Back to Journals » Cancer Management and Research » Volume 11
Pretreatment detection of circulating and tissue CD133+ CD44+ cancer stem cells as a prognostic factor affecting the outcomes in Egyptian patients with colorectal cancer
Authors Zahran AM , Amal Rayan , Fakhry H, Attia AM, Ashmawy AM, Soliman A, Elkady A , Hetta HF
Received 3 October 2018
Accepted for publication 27 December 2018
Published 7 February 2019 Volume 2019:11 Pages 1237—1248
DOI https://doi.org/10.2147/CMAR.S189653
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Asmaa M Zahran,1 Amal Rayan,2 Hussein Fakhry,3 Alia M Attia,4 Ahmed M Ashmawy,5 Ahmed Soliman,6 Azza Elkady,7 Helal F Hetta8,9
1Department of Clinical Pathology, South Egypt Cancer Institute, Assiut, Egypt; 2Department of Clinical Oncology, Assiut University Hospital, Assiut University, Assiut, Egypt; 3Department of Surgical Oncology, South Egypt Cancer Institute, Assiut, Egypt; 4Department of Radiation Oncology, South Egypt Cancer Institute, Assiut, Egypt; 5Department of Internal Medicine, Assiut University Hospital, Assiut, Egypt; 6Department of General Surgery, Faculty of Medicine, Assiut University, Assiut, Egypt; 7Sohag University Medical Administration, Sohag, Egypt; 8Department of Medical Microbiology and Immunology, Faculty of Medicine, Assiut University, Assiut, Egypt; 9Department of Internal Medicine, University of Cincinnati College of Medicine, Cincinnati, OH, USA
Background and aim: Colorectal cancer is one of the most common malignant tumors worldwide. As CD133 and CD44 are notable markers of cancer stem cells (CSCs) identity, it is thought to be a predictive indicator for colorectal cancer. The aim of this study was to investigate the cell cycle state of CD133+ CD44+ and CD133− CD44−cells, isolated from primary human colorectal tumors, and to assess the clinical impact of CD133+ CD44+ CSCs on patients’ outcome regarding disease-free survival (DFS) and overall survival (OS).
Materials and methods: Tissue samples were collected from 50 primary colorectal cancer patients. Flow cytometric analysis was performed to isolate tissue CD133+ CD44+ CSCs and CD133− CD44− tumor cells from primary colorectal cancer tissue to compare the cell cycle of both types of cells. Also circulating CSCs were assessed by flow cytometry.
Results: Higher percentage of tissue CD133+ CD44+ CSCs isolated from colorectal cancer patients was found in G0/G1 phase. However, tissue CD133− CD44− tumor cells were predominantly found in the S phase; there were significant negative correlations between tissue CD133+ CD44+ CSCs and DFS and OS (r=−0.470, P<0.001, respectively and r=−0.487, P<0.001, respectively), also significant negative correlations between tissue CSCs and DFS and OS (r=−0.548, P<0.001, respectively and r=−0.497, P<0.001, respectively). Only the pathological grade (P<0.004) and T stage (P<0.004) had a significant effect on circulating CSC counts.
Conclusion: Tissue CD133+ CD44+ CSCs were more quiescent than tissue CD133− CD44− tumor cells and both circulating CSCs and tissue CSCs were considered independent negative prognostic factors on OS and DFS.
