Back to Journals » Clinical Ophthalmology » Volume 14
Primary Dermis Fat Grafting for Socket Reconstruction: Retrospective Comparison of Electrocoagulation versus Scalpel Dissection for Epidermis Removal
Authors Diab MM , Alahmadawy YA
Received 17 June 2020
Accepted for publication 5 August 2020
Published 29 September 2020 Volume 2020:14 Pages 2925—2933
DOI https://doi.org/10.2147/OPTH.S267085
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mostafa Mohammed Diab,1 Yomna Amr Alahmadawy2
1Department of Ophthalmology, Faculty of Medicine, Fayoum University, Al Fayoum, Egypt; 2Department of Ophthalmology, Faculty of Medicine, Cairo University, Cairo, Egypt
Correspondence: Yomna Amr Alahmadawy 3 Montaser Housing, ALharam, Giza 12512, Egypt
Email [email protected]
Purpose: To evaluate outcomes of the use of electrocoagulation for epidermis removal in dermis fat grafting (DFG) compared to the conventional scalpel dissection in patients who underwent primary anophthalmic socket reconstruction.
Design: Retrospective, observational, and comparative study.
Methods: A retrospective review was performed on patients who underwent primary DFG for socket reconstruction between 2017 and 2019 at tertiary teaching hospitals. Patients with previous orbital surgery, previous radiotherapy to the periocular region, any medical condition that affects healing, cicatrizing ocular surface disease or heavy smokers were excluded. Patients with complete documentation of preoperative and postoperative data only were included. Patients were divided into two groups; group A: epidermis removal by the traditional scalpel dissection and group B: epidermis removal using low power setting electrocoagulation. The main outcome was the timing of complete epithelialization of the dermis layer. Other outcomes included implant motility, prosthesis fitting, patient’s satisfaction, and any complications.
Results: A total of 27 patients met the study criteria, and the mean follow-up period was 24.81 months. There were no differences between both groups regarding preoperative characteristics. The mean duration of complete epithelialization of the DFG implant was 9.15 ± 2.94 weeks in group A compared to 22.29 ± 4.43 weeks in group B (p value < 0.001). Dermal ulceration was noticed in 9 patients (64.3%) in group B compared to none in group A (p value =0.001). Dermal ulceration was significantly associated with long conjunctival healing period (p value < 0.001). Volume loss was more common in group B while graft hirsutism and granuloma were more evident in group A. Final prosthesis fitting was possible in all included patients.
Conclusion: Epidermis removal using the electrocoagulation is related to much more delayed epithelialization of the dermis with a higher rate of dermal ulceration compared to the scalpel dissection technique. However, there was no significant difference between both groups regarding the final prosthesis fitting or the overall patient satisfaction.
Keywords: epidermis removal, electrocoagulation, dermis fat graft, socket reconstruction, anophthalmia
Introduction
Since its resurrection by Smith and Petrelli in 1978,1 dermis-fat grafting (DFG) has been proved to be effective in anophthalmic socket reconstruction as either a primary or a secondary implant.2–4 It augments both volume and surface of the anophthalmic socket while avoiding the disadvantages of allogenic orbital implants. The fat component of these composite grafts restores the orbital volume, while the dermis acts as a scaffold for surface epithelialization and provides a rigid substratum to which the conjunctiva and extraocular muscles can be sutured. Besides, the dermis has been assumed to enhance graft vascularization which makes it less vulnerable to reabsorption.4 The complete removal of the epidermis layer is a critical step to avoid postoperative complications such as hair growth, keratinization, formation of epidermoid cysts and mucoid discharge.5,6 Various surgical methods have been described for epidermis removal including surgical blade dissection, dermatome, dermabrasion device, and diamond burr.1,4,6,7 The scalpel dissection allows even surgical plane between epidermis and dermis with uniform removal of the epidermis; however, it is a slightly lengthy step with the possibility of leaving residual epidermal appendages.7 Trying to avoid the drawbacks of scalpel dissection, some researchers prefer to use a power drill with a diamond burr or an air-driven dermabrader.7,8 On the other hand, in Egypt, it is a common practice to employ a low power setting bipolar electrocautery with saline irrigation for rapid epidermal debridement. One theoretical advantage of this technique is the presumed thermal damage to bases of hair follicles affording complete eradication of epidermis and its appendages. On the down side, we have noticed that significant number of patients in this series had experienced delayed healing with subsequent dermal ulceration.
