Back to Journals » International Medical Case Reports Journal » Volume 13
Primary Osteosarcoma of the Breast with Extensive Chondroid Matrix in a Teenager Female Patient: The Paradoxical Diagnosis in Breast Mastopathy
Received 8 October 2019
Accepted for publication 28 December 2019
Published 10 January 2020 Volume 2020:13 Pages 11—17
DOI https://doi.org/10.2147/IMCRJ.S233674
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
James Joseph Yahaya, 1, 2 Michael Odida 1
1Department of Pathology, Makerere College of Health Sciences (MakCHS), Kampala, Uganda; 2Department of Biomedical Sciences, College of Health Sciences (CHS), the University of Dodoma, Dodoma, Tanzania
Correspondence: James Joseph Yahaya
Department of Pathology, Makerere College of Health Sciences (MakCHS), P.O. Box 7072, Kampala, Uganda
Tel +256 784 847 139
Email [email protected]
Purpose: Non-epithelial tumors of the breast are extremely rare and have an incidence of less than 1%. The most common non-epithelial breast tumor is the phyllodes tumor (PT), which accounts for 61%. Primary osteosarcomas of the breast contribute up to only 12.5% of all breast sarcomas. In young females, osteosarcomas are extremely rare, especially in those without a previous history of primary bone osteosarcoma. A case of a 16-year old female with primary osteosarcoma of the breast (POB) with extensive chondroid matrix involving the left breast is herein presented.
Case Report: This report describes a 16-year old female with neither a previous history of bone osteosarcoma nor family history of breast cancer who was diagnosed with a primary chondroblastic osteosarcoma of the left breast. The mass was shining, warm, firm, and slightly fixed. The excisional biopsy showed a large tumor measuring 11x9x7 cm which was encapsulated, grayish-white, and nodular.
Conclusion: Primary osteosarcomas of the breast carry a poor prognosis by being triple negative and because of being the rarest tumors, they pose a challenge in managing the patients due to lack of established treatment modalities.
Keywords: osteosarcoma, breast, triple negative
Introduction
Primary osteosarcoma of the breast (POB) is a very rare tumor with poor prognosis. Regarding the previous reports, POB has been rarely diagnosed and POB contributes to approximately 1.2% of all soft tissue sarcomas and less than 1% of all sarcomas arising primarily from the mammary glands.1 It is well known that osteogenic osteosarcomas affect mainly young patients but POB commonly affects older patients with a mean age at presentation around 65 years.2 Of the spindle cell neoplasms affecting the breast, both with pure mesenchymal origin and those with an epithelial component which are literally called carcinosarcomatous such as metaplastic carcinomas and malignant phyllodes tumor (MPT), MPT is more prevalent with an incidence of about 30%.3,4
Diagnosis of POB is supposed to be made after excluding metaplastic carcinoma, MPT and metastatic skeletal osteosarcoma. In 2008, Khan et al5 reported that approximately 150 cases of POB had been reported in the literature since 1957. POB is a very aggressive malignancy, with a high possibility of relapse and metastasis especially by hematogenous route instead of lymphatic spread, and the most common distant site for metastasis are the lungs.6 The pathogenesis of POB remains unclear. Some reports have suggested that the tumor develops from totipotent mesenchymal cells of the breast stroma or the transformation of a preexisting fibroadenoma or phyllode tumor.6,7 We present the case of a 16-year-old African female who was diagnosed with an osteosarcoma in her left breast.
Case Report
A 16-year old female was brought to the pathology laboratory for fine needle aspiration cytology (FNAC) after presenting with a painful fast growing left breast mass for 8 months. Before coming to our laboratory, she was taken for mammography examination, which revealed a large mass of about 11x9x6 cm, well circumscribed with cystic degenerative changes. A provisional diagnosis of fibroadenoma was made. An ultrasound scan (USS) was done, w2hich revealed a large irregular predominantly solid mass with cystic areas and large egg shaped calcification (Figure 1). We examined the patient at the pathology laboratory before doing FNAC and we found the left breast was significantly swollen and shining (Figure 2). On palpation the tumor was firm, nodular, ill-defined, and mobile, and it was occupying the lower outer quadrant. The tumor was measuring 11x8x5 cm. The nipple was neither retracted nor inverted and the overlying skin was unremarkable. The contralateral breast was normal and both axillary regions had no palpable lymph nodes. After performing FNAC, the aspirate material was cystically hemorrhagic. The cytomorphological features were of moderate cellular smears indicating few solid clusters of epithelial cells without atypia in a background of cystic hemorrhagic material. A diagnosis of fibrocystic change disease of the breast was made.
