Back to Journals » Patient Preference and Adherence » Volume 16
Primary Non-Adherence to Preventive Drugs and Associations with Beliefs About Medicines in Stroke Survivors
Authors Westberg A, Sjölander M , Glader EL, Gustafsson M
Received 30 November 2021
Accepted for publication 12 January 2022
Published 9 February 2022 Volume 2022:16 Pages 343—352
DOI https://doi.org/10.2147/PPA.S351001
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Annica Westberg,1 Maria Sjölander,1 Eva-Lotta Glader,2 Maria Gustafsson1
1Department of Integrative Medical Biology, Umeå University, Umeå, 901 87, Sweden; 2Department of Public Health and Clinical Medicine, Umeå University, Umeå, 901 87, Sweden
Correspondence: Maria Gustafsson
Department of Integrative Medical Biology, Umeå University, Umeå, SE-901 87, Sweden
, Email [email protected]
Background: Medication non-adherence is a common problem in clinical practice. Little is known about stroke survivors’ primary non-adherence to preventive drugs, and we hypothesised that their beliefs about medicines are associated with primary non-adherence. The objective was to describe primary non-adherence among stroke survivors and to assess associations between primary non-adherence to preventive drugs and beliefs about medicines.
Methods: Questionnaires were sent to 797 individuals 3 months after stroke to assess beliefs about medicines through the Beliefs about Medicines Questionnaire (BMQ). All participants were registered in the Swedish Stroke Register (Riksstroke), and prescriptions for new preventive drugs during the hospital stay were identified through data from Riksstroke. Primary non-adherers were those who failed to fill one or more new prescriptions within 1 month of hospital discharge based on data from the Swedish Prescribed Drug Register. Differences between primary non-adherers and adherers were assessed by 2 tests and associations between the BMQ subscales and primary non-adherence were analysed using independent two-sample t-tests and multivariable logistic regression models.
Results: A total of 594 individuals responded to the survey, of which 452 received new prescriptions of preventive drugs. Overall, 53 (12%) participants were classified as primary non-adherent. Primary non-adherers were more often dependent on help or support from next of kin (p=0.032) and had difficulties with memory more often (p=0.002) than the primary adherent individuals. No statistically significant differences in BMQ subscale-scores were found between the two groups (p> 0.05).
Conclusion: Primary non-adherence to preventive drugs was low, and no associations were found between primary non-adherence and beliefs about medicines. Associations with cognitive impairments such as difficulties with memory and need for help from next of kin suggest that more effort is needed to help stroke survivors to start important preventive drug treatments after discharge from hospital.
Keywords: beliefs about medicines, preventive drugs, primary non-adherence, stroke
Introduction
In 2019, approximately 25,700 people suffered from an ischemic or haemorrhagic stroke in Sweden.1 Next to ischemic heart disease, stroke is the second leading cause of death and accounts for approximately 11% of the total deaths worldwide.2
It has previously been reported that the estimated risk of stroke recurrence is high, with a cumulative risk of approximately 3.1% at 30 days, 11.1% at 1 year and 26.4% at 5 years after the initial event.3 Previous research has confirmed the benefits of using preventive drugs to prevent recurrent stroke.4–6 However, non-adherence and discontinuation of pharmacological treatment can be a problem in clinical practice.6,7 For instance, primary non-adherence may occur, defined as the individual’s failure to fill one or more new prescriptions within a defined number of days after the prescription was initially issued.8 Secondary non-adherence, on the other hand, is often defined as failure to refill a prescription, measured over 6 or 12 months or longer.8 The prevalence of primary non-adherence after discharge from a general internal medicine service has, for instance, been evaluated in a Canadian study by Fallis et al.9 In the study, 66 individuals (28%) displayed primary non-adherence at 7 days post discharge, and 55 individuals (24%) were classified as primary non-adherent at 30 days after discharge. The study population exhibited primary non-adherence to different types of medicines for the management of various conditions, eg, statins and clopidogrel, which were used for stroke prevention.
In a Swedish cross-sectional questionnaire survey, associations between stroke survivors’ beliefs about medicines and self-reported adherence were investigated.10 In the study, negative beliefs were more common among non-adherent individuals, and they also considered their drug treatment as less useful. Different types of validated questionnaires were used in the study, one of them being the Beliefs about Medicines Questionnaire (BMQ), which is a self-report questionnaire developed to assess people’s perceptions about medicines.11
Preventive treatment is an essential part of post-stroke care, and early initiation of preventive treatment is associated with a reduced risk of early stroke recurrence.12,13 Furthermore, assessing associations between people’s primary non-adherence and beliefs about medicines could be of great importance for improving the use of preventive drugs.
