Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Prevalence of Chronic Obstructive Pulmonary Disease and its Associated Factors in Nepal: Findings from a Community-based Household Survey
Authors Adhikari TB , Acharya P , Högman M , Neupane D, Karki A, Drews A , Cooper BG , Sigsgaard T , Kallestrup P
Received 29 June 2020
Accepted for publication 20 August 2020
Published 29 September 2020 Volume 2020:15 Pages 2319—2331
DOI https://doi.org/10.2147/COPD.S268110
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Tara Ballav Adhikari,1,2 Pawan Acharya,1,3 Marieann Högman,4 Dinesh Neupane,1,5 Arjun Karki,6 Arne Drews,7 Brendan G Cooper,8 Torben Sigsgaard,9 Per Kallestrup2
1COBIN Project, Nepal Development Society, Bharatpur, Chitwan, Nepal; 2Center for Global Health, Department of Public Health, Aarhus University, Aarhus, Denmark; 3Hudson College of Public Health, The University of Oklahoma Health Sciences Center, Oklahoma City, OK, USA; 4Department of Medical Sciences, Respiratory, Allergy and Sleep Research, Uppsala University, Uppsala, Sweden; 5Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins University, Baltimore, MD, USA; 6Department of Pulmonary, Critical Care and Sleep Medicine, HAMS Hospital, Kathmandu, Nepal; 7Nepalmed, Leipzig, Germany; 8Lung Function and Sleep, University Hospitals Birmingham, Birmingham, UK; 9Department of Public Health, Section for Environment, Occupation & Health, Aarhus University, Aarhus, Denmark
Correspondence: Tara Ballav Adhikari
Center for Global Health, Department of Public Health, Aarhus University, Aarhus 8000, Denmark
, Email [email protected]
Background: Despite chronic obstructive pulmonary disease (COPD) being the commonest non-communicable disease in Nepal, there is limited research evidence estimating the spirometry-based burden of COPD. This study aims to estimate the prevalence of COPD and its correlates through a community-based survey in Pokhara Metropolitan City, a semi-urban area of Western Nepal.
Methods: A cross-sectional household survey was conducted among 1459 adults ≥ 40 years. COPD was defined according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria as a post-bronchodilator ratio of forced expiratory volume in 1st second (FEV1) to forced vital capacity (FVC) < 0.70 with the presence of symptoms. COPD was also defined by the lower limit of normal (LLN) threshold – FEV1/FVC < LLN cut-off values with the presence of symptoms. Study participants were interviewed about sociodemographic and behavioural characteristics and respiratory symptoms. Descriptive statistics and logistic regression analysis were applied.
Results: Spirometry reports were acceptable in 1438 participants. The mean age of the participants was 55 (± 10) years, and, 54% were female. The prevalence of GOLD-defined COPD was 8.5% (95% CI: 7.1– 10.0) and based on the LLN threshold of 5.4% (95% CI: 4.2– 6.6). The multivariate logistic regression showed that increasing age, low body mass index, illiterate, current or former smoker, and biomass fuel smoke increased the odds of COPD in both the definitions.
Conclusion: COPD is highly prevalent at community level and often underdiagnosed. Strategies aiming at early diagnosis and treatment of COPD, especially for the elderly, illiterate, and reducing exposure to smoking and biomass fuel smoke and childhood lung infection could be effective.
Keywords: COPD, Nepal, prevalence, NCD
Background
Chronic obstructive pulmonary disease (COPD) is a common respiratory disorder characterized by irreversible and gradually progressive airflow limitation.1 In 2017, 3.2 million people died from COPD (5.7% of global mortality).2 The Global Burden of Disease Study estimation ranks COPD as the second most prevalent cause of death in 2017. This estimate already exceeds the World Health Organization’s (WHO) projection of COPD being the third most important cause of death worldwide by 2030.3
More than 90% of COPD-related deaths occur in low- and middle-income countries. COPD associated mortality is projected to increase by 160% in the Southeast Asian region in the coming decades.3 It is also one of the commonest forms of non-communicable diseases (NCDs) among the Nepalese adult population accounting for 43% of NCDs among out-patient visits.4 A recent study by the Nepal Health Research Council reported 11.7% prevalence of COPD in Nepal.5
Tobacco smoking and long-term exposure to smoke from biomass fuel are the major risk factors of COPD. Above 28% of Nepalese men, and nearly 8% of Nepalese women smoke tobacco.6 Nearly 66% of Nepalese households use biomass fuel in cooking and heating.7 The Nepal NCDs risk factor survey 2019 estimated that more than 33% of the Nepalese adult population is exposed to secondhand smoking at home, and 23% are exposed at the workplace.6 Poor air quality in Nepal imposes an additional threat in worsening lung health.8 Similarly, lack of awareness, low health literacy on COPD, educational and economic status are also observed to increase the risk of COPD.9
Despite this high burden of disease and its risk factors in Nepal, there is a paucity of evidence on the prevalence of COPD diagnosed using spirometry applied in community-settings. This study estimates the prevalence of COPD and its correlates in a semi-urban area of western Nepal.
