysis of patients with prior EGFR TKI therapy, there was no significant association between the efficacy of first-line EGFR TKI and the outcome of subsequent PP therapy.
Conclusion: Our results suggested that prior EGFR TKI therapy would not influence the efficacy of subsequent PP therapy in advanced chemonaïve patients with EGFR-mutant lung adenocarcinoma.
Keywords: non-small-cell lung cancer, epidermal growth-factor receptor mutation, epidermal growth-factor receptor tyrosine-kinase inhibitor, pemetrexed
Introduction
In the last decade, many studies focused on defining the individualization of non-small-cell lung cancer (NSCLC) therapy. Both histologic and molecular subtyping have been recognized as important predictive factors in NSCLC treatment.1,2 Several Phase III studies disclosed the significantly higher response rate and longer progression-free survival (PFS) of epidermal growth-factor receptor (EGFR) tyrosine-kinase inhibitor (TKI) therapy compared with chemotherapy as first-line treatment in patients with EGFR-mutant NSCLC.3–6 However, studies on EGFR TKI in the first-line setting did not show significant overall survival (OS) benefit.7
Pemetrexed plus platinum (PP) is one of the standard frontline therapies for chemonaïve patients with advanced lung adenocarcinoma. In 2008, Scagliotti et al compared first-line pemetrexed plus cisplatin to gemcitabine plus cisplatin in chemonaïve patients with advanced NSCLC, and demonstrated a survival benefit of pemetrexed/cisplatin in patients with nonsquamous histology.8 The efficacy of PP for patients with EGFR-mutant lung adenocarcinoma was even better than nonselected patients, as shown in a recent Phase III study.9 However, all the reported efficacy of PP was confined to the treatment-naïve cohort, and whether the results can be applied to patients with prior EGFR TKI therapy is still unclear.
Many recent studies suggest EGFR TKI as first-line therapy for patients with EGFR-mutant lung adenocarcinoma, and PP may be reserved for patients experiencing progression of first line EGFR TKI.10 However, the mechanism of EGFR TKI resistance is complex,11 and the post-EGFR TKI-treatment cancer cells may display different characteristics compared with the treatment-naïve cells. It is unclear if clinical resistance to EGFR TKI might also confer resistance to subsequent PP therapy. We conducted this study to evaluate whether prior EGFR TKI use influences the efficacy of subsequent PP in advanced chemonaïve patients with EGFR-mutant lung adenocarcinoma.
Materials and methods
Patients
This was a retrospective study consisting of patients with advanced lung adenocarcinoma harboring EGFR mutations and treated with PP as first-line chemotherapy regimen at two medical centers of Taiwan (Taichung Veterans General Hospital and National Taiwan University Hospital) from May 2007 to June 2013. We included lung cancer patients with histologically or cytologically confirmed and inoperable lung adenocarcinoma, known EGFR mutations, and clinically measurable disease. Patients were excluded if they had only evaluable lesions, other active malignancy, prior history of other chemotherapies, incomplete data records, or received other treatments, such as radiotherapy, concurrently. Tumor, node and metastases (TNM) staging was done according to the seventh edition of AJCC Cancer Staging Handbook.12 This study was approved by the institutional review board of each institute.
Data records and response evaluation
Clinical data for analysis included patients’ age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS), tumor stage, smoking status, EGFR-mutation status, prior EGFR TKI treatment, and PP-treatment history. Chest computed tomographies, which were followed up with an interval of 8–12 weeks and included the liver and adrenal glands, and other required image studies for response evaluation were reviewed by two chest physicians. Unidimensional measurements as defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 were used in this study.13 The objective of the study was to compare the objective response rate (ORR), disease-control rate (DCR), PFS, and OS of PP treatment in patients with and without prior EGFR TKI therapy. OS was determined from the date of the start of treatment (EGFR TKI in the group with prior EGFR TKI therapy, and PP in the group without prior EGFR TKI therapy) until the date of death, irrespective of cause.
EGFR-mutation tests
EGFR-mutation analyses using either direct sequencing or protein nucleic acid-locked nucleic acid polymerase chain reaction (PNA-LNA PCR) clamp methods were performed in patients with adequate specimens. Different methods were used in this study, because we set up a direct-sequencing method for EGFR-mutation analysis starting in 2007 and then shifted to the PNA-LNA PCR clamp in late 2009. Informed consent was obtained from all subjects.