Until now, little is known about stroke survivors’ primary non-adherence to preventive drugs. Consequently, the objective of this study was to describe primary non-adherence among stroke survivors and to assess whether their personal beliefs about medicines are associated with primary non-adherence to preventive drugs.
Materials and Methods
Study Population and Study Design
In this cross-sectional questionnaire study, all participants were people with stroke who were enrolled in The Swedish Stroke Register (Riksstroke) between December 2011 and March 2012. Riksstroke is a national quality register for stroke care, and the register includes all 74 hospitals that admit patients with acute stroke in Sweden, covering approximately 90% of all stroke patients.14 An invitation to participate in the study was sent to all 74 hospitals included in Riksstroke, and 25 of these volunteered to participate. These 25 hospitals are university hospitals, non-university hospitals and community hospitals, situated in 15 of the 21 different regions in Sweden.
Questionnaires were sent to 797 stroke survivors previously admitted to 1 of the 25 hospitals, who were living at home 3 months after their stroke.
Questionnaire data on adherence, attitudes and beliefs about stroke and medicines were combined with clinical data from Riksstroke and data about dispensed prescriptions from the Swedish Prescribed Drug Register. The three validated questionnaires used in this survey were: The Brief Illness Perception Questionnaire (Brief IPQ), the Beliefs about Medicines Questionnaire (BMQ) and the Medication Adherence Report Scale (MARS). The Brief IPQ addresses questions of the respondent’s perception about their disease, while BMQ is used to assess the respondent’s personal beliefs about medicines.11,15 MARS addresses questions of self-reported medication adherence.16 Of the three validated questionnaires, only BMQ was used in this study.
Information about the individuals’ background characteristics was obtained from Riksstroke through the participants’ personal identity numbers. Riksstroke also provides information about alterations in drug treatments, ie, which types of preventive medicines the patient receives both prior to hospital admission and at hospital discharge. The register was therefore used to identify participants who were prescribed one or more new preventive drugs during the hospital stay. The following drug classes were considered as preventive drugs: statins, acetylsalicylic acid, dipyridamole, clopidogrel, warfarin, and acetylsalicylic acid in combination with dipyridamole.
The Swedish Prescribed Drug Register contains information about all prescribed drugs dispensed at national pharmacies and information about dispensed prescriptions was obtained through each participant’s personal identity number.17 The prevalence of unfilled prescriptions at 1 week, 2 weeks and 1 month after discharge was evaluated for each separate class of drugs. Antihypertensive agents were not included in the analyses since changes were made regarding these register variables in Riksstroke between 2011 and 2012 (previously reported as separate drug classes, in 2012 they were combined into one class of antihypertensive drugs). The novel oral anticoagulants (NOACs) were also excluded since this class of drugs was not yet routinely used in clinical practice at the time of the data collection.
Classification of Study Participants and the Beliefs About Medicines Questionnaire
All participants who received a prescription for a new preventive drug during the hospital stay were identified through Riksstroke. In this study, we defined a new preventive drug as a drug that the participant was prescribed during the hospital stay (ie, the participant did not have a prescription of the drug when admitted to the hospital). Individuals with one or more unfilled prescriptions at 1 month (31 days) after hospital discharge were classified as primary non-adherent, while participants who purchased all the newly prescribed preventive drugs were classified as primary adherent. The number of participants with one or more unfilled prescriptions at 1 and 2 weeks after hospital discharge was also evaluated. The day of discharge was included in all follow-up periods.
BMQ was used to measure beliefs about medicines and the questionnaire is comprised of two different scales: BMQ-General and BMQ-Specific.11 BMQ-General assesses the respondent’s beliefs about drugs in general and BMQ-Specific evaluates the individual’s beliefs about medicines prescribed for their own use (Table 1). BMQ-General consists of three different subscales; harm, overuse, and benefit, while BMQ-Specific consists of necessity and concern. After classifying the participants as primary adherent or non-adherent, the mean scores of the BMQ subscales were compared between the two groups. Participants with one or more missing answers on the subscale questions were excluded in the corresponding subscale analysis, since their total scores would not be comparable to those of the rest of the study population.
 |
Table 1 The Beliefs About Medicines Questionnaire (BMQ) – Description of the General and Specific Scales by Number of Questions, Range of Score and Subscale Themes |
Statistical Analysis
Descriptive statistics were used to present the participants’ characteristics. Frequencies and proportions were calculated for categorical and discrete variables, while continuous variables are presented as mean values ± standard deviations (SD). Groupwise comparisons of background characteristics between primary non-adherers and adherers were assessed using 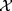 2 tests.