Keywords: cancer stem cells, colorectal cancer, CD133+ CD44+
Introduction
Worldwide, colorectal cancer ranks the second most common cancer in females representing 9.2% of all cancers and the third most common one in males representing 10.0% of the total with marked geographic variation.1 As expected, mortality is highest among developing regions with 52% of patients died from this tumor. Treatment of colorectal cancer, which is rarely diagnosed at early stages, is based largely on the stage of the cancer. Patients in whom colorectal cancer has not spread to distant sites are usually treated with surgery. Treatment with radiation and chemotherapy (CT) may also be used before or after surgery and often not successful in completely eradicating the tumor.2,3
Previous studies have identified subpopulations of colorectal cancer cells that are more resistant to cancer treatments such as chemotherapeutics and radiation.4,5 Elimination of the main tumor bulk without elimination of these highly resistant subpopulations is not considered complete remission. These cells are often referred to as cancer stem cells (CSCs).6
CSCs in cancers have the capacity for self-renewal; the processes involved in self-renewal are deregulated, which leads to CSC overpopulation, driving tumor growth. CSCs are able to resist conventional treatment, such as CT, and cause a tumor relapse and eventually metastasis of the primary tumor mass.7,8 Thereby, the CT-resistant CSCs population causes a tumor relapse and ultimately metastasis of the primary tumor mass. Therefore, a better understanding of CSCs is essential for understanding the biological and clinical consequences of the existing CT regimens and for designing new therapies to improve patient outcome.9
Several cell surface markers have been shown to be expressed in CSCs.6 CD133, CD44, and CD24 are three proposed stem cell markers in colorectal cancer.10 CD133, CD44, CD24, CDCP1, and CXCR4 are five cell surface antigens whose expression is thought to indicate stem cell-like properties.
CD133 is a five-transmembrane domain antigen with a molecular weight of 120 kDa11 and has been identified on stem-like cells of various tissues and cancers like pancreatic, prostate, kidney, and colorectal cancer.12 In colorectal cancer, CD133 is considered as a target for drug therapies as it plays an important role in the progression of colorectal cancer.13,14 CD44 is the main hyaluronan receptor and important for generation, maintenance, and survival of CSCs. Most published data indicated the involvement of CD44 in cancer cells as it promotes tumor growth, survival, migration, and metastasis and has an anti-apoptotic effect.14 CD24, a heat stable antigen, is considered a hallmark of many epithelial cancers as pancreatic, prostate, or breast cancer14,15 and may act as indicator for the likelihood of metastasis.16–18 CD24 has gained considerable interest in cancer research due to its important roles in migration and invasion by improving interactions between integrins and fibronectin.19
DNA ploidy status predicts disease-free intervals and short-term survival in numerous human malignancies.20,21 In colorectal cancer, the negative effect of aneuploidy remains a controversy and an area of much debate for more than 20 years. Numerous studies designed to determine survival–DNA content relationship have reported conflicting results.22 There are limited studies on cell cycle of colorectal CSCs studies.23,24
The present study aimed to detect the frequency of CSCs in primary colorectal cancer and circulating CSCs in peripheral blood. In addition to investigate the cell cycle status of both CD133+ CD44+ and CD133− CD44− cell populations, isolated from human primary colorectal cancer tissue, and to assess the clinical impact of circulating CSCs, tissue CSCs and CD133− CD44− tissue cells on patients’ outcome regarding disease-free survival (DFS) and overall survival (OS).
Materials and methods
This study was a prospective Phase II trial and included all new cases with colorectal cancer eligible for the study that referred from or admitted at Surgical Oncology and Radiotherapy Departments of South Egypt Cancer Institute, and clinical oncology department of Assiut university hospital.
Inclusion criteria
Patients with previously untreated histologically proven colorectal adenocarcinoma, and treated surgically without residual disease as proved histopathologically, were eligible for adjuvant systemic CT ± radiotherapy (RT) according to standardized guidelines with adequate Eastern Cooperative Oncology Group Performance Status (ECOG PS) (≤2), and adequate organ functions.
Exclusion criteria
Exclusion criteria include previous CT or RT, synchronous metastases at time of presentation, and previous malignancy.
Diagnosis
All patients underwent complete clinical examination including history and digital rectal examination, contrasted multisclice CT pelvi-abdomen (with barium enema) and chest, proctosigmoidoscopy and colonoscopy. The diagnosis of CRC was histopathologically confirmed by endoscopic, laparotomy, or surgical biopsies.
Histological diagnosis was based on the microscopic features of adenocarcinoma cells determining the histological subtypes including conventional adenocarcinoma, mucinous carcinoma, and signet ring carcinoma and the histological grade that depends mainly on the number of poorly differentiated clusters that appear in microscopic field of a ×20 objective lens, and colorectal cancer can be graded into grade 1 with PDCs <5, grade 2 with PDCs from 5 to 9, and grade 3 with PDCs ≥10. Confirmatory IHC through CK20 and CDX2 was done in some cases.
