Back to Journals » Journal of Blood Medicine » Volume 13
Primary Bone Marrow Lymphoma: De Novo and Transformed Subtypes
Authors Kimbrough EO , Jiang L , Parent EE, Bourgeois K, Alhaj Moustafa M , Tun HW , Iqbal M
Received 5 August 2022
Accepted for publication 21 October 2022
Published 14 November 2022 Volume 2022:13 Pages 663—671
DOI https://doi.org/10.2147/JBM.S384983
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Martin H Bluth
ErinMarie O Kimbrough,1 Liuyan Jiang,2 Ephraim E Parent,3 Kirk Bourgeois,2 Muhamad Alhaj Moustafa,1 Han W Tun,1 Madiha Iqbal1
1Division of Hematology and Oncology, Mayo Clinic, Jacksonville, FL, USA; 2Department of Pathology, Mayo Clinic, Jacksonville, FL, USA; 3Department of Radiology, Mayo Clinic, Jacksonville, FL, USA
Correspondence: Madiha Iqbal, Division of Hematology and Oncology, Mayo Clinic, 4500 San Pablo Road S, Jacksonville, FL, 32224, USA, Tel +1 904 953 2795, Fax +1 904 953 2315, Email [email protected]
Abstract: Diffuse large B-cell lymphoma (DLBCL) is well known for selectively involving certain extranodal locations such as the central nervous system (CNS), testes, and skin. DLBCL or high-grade B-cell lymphoma selectively involving the bone marrow is rare and has been sparsely reported in the medical literature. We report two cases of lymphoma presenting with primary bone marrow involvement without evidence of involvement of any other sites. The first case represents de novo DLBCL. The patient achieved complete remission with initial treatment, had a bone marrow-only relapse three years later, and achieved a second complete remission following non-transplant salvage therapy. The second case had findings consistent with “double hit” Richter’s transformation of chronic lymphocytic leukemia with translocation of c-MYC and BCL-2. This patient had an aggressive clinical course characterized by rapid progression with CNS involvement within three months resulting in the demise of the patient. These two cases represent two distinct subtypes of primary bone marrow lymphoma: de novo and transformed. Further research is necessary to gain a better understanding of this rare lymphoma entity and develop novel therapies.
Keywords: diffuse large B-cell lymphoma, high-grade B-cell lymphoma with MYC and BCL-2 rearrangements, bone marrow involvement, cytopenias
Plain Language Summary
- Primary bone marrow lymphoma (PBML) is confined to the bone marrow with or without a leukemic component and represents a distinct, aggressive type of extranodal lymphoma.
- PBML is very rare and has been sparsely reported in the medical literature.
- PBML appears to consist of two subtypes: de novo PBML and PBML derived from transformation of bone marrow-based low-grade lymphomas.
- PBML should be distinguished from other aggressive lymphomas which can primarily involve the bone marrow such as Burkitt lymphoma, lymphoblastic lymphoma, blastoid mantle cell lymphoma, and intravascular large B-cell lymphoma.
- PET imaging is useful for monitoring lymphoma activity in the bone marrow.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is well known for selectively involving a number of extranodal anatomic sites including the central nervous system (primary CNS lymphoma), testicles (primary testicular lymphoma), and skin (primary cutaneous DLBCL, leg type). Primary bone marrow lymphoma (PBML) is an exceedingly rare lymphoma and can also be called lymphoma with primary bone marrow involvement.1 It is characterized by lymphomatous bone marrow involvement without focal bone lesions or disease elsewhere except for a leukemic component in the peripheral blood.1–5
The true incidence of PBML is uncertain as definitions have varied, but it appears to represent a distinct, aggressive type of extranodal lymphoma.1–3,6,7 The majority of cases are DLBCLs.1,6,8 Patients with PBML often present with B-symptoms (fevers, night sweats, and weight loss) and cytopenias.1 Diagnosis requires a combination of imaging and bone marrow biopsy which helps distinguish it from primary bone lymphoma (PBL) and leukemias/lymphomas with known primary or secondary bone marrow involvement.1–5,9–11
PBL is defined as either a single bony lesion with or without regional node involvement or multifocal bony disease without nodal or visceral involvement.9 PBL is associated with bone destruction, while PBML does not result in bony destruction but demonstrates diffuse enhancement within the marrow on imaging.1,9 PBML and PBL are also distinct from secondary bone lymphoma and leukemias and lymphomas with known primary bone marrow involvement.1,3,4,9–11
PBML should be differentiated from other lymphomas which can also involve the bone marrow and mimic high-grade B-cell lymphoma (HGBCL) such as Burkitt lymphoma, lymphoblastic lymphoma, and blastoid mantle cell lymphoma.1,4,11 PBML diagnosis also requires exclusion of secondary bone lymphoma which arises from infiltration of the marrow in the setting of disseminated disease in 16–20% of lymphomas.3,9,10
We report two cases of PBML from our institution.