Methods
Study Design
A cross-sectional, community-based household survey was conducted during May–August 2019 in the semi-urban area of Pokhara Metropolitan city of western Nepal. The study site is located in Kaski district, home to 58,816 residents.10
Study Population and Sampling
Our study population consisted of adults ≥40 years - 15,507 according to the latest census.10 Assuming the prevalence of COPD to be 8%, 5% margin of error, a design effect of 1.5, and a response rate of 90%, the sample size determined was 1508.11,12 We used households selected for the Community-based management of non-communicable disease study in Nepal (COBIN).13 These households were selected using systematic random sampling. It was followed by the random selection of one adult per household using a Kish sampling grid, from the household list of adults ≥40 years.14 Interviews were conducted by trained enumerators at participants’ homes using a structured questionnaire. At least three visits were made to contact selected individuals to reduce the non-response rate.
Inclusion criteria included: (i) age ≥40 years; (ii) full-time residency of the study area; and (iii) capacity to consent to the study. Exclusion criteria comprised having active pulmonary tuberculosis or a current respiratory infection or any contraindication performing spirometry such as pregnancy, a recent history of eye, chest or abdominal surgery, myocardial infarction, hemoptysis, and history of pneumothorax.15
Questionnaire
The questionnaire included information on sociodemographic information (age, sex, ethnicity, education, occupation, marital status), lifestyle variables comprised detailed smoking history, physical activity, exposure to secondhand smoking, biomass fuel use, chimney in the kitchen, presence of black smoke mark in the kitchen wall, history of severe childhood lung infection, or tuberculosis infection and occupation exposure to dirt and dust as well as other relevant questions on respiratory symptoms. Pack-years were calculated for all participants with a history of smoking. The questionnaire was adapted from similar past studies.11,16-18 Bodyweight was measured using a digital scale and recorded in kilograms. Height was measured from the head (vertex) to the bottom of the feet and recording the nearest units in centimetres.
Spirometry
Spirometry was performed after completion of the questionnaire using a portable diagnostic spirometer (EasyOne Air, ndd Medizintechnik AG, Switzerland) chosen for its accuracy, portability, and calibration stability. Spirometers were verified regularly using a 3L’s syringe. The American Thoracic Society (ATS) and European Respiratory Society (ERS) task force guidelines of spirometry were adhered to, and the data enumerators were trained for three weeks on the same guidelines.15 The spirometry procedure was explained in the Nepalese language and demonstrated to participants by enumerators. At least three acceptable and reproducible blows were recorded based on the ATS/ERS criteria.15 Two pulmonologists subsequently over-read the spirometry.
As there were no established lung function reference values among Nepalese, an established reference equation of a North Indian population was used.19 Those with Forced Expiratory Volume in 1st second (FEV1) to Forced Vital Capacity (FVC) ratio <0.70 of predicted normal or FEV1/FVC < lower limits of normal (LLN) during the pre-bronchodilator test was given 400 mcg of salbutamol from a metered-dose inhaler followed by spirometry after 15 minutes. Based on the Global Initiative for Chronic Lung Disease (GOLD) criteria of COPD, those participants who demonstrated FEV1/FVC <0.70 or below LLN cut-off values in the post-bronchodilator test with the presence of symptoms were identified as COPD.19 Dyspnoea was graded using the modified Medical Research Council (mMRC) scale (0 to 4).20 COPD severity was classified according to GOLD classification: grade 1–4. Chronic bronchitis was defined as the presence of cough and sputum production for at least three months in each of two consecutive years.