2 tests.
The normally distributed (checked by visual inspection) BMQ subscale-scores are presented as mean values ± SD and independent two-sample t-tests were used to test for differences in subscale-scores between the two groups. Furthermore, multivariable logistic regression models were also computed to test for associations between the BMQ subscales and primary non-adherence. The models were adjusted for potentially confounding factors that displayed a statistically significant difference (p < 0.05) between the two groups in the 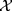 2 tests. For the categorical covariates, “Never or Almost never” regarding memory difficulties and “No” were selected as reference categories whilst the BMQ subscale-scores were included as continuous covariates. Each subscale was included in a separate multivariable analysis. Results were presented as OR with 95% CI. All analyses were performed with IBM SPSS Statistics V.27.0. A p-value <0.05 was considered statistically significant.
2 tests. For the categorical covariates, “Never or Almost never” regarding memory difficulties and “No” were selected as reference categories whilst the BMQ subscale-scores were included as continuous covariates. Each subscale was included in a separate multivariable analysis. Results were presented as OR with 95% CI. All analyses were performed with IBM SPSS Statistics V.27.0. A p-value <0.05 was considered statistically significant.
Results
Baseline Characteristics of Survey Respondents
Questionnaires were sent to 797 people, of whom 594 responded to the survey, corresponding to a response rate of 74.5%. The mean age was 71 years (SD 11.7) and the population consisted of a higher proportion of men (Table 2). Ischemic stroke contributed to 91.8% of all events and 84.8% of the participants suffered from a first-time stroke.
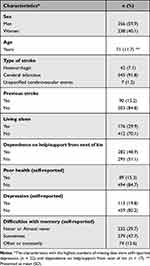 |
Table 2 Baseline Characteristics of the 594 Survey Respondents |
New Prescriptions of Preventive Drugs and Number of Unfilled Prescriptions After Hospital Discharge
Overall, 452 participants received new prescriptions of preventive drugs and together they received a total of 727 prescriptions (Table 3). Statins were the most prevalently prescribed class of drugs, followed by acetylsalicylic acid and clopidogrel. At 1 month after discharge, all drug classes except the fixed-dose combination drugs of acetylsalicylic acid + dipyridamole exhibited a relatively low primary non-adherence rate, ranging from 6% to 13.6%. A total of 66 (9.1%) new prescriptions were unfilled at 1 month after discharge.
 |
Table 3 The Prevalence of New Prescriptions Displayed for Each Separate Class of Drugs. |
Prevalence of Primary Non-Adherence
In total, 452 people received one or more new prescriptions upon discharge, whereas 142 individuals received no new medications during the hospital stay (Table 4). A similar number of participants received one and two new prescriptions, and only a minority of 34 people (5.7%) received three new prescriptions of preventive drugs.
 |
Table 4 Number of People Being Prescribed 0, 1, 2 or 3 New Medicines and the Number of Participants Being Classified as Primary Non-Adherent at 1 Month Post Hospital Discharge |
Primary non-adherent individuals failed to fill at least one new prescription, resulting in 73 (16.2%) non-adherers after 1 week, 65 (14.4%) after 2 weeks and finally 53 primary non-adherers (11.7%) at 1 month post discharge. Among the primary non-adherers, most individuals (n = 41) failed to fill one new prescription, while only one participant presented primary non-adherence to three different medicines (data not shown). A total of 399 (88.3%) individuals were classified as primary adherent at 1 month post hospital discharge, meaning all new prescriptions were filled within 31 days. Additionally, most primary adherers (n = 379, 95%) filled all their new prescriptions within the first week after discharge (data not shown).