Case Reports
Case #1
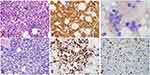
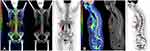
A 71-year-old female was referred for evaluation of leukopenia. She endorsed weight loss related to poor appetite but was otherwise asymptomatic. Her medical history was significant for immune-mediated thrombocytopenia treated with intravenous immunoglobulin, steroids, and platelet transfusions with normalization of her counts 5 years prior to presentation. Laboratory evaluation was significant for mild anemia with a hemoglobin of 10.6 g/dL, leukopenia with a white blood cell (WBC) count of 2.8 × 10 (9)/L, and mild neutropenia with an absolute neutrophil count of 1.11 × 10 (9)/L. The lactate dehydrogenase (LDH) was elevated at 321 U/L (normal 122–222 U/L). Given the leukopenia, unremarkable peripheral smear, and low suspicion for a leukemic component, ancillary studies including peripheral blood flow cytometry (PBFCM), cytogenetics, and molecular studies were deferred. Serum protein electrophoresis demonstrated a monoclonal IgM kappa with an M-spike of 0.2 g/dL. Bone marrow biopsy revealed a hypercellular marrow, 70% cellularity with grade 3+ myelofibrosis secondary to the lymphoma.12 Erythropoiesis and granulopoiesis were decreased, and the lymphocytes were increased. There was diffuse involvement (80%) of the bone marrow with DLBCL. The malignant cells were positive for CD20 (Figure 1A and B) and negative for CD5, CD25, CD30, CD34, BCL-1, and MUM-1. Epstein–Barr virus-encoded RNA (EBER) in situ hybridization (ISH) was also negative. 18F Fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET-CT) demonstrated bone marrow involvement without evidence of other disease (Figure 2A and B). She was diagnosed with stage IV primary bone marrow (PBM)-DLBCL with a revised-international prognostic index (R-IPI) of 3 and central nervous system (CNS)-IPI of 3. She received chemoimmunotherapy with six cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and high-dose (HD) methotrexate (MTX) for CNS prophylaxis in alternate cycles for a total of three cycles. She achieved complete remission (CR) after four cycles (Figure 2C and D). The IgM monoclonal protein resolved with treatment.
Approximately 3 years after completion of therapy, she developed fatigue, anorexia, dyspnea on exertion, and presyncope. Laboratory evaluation revealed anemia, leukopenia, and a recurrent, small monoclonal IgM kappa protein on immunofixation. A bone marrow biopsy confirmed relapsed DLBCL with 60% involvement by a nodular and paratrabecular infiltrate of recurrent B-cell lymphoma. The Ki-67 was 60%. Immunohistochemistry (IHC) studies showed that the neoplastic B lymphocytes were positive for BCL-2 and negative for CD10 and MYC (Figure 1C-F). They were also negative for BCL-6 and MUM1. The immunophenotype was most consistent with a non-germinal center phenotype. Ancillary studies were not performed due to a dry tap with bone marrow biopsy and the absence of circulating neoplastic lymphocytes. FDG PET-CT did not show evidence of extraosseous disease. Given her advanced age and poor performance status, she was deemed ineligible for autologous hematopoietic stem cell transplantation. She was treated with a combination of an experimental Bruton tyrosine kinase (BTK) inhibitor, pomalidomide, and everolimus on a clinical trial and achieved transfusion independence with improvement in her cytopenias. This therapy was discontinued after 2 months due to severe mucositis. She was transitioned to polatuzumab, bendamustine, and rituximab (PBR) and completed 6 cycles of therapy. Bendamustine was not given with the first four cycles. She achieved transfusion independence with excellent blood counts halfway through the treatment. The repeat bone marrow biopsy after PBR treatment showed CR. To date, she has remained in CR and is 4 months post completion of therapy.