The aim of this study was to evaluate the outcomes of the use of gentle electrocoagulation in DFG harvesting compared to the conventional scalpel dissection for primary anophthalmic socket construction.
Patients and Methods
This is a retrospective comparative study approved by Fayoum University Ethics Committee (R115/2020), and the provisions of the Declaration of Helsinki were followed. A written informed consent was obtained from all participants before the surgery. Patients aged 18–40 years who underwent socket reconstruction with primary DFG between April 2017 and April 2019 were included. Patients with previous orbital surgery, history of orbital bone fracture, previous radiotherapy to the periocular region, any medical condition that affects healing, eg, uncontrolled DM, cicatrizing ocular surface disease or those who heavily smoke were excluded. The collected preoperative data included age, sex, laterality, and cause of eye removal. Information on the surgical technique including DFG size, number of muscles attached to the graft, and method of epidermis removal was also gathered.
Surgical Technique
The same surgical steps were used in each study group. Briefly, under general anaesthesia, enucleation of the eyeball was done after tagging the recti muscles and cutting the optic nerve followed by packing of the socket. The dermis fat graft was then harvested from the gluteal region. A 25-mm-diameter circle was marked 5 cm below the middle point of a line between the anterior iliac crest and the ischial tuberosity. The epidermis was incised and removed using one of the two techniques (described below), and a DFG with approximately 20-mm-depth was excised. The donor site was closed with deep 3–0 vicryl and with superficial 4–0 silk sutures in a mattress fashion. The DFG was transferred into the socket with the dermis facing out. The recti were sutured to the edges of the dermis at the four quadrants whenever possible. Tenon’s capsule and the conjunctiva were fixed to the dermis with the underside of the conjunctiva sutured to the anterior surface of the dermis in a circular fashion leaving a small central area (3 to 4 mm) of bare dermis. At the end of surgery, a conformer was placed into the socket for 2 weeks and a temporary tarsorrhaphy was performed.
Epidermis Removal Techniques
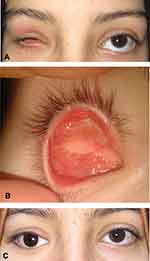
Two techniques were used to remove the epidermis layer before completing the harvesting technique. Group A: the epidermis was dissected using a 15 blade as one piece. Group B: the epidermis was removed using gentle electrocoagulation with saline irrigation using low power setting bipolar diathermy to debride the epidermis from the underlying dermis (Figure 1).
Outcome Assessment
The main outcome parameter was the occurrence of complete conjunctivalization where the dermal surface was completely covered by the conjunctival epithelium. Secondary outcomes included: motility of the implant, assessed and graded as follows: excellent = full motility in 4 directions, reasonable = motility in 1 to 3 directions, poor = no motility.8 Fitting of the prosthesis was graded: excellent = good fit of the prosthesis plus high patient comfort with prosthesis plus good lid-function, reasonable = two of the above criteria are fulfilled, bad = more than one of the above-mentioned criteria not fulfilled. Patients were asked about their satisfaction with the aesthetic result after surgery (graded as highly satisfied, satisfied and not satisfied).8 Finally, complications of this surgery were evaluated; dermal ulcer was defined as a full thickness defect in the dermis layer through which the underlying fat became exposed, graft necrosis (fat liquefaction and shrinkage), graft atrophy and volume loss (detected by deepening of superior sulcus), infections, cyst formation, granuloma, and hair growth.
The whole outcomes were assessed and graded by an oculoplastic fellow at each follow-up examination (after one week, after a month, and every 3 to 6 months). Slit lamp biomicroscopy was used to evaluate the socket. The mean follow-up period was 24.81 months (ranging from 17 to 36 months).
