Back to Journals » OncoTargets and Therapy » Volume 9
Primary gastric anaplastic lymphoma kinase-negative anaplastic large-cell lymphoma
Received 14 April 2016
Accepted for publication 8 August 2016
Published 13 September 2016 Volume 2016:9 Pages 5659—5661
DOI https://doi.org/10.2147/OTT.S110572
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Min Li
Chen Tian, Yizhuo Zhang
Department of Hematology, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center for Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin, People’s Republic of China
Introduction: Most primary stomach lymphomas are now recognized to originate from B-cell. Primary gastric anaplastic lymphoma kinase (ALK)-negative anaplastic large-cell lymphoma (ALCL) as shown in this case is very rare.
Case report: A 59-year-old man presented with a 1-month history of epigastric pain. Computed tomography showed a tumor in the stomach with perigastric lymphadenopathy. Biopsy of the tumor with gastroendoscopy showed ALCL. Bone marrow aspiration and trephine biopsy showed no infiltration. A diagnosis of primary gastric ALK-negative ALCL was made. The patient was first treated with four cycles of cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) regimen, but his condition did not show improvement. Then he was treated with two cycles of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate and cytarabine (Hyper-CVAD/MA) regimen. In spite of these treatments, he still died of disease progression.
Conclusion: The prognosis of ALK-negative ALCLs is usually worse than ALK-positive ALCLs. In this case, the patient was not responsive to a multidrug chemotherapy with CHOP and Hyper-CVAD/MA.
Keywords: ALK-negative ALCL, primary gastric, CHOP, Hyper-CVAD/MA
Introduction
Primary gastrointestinal lymphomas are observed with a male preponderance, most of which are non-Hodgkin lymphomas, with B-cell dominating over T-cell type. Stomach is the commonest site followed by small intestine, and mucosa-associated lymphoid tissue lymphoma is the most common subtype.1,2 Isolated colonic involvement and intestinal perforations at presentation are not infrequent. A few rare variants such as anaplastic large-cell lymphoma (ALCL) and follicular lymphoma are also observed. ALCL is a rare hematological malignancy and a distinct subtype of mature T-cell lymphomas. ALCL is divided into three subtypes: ALK-positive ALCL, anaplastic lymphoma kinase (ALK)-negative ALCL, and primary cutaneous ALCL. Although presenting with a similar morphological spectrum as ALK-positive ALCL, ALK-negative ALCL (15%–50% of all systemic ALCL cases) is only defined as a provisional entity. In addition, ALK-negative ALCL usually occurs in comparatively older people and carries a poorer prognosis. Here, we report a rare case of primary gastric ALK-negative ALCL.
Case report
A 59-year-old man presented with a 1-month history of epigastric pain with low-grade fever. On examination, he had no hepatosplenomegaly or lymphadenopathy. The results of laboratory examinations, including a full blood count, liver function tests, and serum lactate dehydrogenase, were normal. Computed tomography of the neck, chest, abdomen, and pelvis showed a tumor in the stomach (5×6 cm) with perigastric lymphadenopathy (Figure 1). A diagnosis of lymphoma or gastric cancer was considered likely. Biopsy of the tumor with gastroendoscopy was performed and showed ALCL. Lymphoma cells were positive for CD30, CD2, CD43, LCA, EMA, PAX5, and MUM1 and were negative for CD20, CD3, ALK, CD79a, CD10, Bcl6, CD68, MPO, CK, CD34, and CD138 (Figure 2). The positive ratio of Ki67 was ~80%. Bone marrow aspiration and trephine biopsy showed no infiltration. A diagnosis of primary gastric ALK-negative ALCL was made. Although, first, he was treated with four cycles of CHOP regimen (cyclophosphamide, doxorubicin, vincristine, prednisone), the lymphoma lesions increased in size. Then he underwent two cycles of Hyper-CVAD/MA regimen (hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate and cytarabine). After high-dose chemotherapy, the patient had severe bone marrow suppression. Blood transfusion and anti-infection treatment were given. However, he died of disease progression 3 months later. The patient provided written informed consent for publishing this paper and accompanying images.
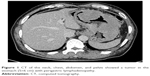  | Figure 1 CT of the neck, chest, abdomen, and pelvis showed a tumor in the stomach (5×6 cm) with perigastric lymphadenopathy. |
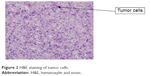  | Figure 2 H&E staining of tumor cells. |
Discussion
ALCL belongs to peripheral T-cell lymphomas, which are characterized with strong expression of CD30.3 According to the expression of ALK, ALCL is divided into three categories: primary systemic ALK-positive ALCL, primary systemic ALK-negative ALCL, and primary cutaneous ALCL. ALK-positive ALCL is sensitive to chemotherapy and has a better prognosis, whereas ALK-negative ALCL usually occurs in older patients with a worse prognosis.