Case #2
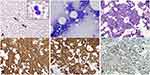
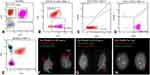
A 75-year-old female presented with a 1-month history of intractable back pain that failed to respond to oral steroids and a corticosteroid injection and more recently fatigue, night sweats, and a 4 kg weight loss. Computed tomography of the chest, abdomen and pelvis was unrevealing. Magnetic resonance imaging (MRI) of the lumbar spine demonstrated multifocal marrow signal abnormalities concerning for malignancy. Laboratory evaluation was significant for thrombocytopenia with a platelet count of 69 × 10 (9)/L and leukocytosis with a WBC count of 10.1 × 10 (9)/L. There was an absolute lymphocytosis with 8% blastoid cells on peripheral smear (Figure 3A and Inset). LDH was elevated at 4890 U/L (normal 122–222 U/L), and ferritin was 9979 mcg/L (normal 11–307 mcg/L). PBFCM revealed two distinct B-cell populations (Figure 4A–E). There was a 1.40 × 10 (9)/L monoclonal kappa restricted B-cell population that was positive for CD10 but negative for CD5, CD23, CD11c, and CD103. This population showed high forward scatter (large size) compatible with a high-grade B-cell neoplasm. The second population consisted of 0.97 × 10 (9)/L monoclonal dim kappa restricted B-cells with co-expression of CD5 and CD23 and low forward scatter (small size), representing a chronic lymphocytic leukemia (CLL) phenotype. Bone marrow biopsy revealed a hypercellular marrow with extensive, near 100%, involvement by HGBCL with a Ki-67 of 80%. IHC studies showed the neoplastic lymphocytes were positive for CD10, CD20, CD79a, PAX-5, BCL-2 (90%), and MYC (90%) (Figure 3B–F). They were negative for CD23, BCL-6, cyclin D1, TdT, CD30, and EBER ISH. Chromosome analysis on peripheral blood revealed a complex karyotype including a balanced t(14;18) consistent with BCL2/IGH rearrangement and an abnormal chromosome 8 consistent with MYC rearrangement. Lymphoma fluorescence in situ hybridization (FISH) testing was consistent with HGBCL with BCL-2 and C-MYC rearrangements, the so-called “double hit” lymphoma (Figure 4F–G).13 FISH for CLL was positive for 13q deletion in 22% of cells (Figure 4H). Cerebrospinal fluid (CSF) cytology was negative for malignancy on 2 occasions. Staging FDG PET-CT demonstrated increased uptake throughout the axial and proximal appendicular skeleton consistent with involvement of the bone marrow (Figure 5A and B). Based on these findings, she was diagnosed with stage IV PBM-HGBCL (“double hit” Richter transformation of CLL with primary involvement of the bone marrow with leukemic component). Her R-IPI score was 3 and CNS-IPI was 3. She initiated treatment with R-CHOP while awaiting the results of FISH testing. The treatment was later changed to dose-adjusted etoposide, doxorubicin, vincristine, cyclophosphamide, prednisone, and rituximab (DA-EPOCH-R) with intrathecal (IT) MTX. Interim bone marrow biopsy demonstrated low-level residual CD5-, CD10+, kappa-light chain restricted B cell population consistent with HGBCL (<5%). The CD5+, CD10- B-cell population was absent. The patient had a rapid deterioration 3 months after her initial diagnosis, and she developed progressive right-sided weakness, gait instability, and numbness in her hands and feet. MRI spine showed diffuse leptomeningeal enhancement in the cervical and thoracic regions. CSF cytology was positive for HGBCL. She was treated with rituximab, HD MTX, and dexamethasone for one cycle. She had a progressive functional decline and was enrolled in hospice, expiring shortly thereafter.