Differences in Background Characteristics Between Primary Non-Adherers and Adherers
Primary non-adherers were more often dependent on the help or support from next of kin and had difficulties with memory more often than primary adherent individuals (Table 5). No other characteristics displayed statistically significant differences between the two groups.
 |
Table 5 Background Characteristics of Study Participants Divided by Level of Primary Adherence |
Associations Between BMQ Subscale-Scores and People’s Primary Non-Adherence
The mean scores of the primary non-adherent and adherent individuals were similar across all BMQ subscales (Table 6). No statistically significant differences were observed between the subscale-scores of the two groups. Furthermore, the multivariable logistic regression models also displayed no associations between the BMQ-subscales and primary non-adherence.
 |
Table 6 Associations Between BMQ Subscale-Scores and People’s Primary Non-Adherence. |
Discussion
In this study, the prevalence of unfilled prescriptions was relatively low for almost all drug classes and approximately 9% of all new prescriptions were unfilled after the first month of hospital discharge. Similar findings were seen in a Spanish study investigating primary adherence in patients with ST-elevated myocardial infarction (STEMI).18 Both stroke and STEMI are severe health conditions associated with high morbidity and mortality. Stroke survivors may therefore initially be motivated to use preventive drugs to reduce the risk of stroke recurrence. However, approximately 12% of the participants in this study were classified as primary non-adherent at 1 month after hospital discharge. In accordance with previous research, primary non-adherers had more difficulties with memory and were more dependent on the help of others, as compared to adherers.10 Forgetfulness has previously been reported as a reason for non-adherence among stroke survivors, and this could perhaps also be applicable regarding primary non-adherence.19 Additionally, in the study by Padilla et al, prior history of a cardiovascular event was associated with primary non-adherence, perhaps due to previous experiences regarding the use of cardiovascular drugs.18 In this study, no associations were found between medical history of stroke and primary non-adherence. However, the number of primary non-adherers with a prior history of stroke was small in this study.
The prevalence of primary non-adherence was low and no significant differences in BMQ subscale-scores were observed between the primary non-adherent and adherent individuals in this study. Factors other than personal beliefs may therefore be associated with people’s primary non-adherence to preventive drugs since no associations were found between primary non-adherence and beliefs about medicines in this study. For instance, non-adherence can be categorised as unintentional or intentional. Unintentional non-adherence occurs due to forgetfulness, lack of understanding of medication instructions or if the individual is unable to afford the drug.20 In contrast, if the individual decides to not follow the recommended medication regimen, intentional non-adherence occurs. Unfilled prescriptions may therefore not be solely the results of active decisions, but rather in some instances perhaps a consequence of unintentional non-adherence. Clifford et al observed no significant differences in beliefs about medicines between adherers and unintentional non-adherers, whereas intentional non-adherers exhibited higher concerns about their medicines than adherers.20 Furthermore, Sjölander et al examined associations between beliefs about medicines and self-reported adherence within the same population of stroke survivors as in this study.10 Conversely, Sjölander et al found that self-reported non-adherers displayed higher scores for negative beliefs, while the scores for positive beliefs were lower, as compared to the adherent individuals. Regarding the results of Sjölander et al,10 self-reported non-adherers are perhaps mainly intentionally non-adherent, and intentional non-adherence is more strongly related to people’s beliefs about medicines, as compared to unintentional non-adherence.20,21 In addition, the results of this study may be in accordance with the observations seen by Clifford et al,20 with primary non-adherers being mainly unintentionally non-adherent to their newly prescribed preventive drugs.
In accordance with previous studies, the prevalence of primary non-adherence was assessed at 1 month post hospital discharge.18,22 A 31-day follow-up period allows time for post-stroke recovery and possible alterations in activities of daily living, for example, the introduction of home-help service. In a previous study, the prevalence of primary adherence was assessed at 7 days post hospital discharge.9 As shown in this study, 16% of the participants had one or more unfilled prescriptions at 1 week after discharge, whereas 12% were classified as primary non-adherent after 1 month post discharge. Selecting a 7-day or 31-day follow-up period would therefore probably not have a considerable impact on the results of this study.
Primary non-adherence was the focus of this study, and long-term adherence was therefore not investigated. Nevertheless, long-term adherence to preventive drugs has previously been reported as suboptimal during the first 2 years after stroke.23,24 Discharge counselling sessions or motivational interviewing have previously been used to improve primary and long-term adherence.25,26 Adherence was assessed after 1 week post hospital discharge or at the end of a 12-month follow-up period and, in both instances, the interventions significantly improved people’s medication adherence. No information was obtained regarding the use of discharge counselling sessions upon hospital discharge in this study. The impact of discharge medication counselling on stroke survivors’ primary adherence therefore warrants further investigation.