Statistical Analysis
Data were entered using Epi Data version 4.0 (The EpiData Association, Odense, Denmark) and analysed using STATA version 15.1 (StataCorp. Texas, USA). Categorical variables were summarized using frequencies and 95% confidence intervals (CI), and continuous variables summarized using the mean and standard deviation. Odds Ratios (ORs) were obtained by unadjusted and adjusted logistic regression analysis. Variables significantly associated in the univariable analysis (p ≤ 0.05) were included in the subsequent multiple logistic regression analysis using the Wald backward elimination method. A value of p<0.05 was considered statistically significant throughout the analysis.
Results
Of 1508 individuals approached, 1459 consented to be part of the survey. Spirometry reports were acceptable (grade A, B, or C) in 1438 and were thus included in the final analysis. The characteristics of included participants are presented in Table 1. The mean age of the participants was 55.5 (± 9.7) years, and mean body mass index was 25.5 kg/m2(± 4.6). Seven out of ten household kitchens were poorly ventilated without a chimney. Similarly, about 35% of the respondents were exposed to the smoke of biomass fuel. Nearly, 60% of participants had a low level of physical activity. A high percentage of respondents were current and former smokers, with statistically significant more males than females (p≤0.001). More than 7 out of 10 ever smokers had a smoking history of more than 10 pack years.
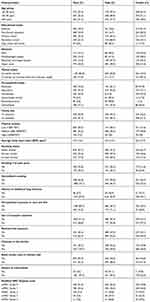 |
Table 1 Characteristics of Study Participants |
Lung Function Parameters
A total of 218 participants presented pre-bronchodilator airflow obstruction and underwent a reversibility test. During post-bronchodilator spirometry 177 (12.3%) participants were found with airflow obstruction according to GOLD criteria. Likewise, out of 109 participants presenting pre-bronchodilator airflow obstruction according to LLN criteria, 95 of them sustained the airflow obstruction during post-bronchodilator spirometry. The average FEV1 and FVC were higher in male participants during pre- and post-bronchodilator spirometry (Table 2).
 |
Table 2 Lung Function Parameters |
Symptoms of COPD
According to mMRC dyspnoea scale, 14.4% of participants had mMRC scores ≥ 2 and 31% had 0 score, which is presented in Table 1. Moreover, more than one-third of the participants self-reported at least one of the respiratory symptoms, ie, either cough, phlegm, breathlessness or wheezing. The presence of symptoms was not significantly between males and females (Figure 1).
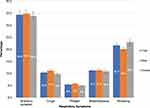 |
Figure 1 Distribution of respiratory symptoms by sex among study participants (n=1438). |
Prevalence of COPD
The prevalence of COPD was 5.4% (95% CI: 4.2 −6.6) based on LLN criteria and 8.5% (95% CI: 7.1–10.0) based on GOLD criteria (Figures 2A and B). The agreement between COPD by the LLN and GOLD criteria for COPD was substantial (kappa 0.76). Likewise, 12.3% and 6.6% of study participants were identified with chronic airflow obstruction based on GOLD criteria and LLN criteria, respectively. Out of 494 participants reporting at least one symptom of COPD, 24.7% (122) of them showed airflow limitation according to GOLD criteria and 15.6% (77) according to the LLN criteria of the case definition.
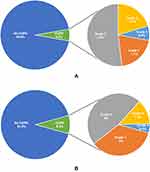 |
Figure 2 (A) Prevalence of COPD and severity classification by LLN criteria (n=1438). (B) Prevalence of COPD and severity classification by GOLD criteria (n=1438). |
The prevalence of COPD was higher in males as compared to females in both GOLD (male-10.9% (95% CI: 8.7–13.5), female-6.4% (95% CI: 4.9–8.4)) and LLN (male-7.6% (95% CI: 5.8–9.9), female-3.5% (95% CI: 2.4–5.0)) criteria. Older age, being unemployed, uneducated, underweight, currently unmarried, currently smoking, coming from underprivileged Dalit caste, having exposure to secondhand smoke and biomass fuel, low physical activity, and having a history of childhood lung infection, were significantly correlated with the prevalence of COPD in both criteria (Table 3).
 |
Table 3 Prevalence of COPD by GOLD Criteria and LLN Criteria |
Determinants of COPD
Variables significantly associated (p<0.05) with having COPD in unadjusted analysis (Supporting table) were included in subsequent multiple logistic regression analysis (Table 4). The adjusted model showed that the odds were highest among ≥60 years age group in both criteria compared to 40–49 years category. Low body mass index, being a current or former smoker, exposed to biomass fuel, and having childhood lung infection were also found to be significantly positively associated with the occurrence of COPD. Likewise, the low level of physical activity was significantly associated with the presence of COPD (Table 4).