Discussion
We report two interesting cases of PBML. It is an exceedingly rare lymphoma entity; however, the true incidence is unknown.1,2,5 PBML has not been recognized in the International Consensus Classification (ICC) or World Health Organization (WHO) classification.5,14,15 Currently, most cases of PBML would be classified as DLBCL not otherwise specified.14 One review suggested that 1.16% of lymphomas are PBM-DLBCLs; however, it included patients with hepatosplenomegaly which would be excluded with the current definition.6
The pathologic and biological understanding of PBML is evolving. There have been no definitive diagnostic criteria established as it is very rare and has been reported sparsely in the medical literature. It is generally defined as primary lymphomatous bone marrow involvement with or without a leukemic component by HGBCL without focal bony lesion or disease elsewhere.1,3–5 A diagnosis of PBML also requires exclusion of intravascular lymphoma, leukemias and other aggressive B-cell lymphomas known for primary bone marrow involvement and secondary bone lymphoma which arises from infiltration of the marrow in the setting of disseminated disease.1,4,5,9–11,16 The majority of reported PBML cases were DLBCLs; however, they were diagnosed before the widespread use of HGBCL as a diagnostic entity.1,6,8 We have used HGBCL confined to the bone marrow with or without leukemic component as the operational diagnostic criteria irrespective of the underlying lymphoma subtype.
Our first case represents de novo PBML without an apparent leukemic component and no evidence of an underlying low-grade lymphoma. Pathologically, it is consistent with DLBCL, non-germinal center phenotype. Additional molecular and FISH testing could not be performed given the dry tap. The interesting finding is a small IgM kappa monoclonal protein in the blood. The relationship between the lymphoma and IgM monoclonal gammopathy is not definitively known. No evidence of lymphoplasmacytic lymphoma was found in the bone marrow. As such, she probably had IgM monoclonal gammopathy of unknown significance (MGUS) related to her age. It has been reported, however, that HGBCL with IgM monoclonal gammopathy is associated with high CNS risk.17 She has not had any CNS involvement. Bone marrow involvement by DLBCL has not been definitively proven to be associated with high CNS risk; however, leukemic manifestation with bone marrow involvement is associated with high CNS risk.18–20 Unlike the second case, this patient did quite well, achieving CR for three years after R-CHOP and getting back into CR following a late bone marrow-only recurrence with non-transplant treatment.
Our second case most likely represents a transformed subtype of PBML (“double hit” Richter’s transformation of CLL confined to the bone marrow) with a leukemic component on initial diagnosis. Richter’s transformation presenting as leukemic HGBCL with extensive bone marrow involvement has been reported in the setting of widely disseminated disease.18 There were two clonal B cell populations in the blood: a CD5+ and CD10- clone likely related to CLL and a CD5- and CD10+ clone related to HGBCL with c-MYC and BCL-2 translocations. These two clonal B-cell populations shared the same kappa light chain restriction. Although the peripheral blood leukemic B-cell count did not meet the criteria for CLL, the immunophenotypic findings and the presence of 13q deletion were suggestive of a CLL clone in the peripheral blood. The CD5+ clonal B-cell population in the blood can be labeled as monoclonal B-cell lymphocytosis with CLL immunophenotype. Interestingly, we did not find any evidence of CLL on the careful examination of the bone marrow. The clinical course was very aggressive with the rapid development of CNS (spinal leptomeningeal) involvement three months following the initial diagnosis. Double-hit lymphoma has been associated with a high CNS risk.21 It is likely that the bone marrow involvement, leukemic manifestation, and Richter’s transformation resulted in higher CNS risk. Leukemic HGBCL carries a high CNS risk.18 Abnormal laboratory findings including very high LDH and ferritin supported the aggressive clinical manifestation.