Laboratory studies included complete blood count, kidney function tests, complete liver functions, random blood sugar, and carcinoembryonic antigen (CEA).
Treatment
Different treatment modalities were given for patients with colorectal cancers including surgery (that was done for all cases), followed by adjuvant CT ± RT for rectal carcinomas and adjuvant CT alone for colonic carcinomas.
Surgery
All patients were subjected to radical resection of the tumor with regional lymphadenectomy proximally up to origin of vascular trunk. Surgical margins of at least 5 cm in colon cancer and 1–2 cm in rectal cancer with sphincter preservation should be done if possible. Mesorectal excision was mandatory in rectal cancer patients. Diverting illeostomy was done when indicated. Histopathologic assessment of the tumor type, margins, site, size, stage, grade, and lymph node metastasis was done.
Chemotherapy
All patients received 6 months of mFOLFOX6 or CAPEOX, mFOLFOX6 which consisted of oxaliplatin 85mg/m2 IV d1 over 2 hour infusion, leucovorin 400mg/m2 IV d1 over 2 hour infusion and 5-FU 400mg/m2 IV bolus on d1 then 1200mg/m2/d for 2 days by continuous infusion, mFOLFOX6 was repeated every 2 weeks for a maximum of 12 cycles, and CAPEOX consisted of oxaliplatin 130mg/m2 IV d1 over 2 hour infusion and capecitabine 1000mg/m2 twice daily PO for 14 days and repeated every 3 weeks
Radiotherapy
RT ± CT was delivered to all patients with cancer rectum according to the stage. The treatment was given by three-dimensional conformal RT in two phases. CTV for Phase I included the tumor, presacral LN, perirectal LN, external and internal iliac LNs, and inguinal LN for tumors extending below the dentate line, whereas CTV for Phase II included the tumor +3 cm safety margin. The dose for Phase I is 45 Gy/25 fractions/5 weeks and for Phase II 5.4 Gy/3 fractions/0.5 week. We limited the dose to the small bowel to 45–50 Gy and the femoral head and neck to 42 Gy. CT was in the form of capecitabine 825 mg/m2 twice daily during RT followed by 4 months of mFOLFOX6.
Flow cytometric detection and isolation of cancer stem cells in primary colorectal cancer tissue
CRC specimens from patients underwent colorectal surgery were obtained, extensively washed in PBS, and mechanically fragmented to prepare single-cell suspension which then filtered through cell strainers (100 µM). Red blood cell lysis solution was added for 5 minutes at 4°C to remove the contaminating red blood cells.
The following antibodies were used to stain the cell suspensions: phycoerythrin (PE)-labeled anti-CD133 (clone AC133/1; Miltenyi Biotec, Bergisch Gladbach, Germany) and APC-labeled anti-CD44 (clone G44–26; BD Biosciences, CA, USA). The expressions of CD133 and CD44 were quantitatively measured by FACSCalibur flow cytometer (BD Biosciences) with CellQuest software. 7-AAD (eBioscience; lot No; 1910559) was used to assess viability of cells. Cell populations were sorted using FACSCalibur flow cytometer. Cells were first gated based on light scatters followed by positive gating of CD133+ CD44+ and CD133− CD44− cells. After collecting CD133+ CD44+ and CD133− CD44− cells, the sorted cells were rerun through the flow cytometer and purity was determined using quality controls. The purity of isolated cells was ranged from 95% to 98%.
Flow cytometric cell cycle analysis
For cell cycle analysis, the sorted CD133+ CD44+ and CD133− CD44− cells were stained with DNA stain according to the manufacturer’s instruction (BD Cycletest™ Plus DNA Kit; BD Biosciences) and analyzed using a flow cytometer (BD FACSCalibur, San Jose, CA, USA) with ModFit software. DNA histograms of at least 50,000 nuclei were analyzed.