 |
Table 4 Factors Associated with COPD (Adjusted Model) |
Discussion
The present study identified an 8.5% prevalence of GOLD-defined COPD and 5.4% prevalence of LLN-defined COPD in the population of this community. This is the first study in Nepal estimating COPD prevalence from the data of a community-based survey defining COPD by spirometry data and symptoms. The prevalence reported in this study is marginally lower than the earlier national-level estimation of 9.8% based on predictive covariates,12 and significantly lower than the 11.7% estimation using the GOLD criteria.9 Likewise, the identified prevalence is also lower than the estimate from neighbouring countries Bangladesh (12.5%)21 and Pakistan (11.3%)22 but similar to the prevalence reported in studies conducted in India (4.1% to 9%)23 and the BOLD study China (8%).11 However, in most of the epidemiological surveys, COPD is defined based upon spirometry data only. The discrepancy in the prevalence estimated by earlier studies in Nepal and the estimates of this study could be explained by the variation in the methods of estimation. This study defined COPD together with airflow obstruction and the presence of symptoms, while earlier studies defined COPD depending on airflow obstruction only. Although spirometry or clinical respiratory symptoms alone are not enough for an accurate diagnosis of COPD.24 The prevalence of chronic airflow obstruction reported in our study (12.3%) is slightly higher than the BOLD study results (11.3%) from 26 sites around the world.25
The variation in the prevalence of COPD reported in this study by GOLD criteria (8.5%), and LLN criteria (5.4%) can be attributed to the point that GOLD criteria tend to over-diagnose in elderly and LLN criteria tend to under-diagnose among elderly.1 Furthermore, only 2.8% of participants reported being clinician-diagnosed COPD suggesting the disease is underdiagnosed and unrecognized despite being largely prevalent in the community.
Similarly, chronic bronchitis was prevalent among 5.3% of participants, indicating diagnosis based on symptoms alone may tend to underestimate the prevalence of COPD. In the Nepalese context symptomatic assessment and diagnosis of COPD is common due to the limited availability of spirometry.26 Though spirometry is recommended only for symptomatic individuals, in our study, nearly 6% of asymptomatic participants were detected having airways obstruction. Also, the traditional norm of ignoring the COPD symptoms especially cough as natural ailment of old age diminishes health care seeking practice, thus leading to high undetected and undiagnosed cases in the community.
Like other studies,16,18 our study indicated a positive correlation between age and COPD. The association of COPD with age may be ascribed to more exposure to risk factors and physiological decline in lung function with age which generally begins at the age of 30–40 years.27 Similarly, our study findings reinforced that being a smoker (former or current) has a higher odds of having COPD.28,29 We found a significantly higher prevalence and also the odds of COPD among current smokers compared to never smokers, in line with previous studies.30,31
Similar to previous studies,16,21,32 biomass fuel consumption was found significantly associated with higher odds of having COPD. Biomass smoke is an important contributor to household air pollution that leads to COPD.33 More than 60% of households use biomass fuel for cooking and heating in Nepal.7 Traditionally, females were predominantly exposed to the emission of biomass fuel, as this study was conducted in a semi-urban area, the majority of women had access to liquid petroleum gas as cooking fuel.
The odds of having COPD was significantly lower among the people who were physically active than the people who had low-level physical activity. This could be ascribed to the fact that physical inactivity is common among people living with COPD. Also, the lower level of physical activity is correlated with a higher risk of exacerbation and all-cause mortality in COPD patients.34 As this is a cross-sectional study, we cannot rule out the reverse association.
Likewise, education and being in an advantaged ethnic category had lower odds of COPD. Contrary to this, being unemployed increased the odds of having COPD. However, only education was found significant in multivariable analysis. Educational status, ethnicity, and employment status are indicators of socioeconomic status. Low socioeconomic status is well known to be concomitant with poor access to healthcare and an increased risk of COPD.9 This association could be explained by the high prevalence of other potential risk factors of COPD among those groups such as lower quality of life and potential exposure to risk factors in early childhood and even in utero.35
A history of childhood lung infection was reported by nearly 7% of study participants, and it was positively associated with COPD. It is well established that early childhood severe lung infection is attributable to degraded lung growth, increasing the likelihood of COPD.36 Similarly, BMI is negatively correlated with COPD. The inverse association between BMI and COPD could be explained by other underlying factors which contribute to loss of muscle mass and overt malnutrition.37 In a meta-analysis, Sun et al also demonstrated low BMI as a risk factor for accelerated decline in lung function.38
This study has highlighted that COPD is highly prevalent but largely undiagnosed at the community level. Limiting to only symptomatic treatment of the disease may lead to a massive rise in the burden of COPD mostly at a severe stage when chances of mortality are high, and quality of life is poor. Early identification and diagnosis are the key to the prevention and control of COPD. Spirometry-based diagnosis enables to detect the presence and severity of COPD in symptomatic and airflow obstruction in the asymptomatic population.