Based on our two cases, it appears that PBML has two subtypes: de novo (d-PBML) and transformed (t-PBML).6,22 The de novo subtype may have tissue tropism for the bone marrow. Our first case remained confined to the bone marrow on relapse without extramedullary manifestations. The transformed subtype can potentially arise from primary bone marrow low-grade lymphomas such as CLL, follicular lymphoma, lymphoplasmacytic lymphoma, and marginal zone lymphoma.1,4,11 Our two cases also demonstrate that PET imaging is an excellent tool for monitoring lymphoma in the bone marrow.
In the pre-PET/CT era, bone marrow biopsy was performed regularly in the staging of lymphoma. Currently, a routine bone marrow biopsy is no longer performed in the absence of abnormal bone marrow metabolic activity on PET/CT. According to the National Comprehensive Cancer Center guidelines, PET/CT may also be used to monitor response to treatment in FDG-avid lymphomas like DLBCL and Hodgkin Lymphoma.23
As a rare lymphoma entity, the best therapeutic approach to PBML is not known. There is no clear standard regimen for the management of PBML. Treatment should be individualized according to Eastern Cooperative Oncology Group performance status, age, histology, and other risk factors. Given the aggressive nature of this lymphoma and its tendency for relapse, several studies have recommended R-CHOP therapy with CNS prophylaxis followed by consolidation with autologous stem cell transplant.7,22,24 R-CHOP chemotherapy has been used with variable success.7,25,26 In a study by the International Extranodal Lymphoma Study Group (IELSG), the majority of PBM-DLBCL patients received CHOP-regimens with or without rituximab with a CR of 33%. Two of the 5 CRs relapsed.3 It has been suggested that poor response to R-CHOP or early relapse following R-CHOP therapy may be associated with elevated LDH, high Ki-67, TP53 mutations, and rearrangements of MYC and BCL-2.26,27 In patients with these high-risk features, more intensive therapy with front-line DA-EPOCH-R or chimeric antigen receptor T-cells could be considered.26,27 Reed et al demonstrated good response to rituximab, cyclophosphamide, vincristine, doxorubicin, and dexamethasone (R-Hyper-CVAD) in a young woman with PBM-DLBCL and high-risk of CNS disease.28 PBML has been reported to have a median overall survival (OS) ranging from 8 months to 1.8 years.3,6,7,11,29 According to the IELSG, prognosis differs by histologic subtype with a median OS <1.5 years for those with PBM-DLBCL.3
While PBML is not included in the ICC or WHO classification of lymphomas, separation of DLBCL or HGBCL confined to extranodal anatomic sites should be considered. This subclassification could enhance research on the biology of these cancers and optimize their management.
Conclusions
PBML is an extranodal lymphoma confined to the bone marrow with or without a leukemic component. It has not been recognized in the most recent ICC or WHO classification. Biologically, it appears to have two subtypes: de novo and transformed. The transformed subtype appears to have a high CNS risk. Further research is necessary to gain a better understanding of this rare lymphoma entity and identify novel therapeutic approaches.
Abbreviations
BTK, Bruton tyrosine kinase; CLL, chronic lymphocytic leukemia; CNS, central nervous system; CNS-IPI, central nervous system-international prognostic index; CR, complete remission; CSF, cerebrospinal fluid; DA-EPOCH-R, dose-adjusted etoposide, doxorubicin, vincristine, cyclophosphamide, prednisone, rituximab; DLBCL, diffuse large B-cell lymphoma; d-PBML, de novo primary bone marrow lymphoma; EBER, Epstein–Barr virus-encoded RNA; FDG, fluorodeoxyglucose; FISH, fluorescence in situ hybridization; HD, high-dose; HGBCL, high-grade B-cell lymphoma; ICC, International Consensus Classification; IESLG, International Extranodal Lymphoma Study Group; IHC, immunohistochemistry; ISH, in situ hybridization; IT, intrathecal; LDH, lactate dehydrogenase; MGUS, monoclonal gammopathy of undetermined significance; MRI, magnetic resonance imaging; MTX, methotrexate; OS, overall survival; PBFCM, peripheral blood flow cytometry; PBL, primary bone lymphoma; PBM-HGBCL, primary bone marrow high-grade B-cell lymphoma; PBR, polatuzumab, bendamustine, rituximab; PET-CT, positron emission tomography-computed tomography; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone; R-Hyper-CVAD, rituximab, cyclophosphamide, vincristine, doxorubicin, dexamethasone; R-IPI, revised-international prognostic index; t-PBML, transformed primary bone marrow lymphoma; WBC, white blood cell; WHO, World Health Organization.
