Back to Archived Journals » Ambulatory Anesthesia » Volume 2
Preoperative testing and risk assessment: perspectives on patient selection in ambulatory anesthetic procedures
Received 17 November 2014
Accepted for publication 3 February 2015
Published 5 August 2015 Volume 2015:2 Pages 67—77
DOI https://doi.org/10.2147/AA.S59819
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Gildasio S De Oliveira Jr.
Tracey L Stierer,1,2 Nancy A Collop3,4
1Department of Anesthesiology, 2Department of Critical Care Medicine, Otolaryngology Head and Neck Surgery, Johns Hopkins Medicine, Baltimore, MD, USA; 3Department of Medicine, 4Department of Neurology, Emory University, Emory Sleep Center, Wesley Woods Center, Atlanta, GA, USA
Abstract: With recent advances in surgical and anesthetic technique, there has been a growing emphasis on the delivery of care to patients undergoing ambulatory procedures of increasing complexity. Appropriate patient selection and meticulous preparation are vital to the provision of a safe, quality perioperative experience. It is not unusual for patients with complex medical histories and substantial systemic disease to be scheduled for discharge on the same day as their surgical procedure. The trend to “push the envelope” by triaging progressively sicker patients to ambulatory surgical facilities has resulted in a number of challenges for the anesthesia provider who will assume their care. It is well known that certain patient diseases are associated with increased perioperative risk. It is therefore important to define clinical factors that warrant more extensive testing of the patient and medical conditions that present a prohibitive risk for an adverse outcome. The preoperative assessment is an opportunity for the anesthesia provider to determine the status and stability of the patient’s health, provide preoperative education and instructions, and offer support and reassurance to the patient and the patient’s family members. Communication between the surgeon/proceduralist and the anesthesia provider is critical in achieving optimal outcome. A multifaceted approach is required when considering whether a specific patient will be best served having their procedure on an outpatient basis. Not only should the patient's comorbidities be stable and optimized, but details regarding the planned procedure and the resources available at the facility also should be ascertained. Equally important to outcome are the resources and support available to the patient during their recovery after they have been discharged from the facility. This article reviews appropriate patient and procedure selection, based on elements of preoperative history and physical examination, supporting testing, and risk assessment.
Keywords: ambulatory, sleep apnea, preoperative, surgery, anesthesia
Introduction
The overarching goal of a preoperative assessment is to minimize perioperative morbidity and mortality, identify and optimize the patient’s medical conditions, and decrease risk for adverse anesthetic or surgical outcomes. In 2009, the Centers for Disease Control and Prevention reported that more than 34 million ambulatory surgical visits were performed in the United States per annum, and the reported safety profile of outpatient surgery has historically been excellent.1–4 However, there have been recent high-profile cases, such as that of Joan Rivers, the late comedian, that have brought into focus the importance of careful evaluation of the patient’s overall health and the appropriateness of venue when planning for an outpatient surgical procedure.
The preoperative assessment is a crucial first step in ensuring the safety and optimal outcome for the ambulatory surgical patient. In addition to providing an opportunity to perform a thorough history and physical examination, the face-to-face preoperative evaluation by an anesthesia provider affords both the patient and the patient’s family members a time to ask questions and voice their concerns. Although in some cases a separate visit to the preoperative clinic may not be convenient or feasible, especially for out-of-town patients, for select patients, a thorough document review combined with a telephone interview may be adequate and result in a similar outcome. Regardless of whether the patient presents to a preoperative clinic or is contacted by telephone, the timing of the preoperative evaluation is key to the success of providing quality care, achieving optimal outcome, and preventing cancelation or delay of the procedure on the day of surgery. The assessment should be scheduled far enough in advance of the surgical procedure to allow adequate time for review of supporting documentation and identification of potential risk factors, as well as discussion of and planning for further diagnostic or therapeutic maneuvers that might lower risk. This may include further consultation with specialists to evaluate the status of specific elements of the patient’s history or physical examination that may affect surgical or anesthetic outcome.
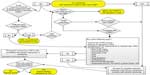
While the surgeon discusses a proposed surgery with the patient, that visit is predominately aimed at addressing the patient’s surgical problem, explaining the planned procedure being contemplated, and discussing alternative treatment options. However, the value of the participation and contribution of the surgeon or proceduralist to the preoperative evaluation process should not be underestimated. A strategy that has been successfully employed is that of a simple screening tool completed while the patient is at the surgeon’s office or at the time of the posting of the case onto the operating room schedule. The answers to this screening tool can be used to help determine whether the patient would benefit from evaluation by an anesthesiologist before the planned procedure, or whether a telephone interview by an anesthesia provider would suffice. The collection of specific information may be performed by ancillary staff members in the surgeon’s office, and personnel from the surgical facility or, alternatively, the patient might be asked to complete a self-reporting questionnaire. Pointed information obtained by the questionnaire can be forwarded for review to the individual or group responsible for triaging the timing and location of preoperative assessments for the particular facility in which the procedure is scheduled (Figure S1). Traditionally, exchange of health care information between providers has involved the use of telephone, fax, or email. However there is increasing use of the shared electronic health record for this purpose.