Statistical Analysis
Data were coded and entered using the statistical package for the Social Sciences (SPSS) version 26 (IBM Corp., Armonk, NY, USA). Categorical data were expressed as frequencies and percentages. Quantitative data were expressed as mean ± standard deviation (SD). Comparison between groups was done using Mann–Whitney U-test for non-parametric data and Chi square (χ2) test for categorical data. Exact test was used instead when the expected frequency is less than 5. Correlations between quantitative variables were done using Spearman correlation coefficient. P-values less than 0.05 were considered as statistically significant.
Results
Medical records involving documented photos of 27 of 69 patients were selected for this study; 42 patients were excluded as they did not fulfill the inclusion criteria or the necessary information was not recorded. Group A included patients who underwent DFG harvesting using the conventional scalpel dissection technique, while group B included patients for whom the electrocoagulation was used. Of the included patients, 53.8% of group A and 50.0% of group B were males. Mean age was 28.46 ± 14.33 years in group A and 33.64 ± 11.45 years in group B. Phthisis bulbi was the most common indication for surgery in both groups (63%), other indications included congenital microphthalmia (29.6%), blind painful eye (3.7%), and self-eviscerated open globe injury (3.7%). Percentage of patients who had 4 rectus muscles attached to the graft was 84.6%, and 71.4% patients in group A, and B, respectively. There were no differences between both groups regarding demographics or preoperative characteristics (Table 1)
 |
Table 1 Baseline Characteristics of Patients in Both Groups |
Primary Outcome (Complete Conjunctivalization)
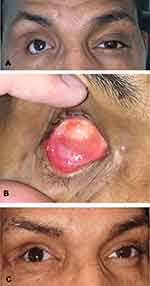
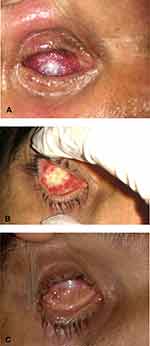
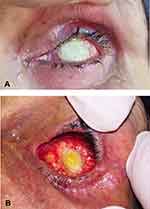
The mean duration of complete epithelialization of the DFG implant was 9.15 ± 2.94 weeks (range: 6–16 weeks) in group A compared to 22.29 ± 4.43 weeks in group B (range; 16–32 weeks) with statistical significant difference (p value <0.001) (Figures 2,3 and 4).
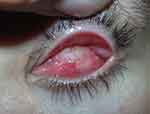 |
Figure 3 Complete epithelialization at postoperative week 8 with hair growth following epidermal scalpel dissection. |
Secondary Outcomes
With respect to graft motility, 84.6% (11/13) of patients in group A and 64.3% (9/14) in group B were able to move the graft in all directions. Prosthesis fitting was excellent in 9 (69.2%) of patients in group A compared to 5 patients (35.7%) in group B (p value = 0.08). Reasonable prosthesis fitting was achieved in 23.1% (3/13) and 57.1% (8/14) in group A and B, respectively (p value = 0.08). Final prosthesis fitting was possible in all patients in both groups. Three patients (23%) in group A vs 6 patients (42.9%) underwent further surgery before final prosthesis fitting (p value =0.226). As regarding patient’s satisfaction, patients were highly satisfied in 76.9% (10/13) in group A compared to 28.6% (4/14) in group B; there was reasonable satisfaction in 15.4% (2/13), 57.1% (8/14) of patients in group A, B, respectively (p= 0.041) (Table 2).
 |
Table 2 Postoperative Outcome |
Complications
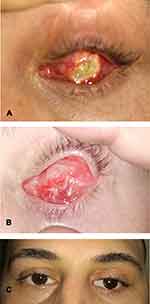
There was a statistically significant higher rate of full thickness dermal ulceration among patients in group B 64.3% (9/14) compared to none in group A (p value = 0.001). Dermal ulceration was significantly associated with prolonged conjunctival healing period (p value <0.001). The conjunctiva was markedly inflamed and heaped up at the margin of the dermal ulcer (Figures 5,6 and 7). All patients with dermal ulceration had persistent mucopurulent discharge with sticky eyes till complete healing of the ulceration. Five of nine patients with dermal ulceration underwent excision of ulcer with amniotic membrane transplantation. The remaining cases declined any further intervention; however, the ulcer showed gradual shrinkage with ultimate closure within weeks.