Consent for Publication
The study participants or their next of kin have given written informed consent to participate as well as written informed consent to publish the case details and accompanying images. Institutional approval was not required to publish the case details.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang G, Chang Y, Wu X, et al. Clinical features and prognostic factors of primary bone marrow lymphoma. Cancer Manag Res. 2019;11:2553–2563. doi:10.2147/CMAR.S187522
2. Yang CF, Hsiao LT, Chang HY, Hsu CY. Large B-cell lymphoma presenting primarily in bone marrow is frequently associated with haemophagocytic lymphohistiocytosis and has distinct cytogenetic features. Pathology. 2020;52(5):561–567. doi:10.1016/j.pathol.2020.04.005
3. Martinez A, Ponzoni M, Agostinelli C, et al. Primary bone marrow lymphoma: an uncommon extranodal presentation of aggressive non-Hodgkin lymphomas. Am J Surg Pathol. 2012;36(2):296–304. doi:10.1097/PAS.0b013e31823ea106
4. Wang HY, Yang CF, Chiou TJ, et al. Primary bone marrow lymphoma: a hematological emergency in adults with fever of unknown origin. Cancer Med. 2018;7(8):3713–3721. doi:10.1002/cam4.1669
5. Zamo A, Johnston P, Attygalle AD, Laurent C, Arber DA, Fend F. Aggressive B-cell lymphomas with a primary bone marrow presentation. Histopathology. 2020;77(3):369–379. doi:10.1111/his.14124
6. Chang H, Hung YS, Lin TL, et al. Primary bone marrow diffuse large B cell lymphoma: a case series and review. Ann Hematol. 2011;90(7):791–796. doi:10.1007/s00277-010-1129-4
7. Kazama H, Teramura M, Yoshinaga K, Masuda A, Motoji T. Long-term remission of primary bone marrow diffuse large B-cell lymphoma treated with high-dose chemotherapy rescued by in vivo rituximab-purged autologous stem cells. Case Rep Med. 2012;2012:957063. doi:10.1155/2012/957063
8. Kim MS, Cho YU, Jang S, Seo EJ, Lee JH, Park CJ. A case of primary bone marrow diffuse large B-cell lymphoma presenting with fibrillar projections and hemophagocytic lymphohistiocytosis. Ann Lab Med. 2017;37(6):544–546. doi:10.3343/alm.2017.37.6.544
9. Messina C, Christie D, Zucca E, Gospodarowicz M, Ferreri AJ. Primary and secondary bone lymphomas. Cancer Treat Rev. 2015;41(3):235–246. doi:10.1016/j.ctrv.2015.02.001
10. Bhagavathi S, Fu K. Primary lymphoma of bone: a review. Semin Diagn Pathol. 2014;31(1):48–52. doi:10.1053/j.semdp.2014.01.004
11. Hong M, Wang S, Zhu H, Wang R, Li J, He G. Primary bone marrow lymphoma initially presenting with cytopenia: a report of eight cases. Blood. 2017;130(Supplement 1):5230. doi:10.1182/blood.V130.Suppl_1.5230.5230
12. Kuter DJ, Bain B, Mufti G, Bagg A, Hasserjian RP. Bone marrow fibrosis: pathophysiology and clinical significance of increased bone marrow stromal fibres. Br J Haematol. 2007;139(3):351–362. doi:10.1111/j.1365-2141.2007.06807.x
13. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi:10.1182/blood-2016-01-643569
14. Alaggio R, Amador C, Anagnostopoulos I, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. 2022;36(7):1720–1748. doi:10.1038/s41375-022-01620-2
15. Campo E, Jaffe ES, Cook JR, et al. The international consensus classification of mature lymphoid neoplasms: a report from the clinical advisory committee. Blood. 2022. doi:10.1182/blood.2022015851