The stomach is a common site of extranodal lymphomas. Most primary stomach lymphomas are recognized to be of the B-cell type, such as mucosa-associated lymphoid tissue lymphoma. In contrast, primary gastric T-cell lymphomas are very rare. Fewer than 100 cases have been reported, most of which are from Japan.4–11
Surgery is not commonly used for the treatment of gastric lymphoma. Surgical resection is frequently performed for accurate diagnosis and staging of the disease. Some patients were reported to undergo surgery because the location of the lesion was difficult to approach.12,13 ALK-positive ALCLs are usually sensitive to a multidrug chemotherapy such as CHOP.14,15 In this case, we first treated the patient with CHOP regimen, but the disease progressed. Then we changed to a high-grade regimen Hyper-CVAD/MA. In spite of these treatments, he still died of disease progression. This suggests that ALK-negative ALCL is not sensitive to chemotherapy, which leads to poor prognosis.
Acknowledgment
The study was funded by grants 31301161 and 81270603 from the National Natural Science Foundation of China.
Disclosure
The authors report no conflicts of interest in this work.
References
Xue Y, Wang Q, He X. Clear cell variant of diffuse large B-cell lymphoma: a case report and review of the literature. Int J Clin Exp Pathol. 2015;8(6):7594–7599. | ||
Molavi O, Samadi N, Wu C, Lavasanifar A, Lai R. Silibinin suppresses NPM-ALK, potently induces apoptosis and enhances chemosensitivity in ALK-positive anaplastic large cell lymphoma. Leuk Lymphoma. 2015;14:1–27. | ||
Armitage JO. The aggressive peripheral T-cell lymphomas: 2015. Am J Hematol. 2015;90(7):665–673. | ||
Mori N, Yatabe Y, Oka K, et al. Primary gastric Ki-1 positive anaplastic large cell lymphoma: a report of two cases. Pathol Int. 1994;44(2):164–169. | ||
Murata T, Nakamura S, Oka K, et al. Granzyme B-positive primary gastric T-cell lymphoma: gastric T-cell lymphoma with the possibility of extrathymic T cell origin. Pathol Int. 2000;50(10):853–857. | ||
Watanabe M, Moriyama Y. Primary gastric T-cell lymphoma without human T-lymphotropic virus type 1: report of a case. Surg Today. 2002;32(6):525–530. | ||
Yatabe Y, Mori N, Oka K, Nakazawa M, Asai J. Primary gastric T-cell lymphoma. Morphological and immunohistochemical studies of two cases. Arch Pathol Lab Med. 1994;118(5):547–550. | ||
Tokunaga O, Watanabe T, Shimamoto Y, Tokudome S. Primary T-cell lymphoma of the gastrointestinal tract associated with human T-cell lymphotropic virus type I. An analysis using in situ hybridization and polymerase chain reaction. Cancer. 1993;71(3):708–716. | ||
Shimada-Hiratsuka M, Fukayama M, Hayashi Y, et al. Primary gastric T-cell lymphoma with and without human T-lymphotropic virus type 1. Cancer. 1997;80(2):292–303. | ||
Horie R, Yatomi Y, Wakabayashi T, et al. Primary gastric T-cell lymphomas: report of two cases and a review of the literature. Jpn J Clin Oncol. 1999;29(3):171–178. | ||
Hatano B, Ohshima K, Katoh A, et al. Non-HTLV-1-associated primary gastric T-cell lymphomas show cytotoxic activity: clinicopathological, immunohistochemical characteristics and TIA-1 expression in 31 cases. Histopathology. 2002;41(5):421–436. | ||
Fleming ID, Mitchell S, Dilawari RA. The role of surgery in the management of gastric lymphoma. Cancer. 1982;49(6):1135–1141. | ||
Kako S, Oshima K, Sato M, et al. Clinical outcome in patients with small-intestinal non-Hodgkin lymphoma. Leuk Lymphoma. 2009;50(10):1618–1624. | ||
Amin AD, Rajan SS, Liang WS, et al. Evidence suggesting that discontinuous dosing of ALK kinase inhibitors may prolong control of ALK+ tumors. Cancer Res. 2015;75(14):2916–2927. | ||
Chen J, Feng X, Dong M. Anaplastic lymphoma kinase-positive diffuse large B-cell lymphoma presenting in nasal cavity: a case report and review of literature. Int J Clin Exp Pathol. 2015;8(2):2123–2130. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