Flow cytometric detection of circulating cancer stem cells
Peripheral blood was drown using stile needle. The first 1 mL of blood draw discarded to avoid contamination by epithelial cells, and the second 5 mL was drown on blood collection tube used for flow cytometric analysis of CSCs. The patient’s peripheral blood mononuclear cells were isolated using Ficoll density gradient centrifugation (Biochrom GmbH, Leonorenstr, Berlin, Germany). However, some cells can be lost through Ficoll preparation. Then the buffy coat layer containing mononuclear cells was washed with PBS, incubated with Red blood cell lysis solution at room temperature for 3 minutes, and washed with PBS again.
About 200,000 isolated mononuclear cells were incubated with PerCP-labeled CD45 (BD biosciences), PE-labeled anti-CD133 clone, and APC-labeled anti-CD44 antibodies for 30 minutes at 4°C. After washing with PBS, the cells were acquired by FACSCalibur flow cytometer with CellQuest software. About 100,000 cells were acquired. CD45 and side scatter histogram was used to select the CD45− cells. Then the expression of CD133 and CD44 in CD45− cells was detected.
Follow-up
All patients were followed-up postoperatively at 3 months intervals for the first 2 years by history and clinical examination, laboratory investigations, and imaging studies (including CT scan pelvis and abdomen) to evaluate the response to CT ± RT and to monitor the toxicity, in addition to CEA level every 3 months for 2 years then every 6 months later on.
Local–regional failure was defined as recurrence within the pelvis, including the tumor bed, regional lymph nodes, anastomosis, or perineal scar. Distant failure was indicated as disease recurrence detected in the liver, lung, brain, and other organs outside the pelvis. The recurrence of disease was confirmed by physical findings, radiological studies, endoscopic examination with biopsy, and surgery.
Statistics
Descriptive data in the form of mean, median, range, SD, and percentages were used. Mann–Whitney U-test was used to find a relation between two groups of ordinal variables, chi-squared test between ≥2 groups of categorical variables, and ANOVA test for the relation between categorical and quantitative variables, and all of these relations were considered significant at P-value <0.05. Spearman’s correlation was used to determine if there was a relation between survival and CSCs and TSCs and to estimate the magnitude of this relation through estimation of r coefficient. Kaplan–Meier for calculation of overall survival (OS) and disease free survival (DFS) plots. DFS was the length of time from enrollment in this study to the time of relapse or death. OS was defined as the interval from enrollment in this study to the date of death from any cause or last follow-up. Log-rank test was used for survival analysis. And all our results were calculated using SPSS, version 21.
Ethical approval
Written informed consent was obtained from all patients included in this study and the study was approved by the institutional ethics committee of faculty of medicine, Assiut University, with approval ID number 17100623. All procedures performed in studies involving human participants were in accordance with the ethical standards of South Egypt Cancer Institute, Faculty of Medicine, Assiut University and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Results
The study involved 50 patients with nonmetastatic colorectal cancers, the characteristics of these patients were shown in Table 1, the median age of the study group was 45.5 years with 52% of them were male while female represented 48% of them, and ECOG PS 1 was the commonest one detected in 42% of patients. Adenocarcinoma was the most common pathologic subtype that was demonstrated in 58% of cases, with pathological grades 2, 4, 3, and 1 found in 54%, 24%, 12%, and 10%, respectively. Fifty-six percent of patients were diagnosed by endoscopic biopsy with 76% of our patients had elevated CEA at time of presentation. Most of our cases had locally advanced disease at the time of surgery with T3 and T4 detected in 44% and 32%, respectively. N1 and N2 were the commonest and represented 38% and 28% of patients, respectively.
Cell cycle analysis of sorted tissue CD133+ CD44+ CSCs and tissue CD133− CD44− tumor cells isolated from the primary tumor
The mean percentage of tissue CD133+ CD44+ CSCs in the primary colon tumor was 43.61±3.606 and that of CD133− CD44− tumor cells was 56.39±6.394. The mean ± SD, range, and significance of CD133+ CD44+ CSCs and CD133− CD44− tumor cells among different cell cycle phases were shown in Table 2.