The prevalence of COPD was alarmingly high among the elderly population. Considering that the life expectancy of Nepal is increasing, and the population is continuously ageing, more people are living longer. They are exposed to growing COPD-related risk factors such as smoking, ambient air pollution. Thus, the burden is likely to surge shortly. This puts early screening and diagnosis as a priority public health measure that needs to be considered. These services should also focus on vulnerable groups such as males, illiterate, and people from disadvantaged ethnicity. Replacing biomass fuel to cleaner fuel alternatives should be prioritized.
The current policy directives for COPD prevention and management in Nepal aim to strengthen national capacity to integrate scaled up services for COPD at primary health care settings, 25% relative reduction of chronic respiratory disease-related mortality, reduction of current smokers by 30% and 50% reduction in the use of biomass fuel for cooking by 2025.39 Our study provides epidemiological evidence of COPD through community-based data. Thus, supplementing our understanding for optimal community tailored prevention and management of COPD, which are the ultimate aims of these aforementioned policies.
Our study has several strengths and limitations. We diagnosed COPD based on both GOLD criteria with a fixed ratio of FEV1/FVC less than 70% and a cut-off based on LLN values together with the presence of symptoms. Contrast to most of the epidemiological studies40 in COPD to diagnose COPD solely on spirometry-based data; we have defined COPD together with results of spirometry and symptoms. The use of these methods makes our results more precise to estimate the true burden of clinical COPD in the community by avoiding false-positive cases. This study is conducted with a representative subset of the adult population (≥40 years) applying the objective measures of lung function parameters measured with high quality diagnostic robust spirometry by well-trained enumerators following standard ERS/ATS guidelines under the guidance of experienced over-readers. Nevertheless, the limitations included the absence of a reference equation for the Nepalese population. However, the North Indian population equation used was the closest acceptable equation to detect predicted percentages of different lung function parameters. Studies have reported that the GOLD criteria overestimate the burden of COPD, especially at the old ages. Since the majority of our participants were below 60 years of age; the bias should be minimum. Although we used a standardized questionnaire with extensive training of enumerators, there might have been a miscalculation in self-reported information such as smoking habits, physical activity, history of severe childhood lung infection, biomass fuel use, and symptoms of COPD. Similarly, some of the participants identified as COPD may have asthma-COPD overlap could be misclassified only as COPD. Due to the lack of overall information on COPD exacerbations GOLD ABCD patient assessment grading was not used. However, the diagnostic evidence used is in this study is sufficient to consider these patients as having COPD.
In conclusion, the first-ever community-based study in western Nepal showed a potentially significant burden of COPD, and often undiagnosed. This finding also suggests the importance of population-based screening in similar low-resource settings to identify undiagnosed COPD patients. As many people are living with COPD, comprehensive actions are needed focusing on prevention and early diagnosis. The already proven strategies like reducing the exposure to biomass fuels, enhancing the use of cleaner fuels, and reducing direct and indirect exposure to tobacco smoke could help to reduce the burden of COPD in Nepal.
Abbreviations
ATS, American Thoracic Society; BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ERS, European Respiratory Society; FEV1, forced expiratory volume in 1st second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease; LLN, lower limit of normal; mMRC, modified Medical Research Council; NCD, non-communicable disease; OR, odds ratio; SD, standard deviation.
Data Sharing Statement
Data are available upon reasonable request to the principal investigator Tara Ballav Adhikari.
Ethical Approval and Informed Consent
The study was conducted in accordance with the Declaration of Helsinki. Ethical approval was obtained from the ethical review board of the Nepal Health Research Council (Approval number: 30-2019). Written informed consent was obtained from study participants.
Consent for Publication
Not Applicable.