Strengths of this study include the use of the validated Beliefs about Medicines Questionnaire, which have been used in previous studies of various health conditions to assess people’s perceptions about medicines.10,20,27 Medication adherence is a multifaceted issue and a gold standard method for measuring adherence does not exist.28 For instance, self-reported methods are generally easy to use, although they are sometimes considered to overestimate people’s adherence.29 In this study, a nationwide database was used to obtain reliable and accurate information of dispensed prescriptions in a large sample of stroke survivors. However, a filled prescription does not provide proof of actual drug intake, or that the individual will use the medicine according to the physicians’ recommendations. Socioeconomic factors such as income or educational level were not included in this study, although socioeconomic status could perhaps serve as a predictor of primary non-adherence in stroke survivors. For example, a previous study performed in individuals with ischemic stroke living in Sweden found that adherence to statins after stroke was less common among people born outside of Sweden and among people with university education. No association was however found between adherence and income.24 Furthermore, no conclusions about causality can be drawn due to the cross-sectional design of this study. Also, it cannot be excluded that beliefs about medicines and primary non-adherence differ between individuals who completed and did not complete the survey, which might influence the results. This study was performed in Sweden, and the results may not be applicable to stroke survivors in other countries due to cultural differences in beliefs about medicines and differences in healthcare systems.
To our knowledge, there are a limited number of studies regarding stroke survivors’ primary non-adherence.
This study found no associations between primary non-adherence and beliefs about medicines and other factors may therefore affect stroke survivors’ primary non-adherence. For instance, patient-healthcare professional communication could perhaps be of great importance. Post-discharge medication adherence may reflect good communication between patient and hospital staffs, whereas insufficient discharge instructions could result in unintentional non-adherence. However, further research is required to estimate the prevalence of unintentional non-adherence among stroke survivors and to evaluate the impact of discharge medication counselling on first-fill prescription adherence to post-stroke medications. Moreover, the impact of primary non-adherence on early stroke recurrence was not investigated in this study, and this issue should be addressed in future studies on stroke survivors.
Conclusion
Primary non-adherence to preventive drugs was low 1 month post hospital discharge in this sample of stroke survivors. No associations were found between primary non-adherence and beliefs about medicines. Rather associations with cognitive impairments such as difficulties with memory and need for help from next of kin suggest that more effort is needed to help stroke survivors to start important preventive drug treatments after discharge from hospital.
Ethics Approval
This project has received ethics approval by the Ethical Review Board at Umeå University, Sweden (Reg. No 2011-375-31M). All study participants provided informed consent. The study was performed in accordance with the principles outlined in the Declaration of Helsinki..
Informed Consent
The study was performed in accordance with the principles outlined in the Declaration of Helsinki.
Funding
This study was financially supported by grants from Apoteket AB and Västerbotten County Council. The funders had no role in the study design, data collection and analysis, or decision to publish.
Disclosure
The authors declare no conflicts of interest for this work.
References
1. Socialstyrelsen. Statistik om stroke 2019 (in Swedish). The Swedish National Board of Health and Welfare, 2019. Stroke statistics; 2019. Available from: https://www.socialstyrelsen.se/globalassets/sharepoint-dokument/artikelkatalog/statistik/2020-11-7047.pdf.
2. World Health Organization. The top 10 causes of death; 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
3. Mohan KM, Wolfe CD, Rudd AG, et al. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke. 2011;42(5):1489–1494. doi:10.1161/STROKEAHA.110.602615
4. Burke JP, Sander S, Shah H, et al. Impact of persistence with antiplatelet therapy on recurrent ischemic stroke and predictors of nonpersistence among ischemic stroke survivors. Curr Med Res Opin. 2010;26(5):1023–1030. doi:10.1185/03007991003670563
5. Yeo SH, Toh MPHS, Lee SH, et al. Impact of medication nonadherence on stroke recurrence and mortality in patients after first-ever ischemic stroke: insights from registry data in Singapore. Pharmacoepidemiol Drug Saf. 2020;29(5):538–549. doi:10.1002/pds.4981
6. Colivicchi F, Bassi A, Santini M, et al. Discontinuation of statin therapy and clinical outcome after ischemic stroke. Stroke. 2007;38(10):2652–2657. doi:10.1161/STROKEAHA.107.487017
7. Hohmann C, Neumann-Haefelin T, Klotz JM, et al. Adherence to hospital discharge medication in patients with ischemic stroke: a prospective, interventional 2-phase study. Stroke. 2013;44(2):522–524. doi:10.1161/STROKEAHA.112.678847
8. Raebel MA, Schmittdiel J, Karter AJ, et al. Standardizing terminology and definitions of medication adherence and persistence in research employing electronic databases. Med Care. 2013;51(8 Suppl 3):S11–21. doi:10.1097/MLR.0b013e31829b1d2a
9. Fallis BA, Dhalla IA, Klemensberg J, et al. Primary medication non-adherence after discharge from a general internal medicine service. PLoS One. 2013;8(5):e61735. doi:10.1371/journal.pone.0061735
10. Sjölander M, Eriksson M, Glader E-L. The association between patients’ beliefs about medicines and adherence to drug treatment after stroke: a cross-sectional questionnaire survey. BMJ Open. 2013;3(9):e003551. doi:10.1136/bmjopen-2013-003551
11. Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14(1):1–24.