Mild volume loss occurred in only one patient (7.7%) in group A, but in 4 patients (28.6%) in group B; however, this difference was not statistically significant (p value = 0.326). Granuloma occurred in 30.8% (4/13) of patients in group A versus none in group B (p value = 0.041). Hirsutism was found in 23.1% of patients in group A versus none in group B (p value= 0.098) (Figure 4). Two patients in group B had mild socket infection that resolved on topical antibiotics.
Discussion
Dermis fat grafting has gained more popularity over the years as a primary implant in anophthalmic socket reconstruction due to its many advantages including low risk of exposure or infection and no risk of extrusion.8–10 Primary grafting is generally thought to be associated with better survival than secondary procedures; therefore, we have excluded all cases of secondary DFG to avoid its negative impact on the current study results.8,11 Successful DFG “take” is being described as complete vascularization and epithelialization of the dermal surface with fat survival and volume retention.12 Initially, the graft is nourished by imbibition of plasma from the recipient bed, followed by vascular ingrowth and conjunctivalization in a few weeks.12,13 Graft vascularization most likely starts from the periphery which can be enhanced by attaching the rectus muscles to the edge of the dermis.14 This assumption was consistent with our observation that dermal ulceration was central. Rapid conjunctivalization of the dermal surface guards against graft ulceration and necrosis. This can be accelerated by advancing the conjunctiva a few millimeters over the edges of the graft leaving a smaller central bare area.4 Additionally, it has been assumed that the use of scleral ring rather than solid conformer helps avoid direct pressure on the dermis face which would impede both vascularization and conjunctivalization.15,16
Following DFG implantation, the persistence of white color for more than 2–3 weeks indicates poor healing14 that matched perfectly with the sequence of events of delayed healing in this study; persistent white dermis followed by superficial dermal ulcer that ended up by a full thickness ulcer with exposure of underlying fat.
Different factors affecting DFG survival were studied in the literature. These factors can be broadly categorized into patient-specific and technique related. Age and general conditions such as concomitant systemic vascular diseases (diabetes, hypertension, or others), immuno-suppression, coagulation problems, or smoking can negatively affect DFG viability.15,17 Technique specific factors include the thickness of the fat pad, attaching extraocular muscles to the graft, the size and integrity of the dermal component.17 A large sized graft may result in ischemia and graft necrosis while a small graft may result in socket retraction due to atrophy. DFG with a fat pad thickness of 20 mm provided adequate volume restoration while affording good take.17
With respect to the dermal portion of the DFG, it serves the following functions; first, it preserves and augments the socket lining helping to maintain deep fornices for prosthesis fitting.1,18,19 Second, it has been hypothesized that the dermis has a vaso-inductive effect with a remarkable potential for capillary ingrowth and would provide nourishment to the fat component.1,20 Third, it affords an ideal matrix for conjunctivalization of the graft surface with its final vascularization.19,21 In view of this, the dermal component seems vital for graft survival and maintenance of its volume. Various surgical methods have been described on epidermis removal during DFG preparation, but there is no current consensus on the optimal one.1,4,6,7 In this study, we aimed to compare the outcomes of low power setting electrocoagulation for epidermis removal, commonly employed in our institutes with no robust evidence to support its use, performed in a consecutive series of patients during the year 2018 versus the conventional free hand scalpel dissection performed in a previous series of patients during the year 2017 and to study the impact of both techniques on GFG take. This sequential grouping explains the significantly longer duration of follow up in group A.
The mean duration of complete healing was significantly shorter in group A than in group B. The time to complete healing for group A was comparable to what was previously reported in the literature, 8–10 weeks.6 On the other hand, healing time was much more prolonged in group B. Delayed healing was significantly associated with dermal ulceration, the most frequent complication encountered in our study.