A significant accumulation of tissue CD133+ CD44+ CSCs was detected in the G0/G1 phase than that of tissue CD133– CD44– tumor cells (P<0.02). Higher significant percentage of tissue CD133– CD44– tumor cells was accumulated in the S phase than tissue CD133+ CD44+ CSCs (P<0.03). There was a higher percentage of tissue CD133− CD44− tumor cells accumulated in the G2/M phase but did not differ significantly from that of tissue CD133+ CD44+ CSCs (P=0.728), demonstrating the different cell cycle pattern of tissue CD133+ CD44+ CSCs and tissue CD133− CD44− tumor cells (Figures 1 and 2). The mean percentage of circulating CSCs per 100,000 cells was 18.657±1.876.
Correlation between circulating CSCs and tissue CD133+ CD44+ CSCs
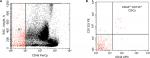
Our results demonstrated moderate significant positive correlation between circulating CSCs and tissue CD133+ CD44+ CSCs (r=+0.677, P<0.001), as shown in Figure 3.
  | Figure 3 Correlation between circulating CSCs and tissue CD133+ CD44+ CSCs (r=+0.677, P<0.001). Abbreviations: CSCs, cancer stem cells; TSC, tissue cancer stem cells. |
Median DFS and OS
The median DFS of 50 patients with nonmetastatic colorectal cancer was 19±2.638 months (95% CI=13.840–24.160), as shown in Figure 4, and the median OS was 23±1.755 months (95% CI=19.560–26.440), as shown in Figure 5.
  | Figure 5 The median OS of patients with colorectal cancers. Note: The median OS of 50 patients with colorectal cancers was 23±1.755 months (95% CI=19.560–26.440). Abbreviation: OS, overall survival. |
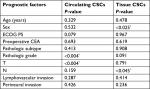
Univariate analysis of the possible prognostic factors influencing the median DFS and OS was represented in Table 3, with a significant effect of ECOG PS (P<0.04), T stage (P<0.002), N stage (P<0.001), and lymphovascular invasion (LVI) (P<0.04) on the median DFS, whereas PS (P<0.04), T stage (P<0.02), N stage (P<0.001), and LVI (P<0.01) had a significant impact on the median OS, as shown in Table 3.
Correlation was done to determine the relations between DFS and OS with the percentage of CD133+ CD44+ CSCs in the primary tumor (tissue CSCs) and circulating CD133+ CD44+ CSCs (circulating CSCs).We found negative correlations between tissue CSCs with DFS and OS with significant effect (P<0.001, r=−0.470 and P<0.001, r=−0.487, respectively). Also negative correlations were declared between circulating CSCs with DFS and OS (P<0.001, r=−0.548 and P<0.001, r=−0.497, respectively) with significant effect. Mann–Whitney U-test was done to assess the relations between the percentages of tissue CD133+ CD44+ CSCs and CD133− CD44− tumor cells in different cell cycle phases with DFS and OS. We found a significant impact of the percentage of tissue CD133+ CD44+ CSCs in different cell cycle phases on DFS (P<0.001, P<0.004, P<0.001 in G0/G1, G2/M, and S phases, respectively). We also found a significant impact of tissue CD133+ CD44+ CSCs in all cell cycle phases on OS (P<0.003, P<0.01, P<0.006 in G0/G1, G2/M, S phases, respectively). While there was insignificant impact of tissue CD133− CD44− tumor cells in different cell cycle phases on DFS and OS except in S phase, which had a significant impact with DFS (P<0.04), as shown in Table 4.
In multivariate analysis of these prognostic factors, the percentages of circulating CSCs and tissue CD133+ CD44+ CSCs were considered independent prognostic factors for the median DFS (P<0.02, P<0.006) and the median OS (P<0.003, P<0.03), as shown in Table 5.