Acknowledgments
The authors would like to thank all the respondents of the study for providing their time and information on the study questionnaire.
Funding
This study is part of research work toward a PhD degree (TBA) at Aarhus University and is funded by a university scholarship. The study is partially supported by Nepalmed, Germany. The funding organizations do not have any role in the study design, the data collection, analysis, interpretation, or in the reporting of the results.
Disclosure
The authors declare that they have no competing interests.
References
1. Global Initiative for Chronic Obstructive Lung Disease Inc. (GOLD). Global Strategy for Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (2020 Report). Fontana, USA; 2020.
2. Li X, Cao X, Guo M, Xie M, Liu X. Trends and risk factors of mortality and disability adjusted life years for chronic respiratory diseases from 1990 to 2017: systematic analysis for the Global Burden of Disease Study 2017. BMJ. 2020;368.
3. World Health Organization (WHO). The Global Burden of Disease: 2004 Update. Switzerland: World Health Organization; 2008.
4. Bhandari GP, Angdembe MR, Dhimal M, Neupane S, Bhusal C. State of non-communicable diseases in Nepal. BMC Public Health. 2014;14(1):23. doi:10.1186/1471-2458-14-23
5. Dhimal M, Karki KB, Sharma SK, et al. Prevalence of selected chronic non-communicable diseases in Nepal. J Nepal Health Res Counc. 2019;17(3):394–401. doi:10.33314/jnhrc.v17i3.2327
6. Nepal Health Reserach Council, World Health Organization. NEPAL–Noncommunicable Disease Risk Factors STEPS Survey 2019 – Tobacco Factsheet. Kathmandu, Nepal; 2019.
7. Ministry of Health-Nepal, New Era, and IFC. Nepal Demographic and Health Survey 2016. Kathmandu, Nepal: Ministry of Health, Nepal; 2017.
8. Kurmi O, Regmi PR, Pant PR. Implication of air pollution on health effects in Nepal: lessons from global research. Nepal j Epidemiol. 2016;6(1):525. doi:10.3126/nje.v6i1.14733
9. Grigsby M, Siddharthan T, Chowdhury MA, et al. Socioeconomic status and COPD among low-and middle-income countries. Int J Chron Obstruct Pulmon Dis. 2016;11:2497. doi:10.2147/COPD.S111145
10. Central Beuaro of Statistics, Government of Nepal. National Population and Housing Census 2011 (National Report). Kathmandu, Nepal 2012.
11. Zhong N, Wang C, Yao W, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007;176(8):753–760. doi:10.1164/rccm.200612-1749OC
12. Adhikari TB, Neupane D, Kallestrup P. Burden of COPD in Nepal. Int J Chron Obstruct Pulmon Dis. 2018;13:583. doi:10.2147/COPD.S154319
13. Neupane D, Shrestha A, Mishra SR, et al. Awareness, prevalence, treatment, and control of hypertension in Western Nepal. Am J Hypertens. 2017;30(9):907–913. doi:10.1093/ajh/hpx074
14. Kish L. A procedure for objective respondent selection within the household. J Am Stat Assoc. 1949;44(247):380–387. doi:10.1080/01621459.1949.10483314
15. Graham BL, Steenbruggen I, Miller MR, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. 2019;200(8):e70–e88. doi:10.1164/rccm.201908-1590ST
16. van Gemert F, Kirenga B, Chavannes N, et al. Prevalence of chronic obstructive pulmonary disease and associated risk factors in Uganda (FRESH AIR Uganda): a prospective cross-sectional observational study. The Lancet Global Health. 2015;3(1):e44–e51. doi:10.1016/S2214-109X(14)70337-7
17. Buist AS, Vollmer WM, Sullivan SD, et al. The Burden of Obstructive Lung Disease Initiative (BOLD): rationale and design. COPD: J Chronic Obstruct Pulmonary Dis. 2005;2(2):277–283. doi:10.1081/COPD-57610
18. Zha Z, Leng R, Xu W, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in Anhui Province, China: a population-based survey. BMC Pulm Med. 2019;19(1):102. doi:10.1186/s12890-019-0864-0
19. Chhabra S, Kumar R, Gupta U, Rahman M, Dash D. Prediction equations for spirometry in adults from northern India. Indian J Chest Dis Allied Sci. 2014;56:221–229.