Tumor specimens were procured for EGFR-mutation analysis as previously described.14 Briefly, deoxyribonucleic acid (DNA) was extracted from the tumors using a QIAmp DNA minikit (Qiagen, Venlo, the Netherlands) following the manufacturer’s protocols. For direct sequencing, the tyrosine-kinase domain of the EGFR-coding sequence – exons 18, 19, 20, and 21 – was amplified by PCR and sequenced bidirectionally with an ABI Prism® 3730 DNA analyzer (Life Technologies, Carlsbad, CA, USA) following standard protocol. For the PNA-LNA PCR clamp, real-time amplification monitoring was done using a SmartCycler® (Cepheid, Sunnyvale, CA, USA) to detect mutations on the tyrosine-kinase domain of the EGFR-coding sequence: exons 18, 19, 20, and 21.
Statistical methods
Patients were divided into without-prior EGFR TKI and with-prior EGFR TKI groups. Student’s t-test was used to compare ages between the two groups. Other univariate analysis comparing clinical characteristics and history of prior EGFR TKI therapy on ORR and DCR was performed by exact test. Multivariate analyses using the logistic regression model with stepwise selection method were performed for ORR and DCR. The Kaplan–Meier method was used to estimate PFS and OS. Differences in survival time regarding prior EGFR TKI therapy were analyzed using the log-rank test. Multivariate analyses using the Cox proportional hazard model with stepwise selection method were performed for PFS and OS. All statistical tests were done with SAS version 9.1 software (SAS Institute, Cary, NC, USA). Two-tailed tests and P-values <0.05 for significance were used.
Results
Patient characteristics
There was a total of 351 patients with advanced lung adenocarcinoma and history of PP treatment from May 2007 to June 2013. A total of 163 patients were excluded, including 85 with prior history of other chemotherapies, 62 without adequate tissue for EGFR-mutation analysis, 12 with incomplete chart records or without measurable lesions, and four who were receiving concurrent treatments (two radiotherapy, one bevacizumab, and one erlotinib). Of the remaining 188 patients, 105 patients had detectable EGFR mutations, and 83 did not. These 105 cases were included for analysis of PP efficacy.
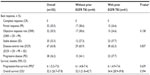
The baseline characteristics are shown in Table 1. Patients without prior EGFR TKI therapy were older than those with prior EGFR TKI therapy (61.6 versus 57.2 years), but this was not statistically significant (P=0.054). Otherwise, there were no significant differences in baseline characteristics between the two groups.
Efficacy of pemetrexed plus platinum chemotherapy
The results of best tumor response and PFS are shown in Table 2. Of the overall 105 patients, 32 patients achieved a partial response, and 35 patients had stable disease. No patients achieved a complete response. The ORR and DCR of the overall population were 30.5% and 63.8%, respectively. Survival data were followed up until the end of November 2013. At the end of data cutoff, five patients (two partial response and three stable disease, total 7.1%) had not experienced progression, and 61 patients were still alive at the last observation, and their data were therefore censored. The median PFS was 6.1 (95% confidence interval [CI] 5.2–7.0) months. The median OS was 32.3 (95% CI 26.7–37.8) months.
ORRs of patients with and without prior EGFR TKI therapy were 24.6% and 38.6%, respectively (P=0.138). DCRs in the two groups were 62.3% and 65.9%, respectively (P=0.837). Univariate analysis for best response regarding patient characteristics and choice of platinum revealed that no factor correlated significantly with ORR or DCR (data not shown), and no covariate reached the significance level to enter the multivariate logistic regression model.