16. Seegobin K, Li Z, Alhaj Moustafa M, et al. Clinical characteristics, prognostic indicators, and survival outcomes in intravascular lymphoma: Mayo Clinic experience (2003–2018). Am J Hematol. 2022;97:1150–1158. doi:10.1002/ajh.26635
17. Cox MC, Di Napoli A, Scarpino S, et al. Clinicopathologic characterization of diffuse-large-B-cell lymphoma with an associated serum monoclonal IgM component. PLoS One. 2014;9(4):e93903. doi:10.1371/journal.pone.0093903
18. Kuhlman JJ, Moustafa MA, Jiang L, et al. Leukemic high grade B cell lymphoma is associated with MYC translocation, double hit/triple hit status, transformation, and CNS disease risk: the Mayo Clinic experience. Clin Lymphoma Myeloma Leuk. 2022;22(8):e815–e825. doi:10.1016/j.clml.2022.04.009
19. Zou D, Yi S, Cui R, et al. BCL-2 and MYC gain/amplification is correlated with central nervous system involvement in diffuse large B cell lymphoma at leukemic phase. BMC Med Genet. 2017;18(1):16. doi:10.1186/s12881-017-0381-z
20. Muringampurath-John D, Jaye DL, Flowers CR, et al. Characteristics and outcomes of diffuse large B-cell lymphoma presenting in leukaemic phase. Br J Haematol. 2012;158(5):608–614. doi:10.1111/j.1365-2141.2012.09209.x
21. Savage KJ, Slack GW, Mottok A, et al. Impact of dual expression of MYC and BCL2 by immunohistochemistry on the risk of CNS relapse in DLBCL. Blood. 2016;127(18):2182–2188. doi:10.1182/blood-2015-10-676700
22. Alvares CL, Matutes E, Scully MA, et al. Isolated bone marrow involvement in diffuse large B cell lymphoma: a report of three cases with review of morphological, immunophenotypic and cytogenetic findings. Leuk Lymphoma. 2004;45(4):769–775. doi:10.1080/10428190310001625746
23. Zelenetz AD, Gordon LI, Chang JE, et al. NCCN guidelines (R) insights: B-cell lymphomas, version 5.2021. J Natl Compr Canc Netw. 2021;19(11):1218–1230. doi:10.6004/jnccn.2021.0054
24. Nishida H, Suzuki H, Hori M, Obara K. Primary isolated bone marrow diffuse large B cell lymphoma with long-term complete remission. Leuk Res Rep. 2018;10:11–15. doi:10.1016/j.lrr.2018.05.004
25. Nabe Y, Kikuchi S, Kamihara Y, Wada A, Murakami J, Sato T. Early complete response of primary bone marrow B-cell lymphoma treated with rituximab-based CHOP therapy, assessed by flow cytometry and immunoglobulin heavy chain rearrangement. Clin Case Rep. 2021;9(8):e04657. doi:10.1002/ccr3.4657
26. Wang W, Zhou GY, Zhang W. Early relapse in a case of primary bone marrow diffuse large B-cell lymphoma treated with rituximab-CHOP. Immunotherapy. 2017;9(5):379–385. doi:10.2217/imt-2017-0005
27. Chen PT, Jorsan K, Avezbakiyev B, Akhtar C, Wang JC. Aggressive diffuse intermediate size B-cell lymphoma with P53 mutation presented as primary bone marrow lymphoma. J Investig Med High Impact Case Rep. 2020;8:2324709620982765. doi:10.1177/2324709620982765
28. Reed A, Sommerhalder D. The use of R-hyper-CVAD in a rare case of primary bone marrow diffuse large B-cell lymphoma. J Hematol. 2019;8(4):165–167. doi:10.14740/jh559
29. Evans MG, Rezk SA, Pinter-Brown LC, Zhao X. Aggressive CD5-positive primary bone marrow diffuse large B-cell lymphoma with leukemic presentation. Case Rep Hematol. 2021;2021:2628100. doi:10.1155/2021/2628100
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.