20. Bestall J, Paul E, Garrod R, Garnham R, Jones P, Wedzicha J. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi:10.1136/thx.54.7.581
21. Sutradhar I, Gupta RD, Hasan M, Wazib A, Prevalence SM. Risk factors of chronic obstructive pulmonary disease in Bangladesh: a systematic review. Cureus. 2019;11:1.
22. Sultana T, Afzal A, Sultana S, et al. Epidemiological estimates of respiratory diseases in the hospital population, Faisalabad, Pakistan. Braz Arch Biol Technol. 2017;60.
23. McKay AJ, Mahesh P, Fordham JZ, Majeed A. Prevalence of COPD in India: a systematic review. Prim Care Respir J. 2012;21(3):313–321. doi:10.4104/pcrj.2012.00055
24. Andreeva E, Pokhaznikova M, Lebedev A, Moiseeva I, Kuznetsova O, Degryse J-M. Spirometry is not enough to diagnose COPD in epidemiological studies: a follow-up study. NPJ Prim Care Respir Med. 2017;27(1):62. doi:10.1038/s41533-017-0062-6
25. Grønseth R, Erdal M, Tan WC, et al. Unemployment in chronic airflow obstruction around the world: results from the BOLD study. Eur Respir J. 2017;50:3. doi:10.1183/13993003.00499-2017
26. Bolton CE, Binaya K, Koju R, Hall IP. Challenges of chronic obstructive pulmonary disease in rural Nepal. The Lancet Respir Med. 2019;7(6):476–478. doi:10.1016/S2213-2600(19)30079-7
27. Wahba W. Influence of aging on lung function-clinical significance of changes from age twenty. Anesth Analg. 1983;62(8):764–776. doi:10.1213/00000539-198308000-00011
28. Laniado-Laborín R. Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21st century. Int J Environ Res Public Health. 2009;6(1):209–224. doi:10.3390/ijerph6010209
29. Forey BA, Thornton AJ, Lee PN. Systematic review with meta-analysis of the epidemiological evidence relating smoking to COPD, chronic bronchitis and emphysema. BMC Pulm Med. 2011;11(1):36.
30. Eisner MD. Secondhand Smoke and Obstructive Lung Disease: A Causal Effect?: American Thoracic Society; 2009.
31. Hagstad S, Bjerg A, Ekerljung L, et al. Passive smoking exposure is associated with increased risk of COPD in never smokers. Chest. 2014;145(6):1298–1304. doi:10.1378/chest.13-1349
32. Hu G, Zhou Y, Tian J, et al. Risk of COPD from exposure to biomass smoke: a metaanalysis. Chest. 2010;138(1):20–31. doi:10.1378/chest.08-2114
33. Shrestha IL, Shrestha SL. Indoor air pollution from biomass fuels and respiratory health of the exposed population in Nepalese households. Int J Occup Environ Health. 2005;11(2):150–160. doi:10.1179/oeh.2005.11.2.150
34. Lee SH, Kim KU, Lee H, Kim YS, Lee MK, Park H-K. Factors associated with low-level physical activity in elderly patients with chronic obstructive pulmonary disease. Korean J Intern Med. 2018;33(1):130–137. doi:10.3904/kjim.2016.090
35. Kant S, Gupta B. Role of lifestyle in the development of chronic obstructive pulmonary disease: a review. Lung India. 2008;25(2):95–101. doi:10.4103/0970-2113.59591
36. Anda RF, Brown DW, Dube SR, Bremner JD, Felitti VJ, Giles WH. Adverse childhood experiences and chronic obstructive pulmonary disease in adults. Am J Prev Med. 2008;34(5):396–403. doi:10.1016/j.amepre.2008.02.002
37. Agusti A, Soriano JB. COPD as a systemic disease. Copd. 2008;5(2):133–138. doi:10.1080/15412550801941349
38. Sun Y, Milne S, Jaw JE, et al. BMI is associated with FEV 1 decline in chronic obstructive pulmonary disease: a meta-analysis of clinical trials. Respir Res. 2019;20(1):236. doi:10.1186/s12931-019-1209-5
39. Ministry of Health and Population; Government of Nepal. Multisectoral Action Plan for the Prevention and Control of Non Communicable Diseases (2014-2020). Kathmandu, Nepal: Government of Nepal, World Health Organization, Country office of Nepal; 2014.
40. Bakke PS, Rönmark E, Eagan T, et al. Recommendations for epidemiological studies on COPD. Eur Respir J. 2011;38(6):1261–1277. doi:10.1183/09031936.00193809
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