The results of survival analysis are shown in Table 2 and Figure 1. The median PFS (6.1 versus 6.1 months, P=0.639) and OS (34.4 versus 32.3 months, P=0.394) were comparable between the groups with and without prior EGFR TKI therapy. Univariate analysis for survival regarding patient characteristics and choice of platinum revealed that no factor correlated significantly with PFS or OS (data not shown), and no covariates reached the significance level to enter the multivariate Cox proportional hazard model.
  | Figure 1 Kaplan–Meier plot showing progression-free survival (A) and overall survival (B) (n=105). |
Association between efficacies of prior EGFR TKI therapy and subsequent pemetrexed-plus-platinum chemotherapy
In the EGFR-mutation analysis, 55 patients (52.4%) had exon 19 deletion, and 40 patients (38.1%) had L858R. The EGFR statuses of the remaining ten patients were heterogeneous, including G719A/S, L861Q, T790M, and other complex mutations. In 55 patients harboring exon 19 deletion, seven had complex mutations, including four with T790M. In 40 patients harboring L858R, five revealed complex mutations, including two with T790M. In 61 patients with EGFR TKI as first-line therapy, 46 received gefitinib, 13 received erlotinib, and two received afatinib. The ORR and DCR of EGFR TKI were 67.2% (47 of 61) and 90.2% (55 of 61), respectively. The median PFS of EGFR TKI was 8.0 (95% CI 5.6–10.4) months. EGFR TKI therapy was stopped in 58 patients (95.1%) due to disease progression. Another three patients withdrew from EGFR TKI therapy because of hepatotoxicity (n=2) and financial considerations (n=1), respectively.
Results of univariate analysis for ORR and DCR in subgroups of patients with prior EGFR TKI therapy are shown in Table 3. Neither responses nor PFS of prior EGFR TKI therapy significantly influenced the efficacy of subsequent PP therapy.
Subsequent treatments after pemetrexed-plus-platinum chemotherapy
In the group treated with PP initially (n=44), two patients did not receive further treatment, one patient received other chemotherapies and 41 patients received EGFR TKI as second-line therapy. Eight of the 41 patients were still undergoing EGFR TKI therapy without PD, and 27 of the remaining 33 patients (81.8%) received at least one subsequent therapy after progression to second-line EGFR TKI. In the group with prior EGFR TKI therapy (n=61), five patients were still undergoing PP therapy without PD, and 53 of the remaining 56 patients (94.6%) received at least one subsequent therapy after progression to PP.
Discussion
Chemotherapy with a PP regimen is one of the standard frontline therapies for patients with advanced nonsquamous NSCLC. Mutation of the EGFR gene, a member of the ErbB-receptor family, is the most common genetic alteration in lung adenocarcinoma of East Asians.15 Therefore, several studies have tried to evaluate the association between EGFR status and the efficacy of pemetrexed.16–18 However, the baseline characteristics of most studies, including histology and concurrent platinum and treatment lines, were not homogeneous and the results were inconsistent. Furthermore, few studies have looked at the effectiveness of chemotherapy after EGFR TKI in EGFR-mutant NSCLC.19,20 In the present study, our patients were relatively homogeneous, because all were chemonaïve, had EGFR-mutant adenocarcinoma, and received platinum-containing doublet chemotherapy. Our results suggested that prior EGFR TKI therapy would not influence the efficacy of subsequent PP therapy in chemonaïve patients with advanced EGFR-mutant lung adenocarcinoma.
In the present study, we showed an ORR of 30.5%, a DCR of 63.8%, a median PFS of 6.1 months, and a median OS of 32.3 months with PP therapy in the 105 advanced chemonaïve patients with EGFR-mutant adenocarcinoma. The efficacy was similar to that of a Phase III study conducted by Scagliotti et al, who reported the ORR was 30.6% and the PFS was 4.8 months for all NSCLC.8 In comparison, our cohort comprised only EGFR-mutant adenocarcinoma, and half of them received EGFR TKI prior to PP. The efficacy of PP was similar in both groups, and patients who received PP as first-line therapy (n=44) had an ORR of 38.6% and a median PFS of 6.1 months. The results were comparable with those reported in LUX-Lung 3 study, which compared the efficacy of afatinib and pemetrexed plus cisplatin as first-line treatment for patients with advanced lung adenocarcinoma harboring EGFR mutations.9 In the chemotherapy arm, the ORR was 23% and the median PFS was 6.9 months.