Dermal ulceration was reported in 1.9–25% of cases in most studies.6,15 Central dermal ulceration was reported in five out of sixteen patients who underwent primary DFG in Shore et al study;15 however, all these five cases showed coexisting severe conjunctival disease that was excluded in our study. They suppose that when conjunctival epithelialization is delayed beyond 8–10 weeks, ulceration becomes more likely.15
Given the information that both study groups were comparable regarding factors that could affect healing, the technique used in epidermal removal is most probably the cause of delayed healing and occurrence of dermal ulceration. We hypothesize that it causes thermal damage to the underlying dermis and its blood vessels impeding its early survival. Bipolar diathermy uses a low current density to rise tissue temperatures to 50–70°C which are sufficient to cause intracellular protein denaturation and coagulation. Experimental data have shown the effect of bipolar electro cautery extends beyond the tissue between the two electrodes exposing surrounding tissue to potential thermal damage.22 Trying to minimize this expected effect, we used a low power setting with saline irrigation. Other factors that have been cited as causes of dermal ulceration include vascular insufficiency, oversized grafts, aggressive handling of the graft, use of excessive cautery to the graft bed and high pressure patching causing pressure necrosis;23–25 however, these factors were homogeneous in both study groups. The exposed fat lobules in the bed of the dermal ulcer are not appropriate substrate for epithelial overgrowth leading to delayed healing.2,6 This delayed healing increases the risk of fat atrophy and deep infections and may impede comfortable prosthesis fitting15 In our study, five patients having dermal ulcer and delayed healing then experienced volume loss; nonetheless, it did not cause significant asymmetry or patient dissatisfaction.
We treated some of patients with dermal ulceration by excision of the ulcer together with amniotic membrane grafting, while the remaining patients refused any further intervention and were treated conservatively with eventual complete healing. Other researchers tried the use of autologous serum tears and platelet-rich plasma injection to address this complication with positive results in accelerating healing.21,25 Graft hirsutism was noticed in 3 patients in scalpel dissection versus none in the electrocoagulation group. This can be attributed to the fact that the bases of hair follicles were destroyed by the thermal effect of diathermy. Fitting a prosthesis is best done after complete epithelialization of the dermal surface; however, we did not wait till the complete healing in some patients due to prolonged healing period.
This study has limitations related to being retrospective. We did not routinely perform Hertel exophthalmometry on our patients; therefore, only the asymmetrical deepening of the superior sulcus deformity was considered as evidence of volume loss.
This study highlights the importance of the used technique for epidermal removal during DFG preparation and its influence on the graft take the use of electrocoagulation for epidermal removal is associated with delayed healing and higher risk of dermal ulceration compared to the conventional scalpel dissection; however, there was no significant impact on final prosthesis fitting or overall patient satisfaction.
Acknowledgment
We would like to thank Dr Fatma ALzahraa Ahmed resident of ophthalmology at Cairo University for her sincere help in data collection.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Smith B, Petrelli R. Dermis-fat graft as a movable implant within the muscle cone. Am J Ophthalmol. 1978;85(1):62–66. doi:10.1016/S0002-9394(14)76666-8
2. Smith B, Bosniak SL, Lisman RD. An autogenous kinetic dermis-fat orbital implant: an updated technique. Ophthalmology. 1982;89(9):1067–1071. doi:10.1016/S0161-6420(82)34690-4
3. Aryasit O, Preechawai P. Indications and results in anophthalmic socket reconstruction using dermis-fat graft. Clin Ophthalmol. 2015;9:795–799. doi:10.2147/OPTH.S77948
4. Jovanovic N, Carniciu AL, Russell WW, Jarocki A, Kahana A. Reconstruction of the orbit and anophthalmic socket using the dermis fat graft: a major review [published online ahead of print]. Ophthalmic Plast Reconstr Surg. 2020;10:16–45.
5. Korn BS, Kikkawa DO, Cohen SR, Hartstein M, Annunziata CC. Treatment of lower eyelid malposition with dermis fat grafting. Ophthalmology. 2008;115(4):4. doi:10.1016/j.ophtha.2007.06.039
6. Starks V, Freitag SK. Postoperative complications of dermis-fat autografts in the anophthalmic socket. Semin Ophthalmol. 2018;33(1):112–115. doi:10.1080/08820538.2017.1353830
7. Migliori ME, Putterman AM. The domed dermis-fat graft orbital implant. Ophthalmic Plast Reconstr Surg. 1991;7(1):23–30. doi:10.1097/00002341-199103000-00003
8. Nentwich MM, Schebitz-Walter K, Hirneiss C, Hintschich C. Dermis fat grafts as primary and secondary orbital implants. Orbit. 2014;33(1):33–38. doi:10.3109/01676830.2013.844172
9. Hintschich CRB-MC, Beyer-Machule CK. Dermis fatty tissue transplant as primary and secondary orbital implant. Complications and results. Ophthalmologe. 1996;93(5):617–622. doi:10.1007/s003470050048
10. Yu DK, Lee JCK, Chan E, Khoo JLS, Chan CW. Primary dermis fat graft as orbital implant after evisceration. Hong Kong J Ophthalmol. 2010;16(1):14–18.