ANOVA test was applied to determine the impact of different clinical characteristics on circulating CSCs and tissue CD133+ CD44+ CSCs, and only pathological grade (P<0.004) and T stage (P<0.004) had a significant effect on CSC counts; however, in tissue CSCs, male had a significantly higher count than females (P<0.025) and those with higher N stage had a significantly higher count (P<0.045) than those of lower N stage, as shown in Table 6.
Discussion
Colorectal cancer is a common and deadly tumor with several environmental and genetic factors influencing its development and rarely diagnosed early, with subsequent failure of surgery and CT ± RT to completely eradicate the tumor.
Colorectal cancer is hierarchically organized, and this hierarchical arrangement is maintained by many epigenetic mechanisms that involve CSCs.25
The existence of CSCs in colorectal cancers is well established, which can be defined by self-renewal ability, asymmetric division, and functional heterogeneity.26 Therapy-resistant CSCs represent a major challenge in the success of treatment to colorectal cancers and they can reestablish the whole tumor at the primary or secondary sites,27 wherefore better understanding of CSCs helps to detect remote metastasis earlier, target CSC subpopulations, and subsequently eradicate tumors.28 Plasticity is a fundamental aspect of colorectal CSCs, which means their capability to shift between different functional states including quiescence/nonquiecence, drug resistance/sensitivity, epithelial–mesenchymal/mesenchymal–epithelial, and stemness/nonstemness.29
This study tried to assess the correlations between CSCs expressing both CD133 and CD44 and patient outcomes. Although some studies reported no correlations existed,30 others reported the rarity of both expressions in CSCs.30
Our results demonstrated that CD133+ CD44+ CSCs were more quiescent than CD133− CD44− tumor cells (G0 phase is a period in the cell cycle in which cells are neither dividing nor preparing to divide, ie, quiescent, and can be considered as extended G1 phase; cells remain in G1 phase for a bit less than half of the total cell cycle time. This is the longest phase and microenvironmental conditions and signals received from other cells can shorten or lengthen G1 so considered quiescent comparing to actually dividing cells in M phase) with significant higher count of the former in G0/G1 phase and significant lower count in S phase, which agreed with the slow cycling nature of CSCs and this was comparable to a previous study.31
This study was comparable to several studies that reported the significant impact of good ECOG PS32 and positive LNs33 on patients’ survival; in addition, the outcome differs significantly within the same TNM stage. Although the median ages for colorectal cancers were globally reported to be 68 years for males and 72 years for females, in Egypt, colorectal cancer had no specific age predilection and more than one-third of patients were below the age of 40 years that cannot be attributed to bilharziasis and hereditary basis.34
In spite of radical surgery, 25%–50% of patients experienced recurrence with only 50% of them cured by surgery alone indicating the significant survival benefit of adjuvant treatment;35 also 25%–40% of patients with LNs negative tumors develop liver metastasis.36 This means that distant spread can occur at all colorectal cancer stages.37
High CD133+ CSC count was associated with poor clinical outcomes including poor 5-year survival in cancer pancreas,38 cancer ovary, and worse prognosis in non-small cell lung cancer.39
Mulder et al40 also showed that CD44 has prognostic value independent of staging in colorectal cancer patients, and it may predict its tendency to metastasize after curative surgery.
In this study, circulating CD133+ CD44+ CSCs and tissue CD133+ CD44+ CSCs were negatively correlated with the median DFS and median OS with significant effect (P<0.000, r=−0.470; P<0.000, r=−0.487 for CSC and P<0.000, r=−0.548; P<0.000, r=−0.497 for TSC).
Few studies demonstrated that CD133+ CSC had a role in predicting patients’ survival of colorectal cancers.41 Horst et al42 showed that 5- and 10-year survivals were negatively correlated with CD133+ CSCs, and Kojima et al43 showed that both negative and positive CD133 tumors had no difference in survival time while Gazaniga et al44 found no relationship between CD133 expression on tumor cells and survival in colorectal cancer patients.
Jing et al45 suggested that CD44 and CD133 are putative CSCs markers that are highly coexpressed in CRC with hepatic metastases, and CD44 expression was an independent factor associated with patient survival, while CD133 did not show this pattern.