Whether EGFR TKI should precede or be followed by chemotherapy is still unclear. Several studies have suggested that chemotherapy and EGFR TKI may influence the efficacy of each other, and drugs used in frontline therapy, whether EGFR TKI or chemotherapy, may destroy more tumor cells. In 2006, Chang et al showed that chemonaïve patients had a higher response rate to gefitinib than chemotherapy-treated patients, and hypothesized that tumor cells will evolve into a more heterogeneous and resistant phenotype with a longer time after diagnosis.21 Recently, Bai et al also showed that chemotherapy may reduce EGFR-mutation frequency in both plasma and tumor tissue, and suspected a reduction of overall clinical benefit of subsequent EGFR TKI after chemotherapy.22 However, in the present study, we showed that neither responses nor survival time of PP therapy were significantly different between patients with and without prior EGFR TKI therapy. As PFS represents the duration of tumor control of an investigational therapy, PFS together with ORR and DCR are important indicators to demonstrate the effectiveness of the study regimen irrespective of subsequent treatment,23 and all these outcome parameters were similar in each group of the present study. By contrast, OS represents the greatest clinical benefit but could be skewed by the effects of subsequent therapies. In the group with PP as first-line therapy, 41 of 44 (93.2%) received EGFR TKI in subsequent therapies, which might explain the similar OS of both groups. The ORR of second-line EGFR TKI among 35 patients with measurable disease was 62.9%, which was similar to that of patients receiving EGFR TKI as first-line therapy (P=0.663).
In 2008, Deng et al used the lung adenocarcinoma cell lines PC9 and PC9/G with acquired resistance to gefitinib to explore the influence of acquired resistance of EGFR TKI on the sensitivity of tumor cells to chemotherapeutic drugs, and showed that no significant difference of sensitivity to pemetrexed was found between these two cell lines.24 Furthermore, a study by Maemondo et al that compared the efficacy of gefitinib and carboplatin plus paclitaxel as first-line treatment for patients with advanced NSCLC harboring EGFR mutations also showed a similar response rate of carboplatin plus paclitaxel either in the first-line setting or as subsequent therapy after progression to first-line gefitinib (30.7% versus 28.8%, respectively).3 In the gefitinib group, 67.5% of patients received carboplatin plus paclitaxel as second-line therapy. We suggest that there might be no clinically meaningful interference between EGFR TKI and chemotherapy.
In a study by Sun et al, there was no significant association between the efficacy of prior EGFR TKI and subsequent pemetrexed therapy.25 However, the results might have been limited by the diversity of previous treatments and the lack of EGFR-mutation analysis. The present study may provide a more solid result, because all patients with prior EGFR TKI therapy received PP therapy as second-line therapy, and all of them harbored EGFR mutations. Our results suggested that neither efficacy of prior EGFR TKI therapy nor the EGFR-mutation type would influence the outcome of subsequent PP therapy.
There are three limitations of this study. First, this was a retrospective study. Although data were collected retrospectively, we tried to ensure the validity of patients’ characteristics, and excluded patients with confounding factors that could lead to incorrect response evaluation. A prospective trial is needed to evaluate the extent of the impact of interaction between EGFR TKI and PP in advanced EGFR-mutant lung adenocarcinoma patients. Second, the EGFR status in this study was not assessed by the same method. However, both direct sequencing and PNA-LNA PCR clamp are standard methods for EGFR testing, and this did not likely influence our results. The relatively shorter PFS of EGFR TKI in this study might be explained by the effect of complex and uncommon mutations.26 Third, there was no biological analysis in the present study, such as thymidylate synthase-expression level between the treatment-naïve and post-EGFR TKI tumor tissue, which has been recognized to be associated with pemetrexed efficacy.27
Conclusion
In conclusion, our results suggested that the efficacy of PP therapy in chemonaïve patients with EGFR-mutant adenocarcinoma was comparable between patients with and without prior EGFR TKI therapy. Furthermore, in patients with prior EGFR TKI therapy, there was no significant association between the efficacy of first-line EGFR TKI and the outcome of subsequent PP therapy.
Acknowledgments
We would like to thank the Comprehensive Cancer Center and Clinical Informatics Research and Development Center of Taichung Veterans General Hospital for the assistance of data collection and management, and acknowledge the technical services provided by the Pharmacogenomics Lab of the National Research Program for Biopharmaceuticals (NRPB) (TR6-3) and Integrated Core Facility for Functional Genomics of the National Core Facility Program for Biotechnology (NCFPB) (C5), which were supported by the National Science Council (NSC102-2325-B-002-078 and NSC102-2319-B-002).
Disclosure
The authors have no conflicts of interest in this work.