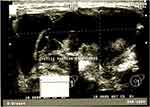 |
Figure 1 Ultrasound scan appearance of the involved left breast. Both upper quadrants are involved by the tumor, with marked cystic changes as well as marked areas of the lesion with calcification. |
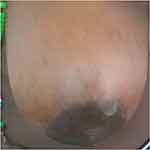 |
Figure 2 The breast is swollen, shining, and somewhat paler at the center above the nipple. |
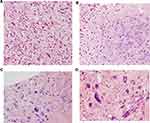
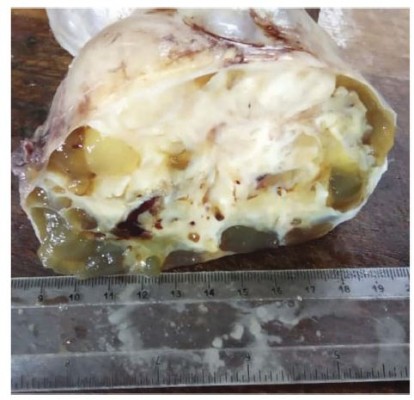
Following this deviation from usual fibroadenoma as it was suggested by mammography, an open biopsy was requested. The submitted lumpectomy specimen was measuring 11×9×7 cm, encapsulated, grayish-white, and nodulated. Cut-surface showed heterogeneous tissue with numerous cystic and a few whitish and fibrous solid lesions (Figure 3). Microscopically, using Hematoxylin and Eosin (H&E) stained sections, the tumor was composed of atypical spindle cells and large pleomorphic epithelioid cells and multinucleated cells of osteoclastic type which were forming a new bone matrix (osteoid). About 40 atypical mitoses per 10 HPF were seen. Therefore, a diagnosis of primary osteosarcoma was made (Figures 4A–D).
 |
Figure 3 The tumor is heterogeneous with marked cystic changes. Other areas show extensive fibrosis and gritty sensation on sectioning. |
For immunohistochemical (IHC) staining for alpha-SMA and vimentin, sections of thickness of 4 μm were prepared from the FFPE tissue block using a microtome. The sections were de-waxed by being microwaved at 60°C for 50 minutes, and then cleared in two changes of xylene using 10 dips in each change. Hydration was done by dipping the sections in 95%, 80%, and 70% ethanol and then rinsed in running tap water. Two drops of 3% H2O2 solution were added to each section for 20 minutes to block endogenous peroxidase and, then, the slides were rinsed in distilled water. The slides were incubated in the antigen retrieval solution of 10 mmol/L citrate buffer of pH 6.0 within a pressure cooker at 95°C for 30 minutes, from which the slides were removed after 2 minutes of full pressure.
The slides were placed in distilled water waiting for addition of phosphate buffer solution (PBS, Dako, Denmark) to the sections for 3 minutes. PBS was then drained from slides before adding a ready to use primary monoclonal antibody of alpha-SMA (MA1106, BosterBio, CA, USA) and allowed to incubate for 1 hour. The slides were washed with PBS for 5 minutes, and, then, two drops of horse rabbit peroxidase were added to each section for 30 minutes. The slides were again washed with PBS for 3 minutes. PBS was drained from the sections; then, chromogen diaminobenzidine (DAB) (Dako, Denmark) was added to the sections for 3 minutes.
The sections were washed in distilled water, counterstained in Harris hematoxylin solution for 10 seconds, and differentiated by 2 dips into 1% of HCL-Ethanol solution. The sections were blued in warm water for 5 minutes, dehydrated through 70%, 80%, and 95% ethanol, and then cleared in two changes of xylene for 10 minutes. The sections were finally cover-slipped using Distyrene Plasticizer Xylene (DPX) and were ready for assessment and interpretation.