Improvement of clinical outcomes after treatment of CSC in many cancers, with targeted therapies in the adjuvant settings, by all the odds, supports the stem cell hypothesis, and this is true for colorectal cancers and explains, in part, the independent impact of CSC and TSC counts on DFS and OS in our study.
Large meta-analysis of CD133 expression in colorectal cancer CSCs confirmed that higher levels of CD133+ CSCs were associated with several clinicopathologic characteristics and CD133+ CSCs can be used as an independent negative prognostic factor; in our study, higher counts of CD133+ CD44+ CSCs were significantly associated with T3 and T4 lesions and higher grades, which were considered comparable to Chen et al.46 Also our results were comparable to Chen et al in confirming the independent significant impact of CSCs on OS and DFS.
Conclusion
Tissue CD133+ CD44+ CSCs were more quiescent than tissue CD133− CD44− tumor cells, and both circulating CSCs and tissue CD133+ CD44+ CSCs were considered independent negative prognostic factors on OS and DFS.
Acknowledgments
All authors acknowledged all participating patients and supporting colleagues. No funds and grants were received for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | ||
Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J Clin Invest. 2010;120(1):41–50. | ||
Zhou BB, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8(10):806–823. | ||
Rich JN. Cancer stem cells in radiation resistance. Cancer Res. 2007;67(19):8980–8984. | ||
Abdullah LN, Chow EK. Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med. 2013;2(1):3. | ||
Baumann M, Krause M, Thames H, Trott K, Zips D. Cancer stem cells and radiotherapy. Int J Radiat Biol. 2009;85(5):391–402. | ||
Dylla SJ, Beviglia L, Park IK, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3(6):e2428. | ||
Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–768. | ||
Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–1261. | ||
Chu P, Clanton DJ, Snipas TS, et al. Characterization of a subpopulation of colon cancer cells with stem cell-like properties. Int J Cancer. 2009;124(6):1312–1321. | ||
Mikhail S, Zeidan A. Stem cells in gastrointestinal cancers: the road less travelled. World J Stem Cells. 2014;6(5):606. | ||
Schneider M, Huber J, Hadaschik B, Siegers GM, Fiebig HH, Schüler J. Characterization of colon cancer cells: a functional approach characterizing CD133 as a potential stem cell marker. BMC Cancer. 2012;12(1):96. | ||
Mizrak D, Brittan M, Alison M. CD133: molecule of the moment. J Pathol. 2008;214(1):3–9. | ||
Keysar SB, Jimeno A. More than markers: biological significance of cancer stem cell-defining molecules. Mol Cancer Ther. 2010;9(9):2450–2457. | ||
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. | ||
Lim SC. CD24 and human carcinoma: tumor biological aspects. Biomed Pharmacother. 2005;59(Suppl 2):S351–S354. | ||
Sano A, Kato H, Sakurai S, et al. CD24 expression is a novel prognostic factor in esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16(2):506–514. | ||
Aigner S, Sthoeger ZM, Fogel M, et al. CD24, a mucin-type glycoprotein, is a ligand for P-selectin on human tumor cells. Blood. 1997;89(9):3385–3395. | ||
Baumann P, Cremers N, Kroese F, et al. CD24 expression causes the acquisition of multiple cellular properties associated with tumor growth and metastasis. Cancer Res. 2005;65(23):10783–10793. | ||
Friedlander ML, Hedley DW, Taylor IW. Clinical and biological significance of aneuploidy in human tumours. J Clin Pathol. 1984;37(9):961–974. | ||
Barlogie B, Raber MN, Schumann J, et al. Flow cytometry in clinical cancer research. Cancer Res. 1983;43(9):3982–3997. | ||