References
West H, Harpole D, Travis W. Histologic considerations for individualized systemic therapy approaches for the management of non-small cell lung cancer. Chest. 2009;136(4):1112–1118. | |
Moreira AL, Thornton RH. Personalized medicine for non-small-cell lung cancer: implications of recent advances in tissue acquisition for molecular and histologic testing. Clin Lung Cancer. 2012;13(5):334–339. | |
Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. | |
Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. | |
Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. | |
Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. | |
Mok T, Yang JJ, Lam KC. Treating patients with EGFR-sensitizing mutations: first line or second line – is there a difference? J Clin Oncol. 2013;31(8):1081–1088. | |
Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3543–3551. | |
Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. | |
Leighl NB. Treatment paradigms for patients with metastatic non-small-cell lung cancer: first-, second-, and third-line. Curr Oncol. 2012; 19 Suppl 1:S52–S58. | |
Oxnard GR, Arcila ME, Chmielecki J, Ladanyi M, Miller VA, Pao W. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res. 2011;17(17):5530–5537. | |
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Handbook. 7th ed. New York: Springer; 2009. | |
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. | |
Tseng JS, Yang TY, Chen KC, Hsu KH, Chen HY, Chang GC. Retrospective study of erlotinib in patients with advanced squamous lung cancer. Lung Cancer. 2012;77(1):128–133. | |
An SJ, Chen ZH, Su J, et al. Identification of enriched driver gene alterations in subgroups of non-small cell lung cancer patients based on histology and smoking status. PloS One. 2012;7(6):e40109. | |
Wu SG, Yang CH, Yu CJ, et al. Good response to pemetrexed in patients of lung adenocarcinoma with epidermal growth factor receptor (EGFR) mutations. Lung Cancer. 2011;72(3):333–339. | |
Camidge DR, Kono SA, Lu X, et al. Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol. 2011;6(4):774–780. | |
Kim YH, Hirabayashi M, Togashi Y, et al. Phase II study of carboplatin and pemetrexed in advanced non-squamous, non-small-cell lung cancer: Kyoto Thoracic Oncology Research Group Trial 0902. Cancer Chemother Pharmacol. 2012;70(2):271–276. | |
Gridelli C, Ciardiello F, Gallo C, et al. First-line erlotinib followed by second-line cisplatin-gemcitabine chemotherapy in advanced non-small-cell lung cancer: the TORCH randomized trial. J Clin Oncol. 2012;30(24):3002–3011. | |
Wu JY, Shih JY, Yang CH, et al. Second-line treatments after first-line gefitinib therapy in advanced nonsmall cell lung cancer. Int J Cancer. 2010;126(1):247–255. | |
Chang GC, Tsai CM, Chen KC, et al. Predictive factors of gefitinib antitumor activity in East Asian advanced non-small cell lung cancer patients. J Thorac Oncol. 2006;1(6):520–525. | |
Bai H, Wang Z, Chen K, et al. Influence of chemotherapy on EGFR mutation status among patients with non-small-cell lung cancer. J Clin Oncol. 2012;30(25):3077–3083. | |
Soria JC, Massard C, Le Chevalier T. Should progression-free survival be the primary measure of efficacy for advanced NSCLC therapy? Ann Oncol. 2010;21(12):2324–2332. | |
Deng QF, Su B, Zhao YM, Zhou CC. [Sensitivity of two cell lines with acquired resistance to gefitinib to several chemotherapeutic drugs]. Zhonghua Zhong Liu Za Zhi. 2008;30(11):813–816. Chinese. | |
Sun JM, Oh DY, Lee SH, et al. The relationship between response to previous systemic treatment and the efficacy of subsequent pemetrexed therapy in advanced non-small cell lung cancer. Lung Cancer. 2010;68(3):427–432. | |
Wu JY, Yu CJ, Chang YC, Yang CH, Shih JY, Yang PC. Effectiveness of tyrosine kinase inhibitors on “uncommon” epidermal growth factor receptor mutations of unknown clinical significance in non-small cell lung cancer. Clin Cancer Res. 2011;17(11):3812–3821. | |
Christoph DC, Asuncion BR, Hassan B, et al. Significance of folate receptor alpha and thymidylate synthase protein expression in patients with non-small-cell lung cancer treated with pemetrexed. J Thorac Oncol. 2013;8(1):19–30. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.