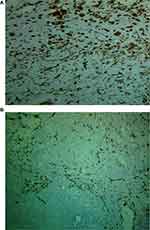
The similar protocol according to the manufacturer was used for vimentin (Monoclonal antibody, PB93559, CA, USA). Vimentin was strongly positive (Figure 5A) and SMA was focally positive (Figure 5B). ER/PR and HER-2/neu were all negative (triple negative) and also AE1/AE3 was negative. This confirmed the histological diagnosis of primary osteosarcoma. After the wide excisional biopsy treatment of the patient, the patient was sent for a survey of metastasis by means of computer tomography (CT) scan which revealed no metastasis. Following evaluation of the patient for metastasis, the patient was supposed to start chemotherapy; however, before starting chemotherapy, the parents decided to take her for herbals. The patient has not developed either recurrence or metastasis for 10 months since diagnosis and she was reported to have no complaint regarding the previous diagnosis.
Discussion
Encountering of osteosarcoma of the breast in a patient at the age of 16 years is something that raises questions that may not get answers easily. This is because development of the commonest epithelial breast malignancies in patients aged below 18 years is unusual. Then the development of POB in the current case remains a paradox. Risk factors have been identified for some extraskeletal osteosarcomas and include prior local irradiation, trauma, and a foreign body. The mechanism of epithelial cells transforming into mesenchymal cells (EMT) has been described and various EMT-regulating transcription factors such as Snail, Slug, Twist, and zinc finger E-box-binding homeobox 1 (ZEB1) and OVol2 protein genes, have been widely recognized as important players in tumor development and progression.8
Involvement of these transcription factors in the complex pathogenesis of OS has been reported in the literature. The current communication of Crevecoeur et al9 analyzed the overexpression of Ovol2 in patients with metastatic osteosarcoma from different sites including breast, and they found that overexpression of Ovol2 gene protein was associated with poor prognosis (P˂0.05). Another school of thought regarding the pathogenesis of POB is the linking with fibroadenomas based on the argument that both fibroadenomas and POBs contain bone and osteoid cells. Both disease entities share a similar mammographic appearance of being well-circumscribed dense masses with numerous calcifications, as was the case in the present patient.10 However, these two hypotheses do not give a causal relationship, hence they still remain as suggestions.
The presentation of POB is that of either any other breast cancer or like any other benign breast lesions such as fibroadenoma or phyllodes tumors. Clinically most patients with POB tumors present with pain and a fast growing mass which is firm, mobile, and circumscribed without involving axillary lymph nodes.6 Because this disease presents with mammographic features resembling those of benign breast lesions, this contributes greatly to the decrease of the clinical index of suspicion. This in turn may lead to a delay for initiation of proper treatments. Because POB is very rare, this contributes greatly to the lack of defined comprehensive and reliable systemic treatment modalities. Studies have shown that the treatments for POB are excision, tylectomy, and mastectomy depending on the status of the patient.11 Excisional biopsy stands as the initial treatment. The current patient underwent excisional biopsy as a means of treatment. This was followed by scanning for presence of metastasis using computer tomography (CT) scan which revealed no metastasis to any site. Her condition has continued to be uneventful for 10 months since she underwent excisional biopsy.
Studies have shown that the prognosis of POB is very poor. The high propensity of metastasizing to the lungs followed by bones, more through a hematogenous route than lymphatic pathway, gives the disease a poor prognosis. The nature of being osteosarcoma is another reason for the poor diagnosis due to the fact that osteosarcomas are resistant to chemotherapy as well as radiotherapy. A number of prognostic factors regarding POB have been reported, including the number of mitotic figures, tumor size, and presence of stromal atypia.12 In the series of Silver et al11 which included 50 patients with POB, it was found that the overall survival for the 39 patients with complete follow-up data was only 38%. During follow-up of the patients, 15 patients developed metastasis to the lungs and bones after 20 months and 11 of them had recurrence of the disease. The argument of if adjuvant radiotherapy should be used remains unclear, albeit several studies reporting on a small number of patients suggest that adjuvant chemotherapy may be of value in patient management.13
Conclusion
In summary, POB is a rarely reported disease entity with a very poor prognosis and it has not been studied extensively for different treatment regimens to be established. The mimicking of mammographic findings poses another challenge for timely treatment of the disease. Therefore, it should be understood that POB may involve very young patients as it has been for the index case contrary to other mammary sarcomas.