Quirke P, Fozard JB, Dixon MF, Dyson JE, Giles GR, Bird CC. DNA aneuploidy in colorectal adenomas. Br J Cancer. 1986;53(4):477–481. | ||
Ieta K, Tanaka F, Haraguchi N, et al. Biological and genetic characteristics of tumor-initiating cells in colon cancer. Ann Surg Oncol. 2008;15(2):638–648. | ||
Tirino V, Desiderio V, D’Aquino R, et al. Detection and characterization of CD133+ cancer stem cells in human solid tumours. PLoS One. 2008;3(10):e3469. | ||
Ricci-Vitiani L, Fabrizi E, Palio E, de Maria R. Colon cancer stem cells. J Mol Med. 2009;87(11):1097–1104. | ||
Greaves M. Cancer stem cells as “units of selection”. Evol Appl. 2013;6(1):102–108. | ||
Verga Falzacappa MV, Ronchini C, Reavie LB, Pelicci PG. Regulation of self-renewal in normal and cancer stem cells. Febs J. 2012;279(19):3559–3572. | ||
Galizia G, Gemei M, Del Vecchio L, et al. Combined CD133/CD44 expression as a prognostic indicator of disease-free survival in patients with colorectal cancer. Arch Surg. 2012;147(1):18–24. | ||
Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14(3):275–291. | ||
Du L, Wang H, He L, et al. CD44 is of functional importance for colorectal cancer stem cells. Clin Cancer Res. 2008;14(21):6751–6760. | ||
Gharagozloo M, Mirzaei H, Bagherpour B, et al. Cell cycle analysis of the CD133 + and CD133– cells isolated from human colorectal cancer. J Can Res Ther. 2012;8(3):399–403. | ||
Mol L, Ottevanger PB, Koopman M, Punt CJ. The prognostic value of WHO performance status in relation to quality of life in advanced colorectal cancer patients. Eur J Cancer. 2016;66:138–143. | ||
Baxter NN. Is lymph node count an ideal quality indicator for Cancer care? J Surg Oncol. 2009;99(4):265–268. | ||
Abou-Zeid AA, Khafagy W, Marzouk DM, Alaa A, Mostafa I, Ela MA. Colorectal cancer in Egypt. Dis Colon Rectum. 2002;45(9):1255–1260. | ||
André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27(19):3109–3116. | ||
Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg. 2008;247(1):125–135. | ||
Maeda S, Shinchi H, Kurahara H, et al. CD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancer. Br J Cancer. 2008;98(8):1389–1397. | ||
Zhang J, Guo X, Chang DY, Rosen DG, Mercado-Uribe I, Liu J. CD133 expression associated with poor prognosis in ovarian cancer. Mod Pathol. 2012;25(3):456–464. | ||
Qu H, Li R, Liu Z, Zhang J, Luo R. Prognostic value of cancer stem cell marker CD133 expression in non-small cell lung cancer: a systematic review. Int J Clin Exp Pathol. 2013;6(11):2644–2650. | ||
Mulder JW, Kruyt P, Sewnath M, et al. Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet. 1994;344(8935):1470–1472. | ||
Kemper K, Versloot M, Cameron K, et al. Mutations in the Ras-Raf axis underlie the prognostic value of CD133 in colorectal cancer. Clin Cancer Res. 2012;18(11):3132–3141. | ||
Horst D, Kriegl L, Engel J, Kirchner T, Jung A. CD133 expression is an independent prognostic marker for low survival in colorectal cancer. Br J Cancer. 2008;99(8):1285–1289. | ||
Kojima M, Ishii G, Atsumi N, Fujii S, Saito N, Ochiai A. Immunohistochemical detection of CD133 expression in colorectal cancer: a clinicopathological study. Cancer Sci. 2008;99(8):1578–1583. | ||
Gazzaniga P, Gradilone A, Petracca A, et al. Molecular markers in circulating tumour cells from metastatic colorectal cancer patients. J Cell Mol Med. 2010;14(8):2073–2077. | ||
Jing F, Kim HJ, Kim CH, Kim YJ, Lee JH, Kim HR. Colon cancer stem cell markers CD44 and CD133 in patients with colorectal cancer and synchronous hepatic metastases. Int J Oncol. 2015;46(4):1582–1588. | ||
Chen S, Song X, Chen Z, et al. CD133 expression and the prognosis of colorectal cancer: a systematic review and meta-analysis. PLoS One. 2013;8(2):e56380. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.