11. Smith B, Bosniak S, Nesi FLR, Lisman R. Dermis-fat orbital implantation: 118 cases. Ophthalmic Surg. 1983;14(11):941–943.
12. Smith BR, Beyer-Machule CK, Cheng HM, Pitts WC. Fate of primary orbital dermis-fat grafts in guinea pigs. Ophthalmic Plast Reconstr Surg. 1988;4(4):193–201. doi:10.1097/00002341-198804040-00001
13. Hynes W. The early circulation in skin grafts with a consideration of methods to encourage their survival. Br J Plast Surg. 1953;6(C):257–263. doi:10.1016/s0007-1226(53)80038-x
14. Guberina C, Hornblass A, Meltzer MA, et al. Autogenous dermis-fat orbital implantation. Arch Ophthalmol. 1983;101(10):1586–1590. doi:10.1001/archopht.1983.01040020588018
15. Shore JW, McCord CD, Bergin DJ, Dittmar SJ, Maiorca JP, Burks WR. Management of complications following dermis-fat grafting for anophthalmic socket reconstruction. Ophthalmology. 1985;92(10):1342–1350. doi:10.1016/S0161-6420(85)33865-4
16. Przybyla VA
17. Sihota R, Sujatha Y, Betharia SM. The fat pad in dermis fat grafts. Ophthalmology. 1994;101(2):231–234. doi:10.1016/S0161-6420(94)31342-X
18. Kuzmanović Elabjer B, Bušić M, Miletić D, Bjeloš M, Šarić B, Bosnar D. Single-stage orbital socket reconstruction using the oversized dermis fat graft and the 22 mm silicone orbital implant after an extended enucleation. Case Rep Ophthalmol Med. 2018;2018:1–4. doi:10.1155/2018/8954193
19. Vagefi MR, McMullan TF, Burroughs JR, et al. Autologous dermis graft at the time of evisceration or enucleation. Br J Ophthalmol. 2007;91(11):1528–1531. doi:10.1136/bjo.2007.115543
20. Mitchell KT, Hollsten DA, White WL, O’Hara MA. The autogenous dermis-fat orbital implant in children. J AAPOS. 2001;5(6):367–369. doi:10.1067/mpa.2001.118870
21. Bengoa-González Á, Dolores Lago-Llinás M, Martín-Clavijo A, Ling-Tan S. The use of autologous dermis grafts for the reconstruction of the anophthalmic socket. Orbit. 2010;29(4):183–189. doi:10.3109/01676831003695347
22. Ricks R, Hopcroft S, Powari M, Carswell A, Robinson P. Tissue penetration of bipolar electrosurgery at different power settings. Br J Med Med Res. 2017;22(1):1–6. doi:10.9734/bjmmr/2017/33773
23. Shore JW, Col L, Mc U, Mccord CD, Maiorca JP, Burks WR. Management of complications following dermis-fat grafting for anophthalmic socket reconstruction. Ophthalmology. 1985;92(10):1342–1350. doi:10.1016/S0161-6420(85)33865-4
24. Chaparro Tapias TA, Díaz Díaz AL, Secondi R, Coy Villamil H, Sánchez España JC. Platelet-rich plasma to rescue an ulcerated orbital dermal fat graft. Eur J Ophthalmol. 2019;29(6):654–658. doi:10.1177/1120672118805299
25. Romera MA, Fernández E, Martínez G, Torres JJ, Alonso T, Andreu D. Amniotic membrane transplantation for conjunctival epithelization of exposed dermitis-fat graft. Orbit. 2007;26(2):133–135. doi:10.1080/01676830600977400
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.