Ethical Approval
We confirm that publication of the case details required institutional approval and we also confirm that we obtained ethical clearance from the institution review board (IRB) of the School of Biomedical science, Makerere College of Health Sciences (MakCHS).
Informed Consent
We confirm that a written informed consent was provided by the parent to have the case details and any accompanying images published.
Acknowledgment
An abstract of this paper was presented at the 2019 American Society of Clinical Pathology (ASCP) Annual Meeting in Phoenix, AZ, USA, as a poster presentation with interim findings. The poster’s abstract was published in “Poster’s abstracts” in the American Journal of Clinical Pathology (AJCP).
Author Contributions
Both authors contributed equally towards collection of information regarding the case reported and organizing and drafting the first version of the paper. Additionally, both authors critically revised the final paper and gave approval of the final version to be published, as well as accepted to be accountable for all aspects of the paper.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sarkar S, Kapur N, Mukri HM, Saurabh A, Kumar N. Chondroblastic osteosarcoma of breast in a case of phyllodes tumour with recurrence, a rare case report. Int J Surg Case Rep. 2016;27(3):189–191. doi:10.1016/j.ijscr.2016.08.035
2. Vorobiof G, Hariparsad G, Freinkel W, Said H, Vorobiof DA. Primary osteosarcoma of the breast: a case report. Breast J. 2003;9(3):231–233. doi:10.1046/j.1524-4741.2003.09320.x
3. Pietruszka M, Barnes L. Cystosarcoma phyllodes. A clinicopathologic analysis of 42 cases. Cancer. 1978;41(5):1974–1983. doi:10.1002/(ISSN)1097-0142
4. Zhao J, Zhang X, Liu J, Li J. Primary osteosarcoma of the breast with abundant chondroid matrix and fibroblasts has a good prognosis: A case report and review of the literature. Oncol Lett. 2013;6(3):745–747. doi:10.3892/ol.2013.1446
5. Khan S, Griffiths EA, Shah N, Ravi S. Primary osteogenic sarcoma of the breast: A case report. Cases J. 2008;1(1):148. doi:10.1186/1757-1626-1-148
6. Yoon CS, Kang SS. Primary osteosarcoma of the breast: a case report. Ann Surg Treat Res. 2017;93(1):57–60. doi:10.4174/astr.2017.93.1.57
7. El Ochi MR, Zouaidia F, Kabaj R, et al. Primary chondroblastic osteosarcoma of the breast. Turk Patoloji Derg. 2014;30(3):225–227. doi:10.5146/tjpath.2014.01249
8. Liu J, Wu Q, Wang Y, et al. Ovol2 induces mesenchymal-epithelial transition via targeting ZEB1 in osteosarcoma. Onco Targets Ther. 2018;11:963–2973.
9. Crevecoeur J, Jossa V, Gennigens C, Parmentier JC, Crevecoeur A. Primary osteosarcoma of the breast: a case report. Clin Case Rep. 2016;4(1):62–66. doi:10.1002/ccr3.450
10. Uner A, Ozturk B, Benekli M, et al. Synchronize primary breast osteosarcoma and contralateral benign cystosarcoma phylloides: radiologic and pathologic imaging. Breast J. 2008;14(1):109–110. doi:10.1111/j.1524-4741.2007.00533.x
11. Silver SA, Tavassoli FA. Primary osteogenic sarcoma of the breast: a clinicopathologic analysis of 50 cases. Am J Surg Pathol. 1998;22(8):925–933. doi:10.1097/00000478-199808000-00002
12. Bhatta S, Aryal G. Primary osteosarcoma of the breast. JNMA J Nepal Med Assoc. 2013;52(192):631–633.
13. Kallianpur AA, Gupta R, Muduly DK, Kapali A, Subbarao KC. Osteosarcoma of breast: a rare case of extraskeletal osteosarcoma. J Cancer Res Ther. 2013;9(2):292–294. doi:10.4103/0973-1482.113392
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.