12. Rothwell PM, Giles MF, Chandratheva A, et al. Early use of Existing Preventive Strategies for Stroke (EXPRESS) study. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370(9596):1432–1442. doi:10.1016/S0140-6736(07)61448-2
13. Rothwell PM, Algra A, Chen Z, et al. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials. Lancet. 2016;388(10042):365–375. doi:10.1016/S0140-6736(16)30468-8
14. Asplund K, Hulter Åsberg K, Appelros P, et al. The Riks-Stroke story: building a sustainable national register for quality assessment of stroke care. Int J Stroke. 2011;6(2):99–108. doi:10.1111/j.1747-4949.2010.00557.x
15. Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60(6):631–637. doi:10.1016/j.jpsychores.2005.10.020
16. Mahler C, Hermann K, Horne R, et al. Assessing reported adherence to pharmacological treatment recommendations. Translation and evaluation of the Medication Adherence Report Scale (Mars) in Germany. J Eval Clin Pract. 2010;16(3):574–579. doi:10.1111/j.1365-2753.2009.01169.x
17. Wettermark B, Hammar N, Fored CM, et al. The new Swedish Prescribed Drug Register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–735. doi:10.1002/pds.1294
18. Padilla López A, Alós-Almiñana M, Peris JE. Health outcomes and primary adherence to secondary prevention treatment after St-Elevation myocardial infarction: a Spanish cohort study. J Cardiovasc Transl Res. 2021;14(2):308–316. doi:10.1007/s12265-020-10045-0
19. Chambers JA, O’Carroll RE, Hamilton B, et al. Adherence to medication in stroke survivors: a qualitative comparison of low and high adherers. Br J Health Psychol. 2011;16(3):592–609. doi:10.1348/2044-8287.002000
20. Clifford S, Barber N, Horne R. Understanding different beliefs held by adherers, unintentional nonadherers, and intentional nonadherers: application of the Necessity-Concerns Framework. J Psychosom Res. 2008;64(1):41–46. doi:10.1016/j.jpsychores.2007.05.004
21. Wroe AL. Intentional and unintentional nonadherence: a study of decision making. J Behav Med. 2002;25(4):355–372. doi:10.1023/a:1015866415552
22. Mitchell B, Chong C, Lim WK. Medication adherence 1 month after hospital discharge in medical inpatients. Intern Med J. 2016;46(2):185–192. doi:10.1111/imj.12965
23. Glader EL, Sjölander M, Eriksson M, Lundberg M. Persistent use of secondary preventive drugs declines rapidly during the first 2 years after stroke. Stroke. 2010;41(2):397–401. doi:10.1161/STROKEAHA.109.566950
24. Sjölander M, Eriksson M, Glader EL. Inequalities in medication adherence to statin treatment after stroke: a nationwide observational study. Eur Stroke J. 2016;1(2):101–107. doi:10.1177/2396987316646026
25. Leguelinel-Blache G, Dubois F, Bouvet S, et al. Improving patient’s primary medication adherence: the value of pharmaceutical counseling. Medicine (Baltimore). 2015;94(41):e1805. doi:10.1097/MD.0000000000001805
26. Hedegaard U, Kjeldsen LJ, Pottegård A, et al. Improving medication adherence in patients with hypertension: a randomized trial. Am J Med. 2015;128(12):1351–1361. doi:10.1016/j.amjmed.2015.08.011
27. Brandstetter S, Finger T, Fischer W, et al. Differences in medication adherence are associated with beliefs about medicines in asthma and COPD. Clin Transl Allergy. 2017;7:39. doi:10.1186/s13601-017-0175-6
28. Anghel LA, Farcas AM, Oprean RN. An overview of the common methods used to measure treatment adherence. Med Pharm Rep. 2019;92(2):117–122. doi:10.15386/mpr-1201
29. Zeller A, Ramseier E, Teagtmeyer A, et al. Patients’ self-reported adherence to cardiovascular medication using electronic monitors as comparators. Hypertens Res. 2008;31(11):2037–2043. doi:10.1291/hypres.31.2037
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