Not unlike a preoperative evaluation before an inpatient procedure, the necessary components of the patient’s history that should be gathered before ambulatory surgery include the nature and extent of the surgical procedure; allergies; an up-to-date and accurate medication list; prior medical and surgical history, including a history of problems with anesthesia; a current problem list; and a review of systems. The focused physical examination incorporates a thorough description of the airway, as well as evaluation of the cardiopulmonary systems. Additional information that is particularly relevant to the outpatient is their postoperative social support system. Details as to who will accompany the patient home from the facility after the procedures, as well as the resources available to the patient for home care, are critical to ensuring the safety of the patient and to a smooth perioperative transition of care. All too frequently, adequate consideration is not given to these issues, and the patient subsequently suffers the risk for insufficient postoperative support or, alternatively, the patient’s family incurs an undue burden of care that they may be unwilling or ill-equipped to handle.
It is during the preoperative assessment that a determination can be made as to whether the patient has been scheduled in the appropriate location of care. Outpatient surgery is performed in a variety of venues, including inpatient operating rooms, hospital-based outpatient units, freestanding ambulatory surgery centers (ASCs), and private physician offices. There is an expectation that an accredited ASC or office will have airway and resuscitation equipment equivalent to that found in a hospital-based inpatient operating room. However, the availability of special equipment and consultation services may vary between the different venues. Basic cardiology and electrophysiology services, radiology, respiratory therapy, and providers skilled at obtaining a surgical airway are examples of resources that may be required for special patient populations, yet these services may not be available at all venues. Part of the preoperative assessment is the determination of whether or not the location of the planned procedure is adequately equipped to deal with an emergency that may stem from the surgical procedure or decompensation of the patient’s preexisting medical condition. With recent advances in technology, the range and complexity of outpatient surgical procedures has markedly increased. In addition, intensified scrutiny by third-party payers as well as changes in payment arrangements have contributed to the sharp increase in the number of surgical procedures performed in freestanding ASC’s and private offices during the last decade. Guidelines and recommendations for appropriate patient selection exist but are variable- and facility-dependent.
With regard to laboratory testing, there is little to no evidence that supports the concept of “routine testing” before ambulatory surgery, or any surgery for that matter.5 It has long been accepted that preoperative testing without a specific indication does nothing to enhance the safety of surgery or anesthesia or to improve outcomes. Unfortunately, there remain surgeons who persist in ordering a plethora of unnecessary tests in the hope of avoiding having their case canceled or delayed. In 2001, the American Society of Anesthesiologists convened a task force to assess the evidence pertaining to the content and timing of the preoperative assessment.6 However, after extensive review of the existing literature and opinion survey, the task force concluded that there was insufficient evidence to define explicit decision parameters or rules for ordering tests on the basis of clinical conditions, and that specific characteristics of the patient’s medical history and diagnoses should be used in conjunction with clinical judgment to guide testing. The overwhelming majority of surgical procedures performed on an ambulatory basis are classified as low risk with regard to invasiveness, anticipated blood loss, and fluid shifts.
In accordance with the published recommendations of the task force, there is no evidence to support obtaining serum chemistries, coagulation studies, or even hemoglobin levels unless indicated by a specific patient condition. Furthermore, a given test is indicated only if the results will be reviewed before the procedure and if it has the potential to affect the perioperative management of the patient. Similarly, the task force was unable to determine unambiguously the required timing of the testing. Responses of survey opinion among practicing anesthesiologists, when asked about acceptable timing of testing, ranged from 4 weeks to 6 months before the procedure. Clinical judgment is often required to determine the optimal timing of a preoperative assessment, and the decision should be tailored for the individual patient. Local policies and procedures will frequently dictate the timing; however, the optimal interval for testing before a procedure allows the opportunity to obtain additional required studies, but it is not so far in advance that the patient’s medical status is likely to have changed.
Therefore, the healthy American Society of Anesthesiologists I and II patients may best be triaged to a preoperative telephone interview, with concise documentation in the patient’s record, to minimize unnecessary use of clinic resources and to maximize patient satisfaction. The documentation should include, in addition to the patient’s responses to questions, any advice, recommendations, or instructions dispensed to the patient during the telephone encounter. There are specific patient conditions, however, that pose substantial challenges in the perioperative management for those undergoing same-day surgery. The patient with significant comorbidities is most likely to benefit from a face-to-face visit with anesthesia staff.
Cardiovascular disease
As alluded to earlier, perioperative mortality associated with ambulatory surgery is low, and adverse outcomes attributed to a cardiac etiology are infrequent. With respect to the patient with cardiac disease, the function of the preoperative assessment is twofold. First, the evaluation is meant to identify and modify cardiac risk, and second, it is meant to determine whether or not the patient would be better served in an inpatient setting with a higher level of resources than the standard ASC. Unfortunately, there remains a relative paucity of high-quality evidence directed at determining the risk of a major adverse cardiac event (MACE) in patients undergoing ambulatory procedures. Although guideline-directed medical therapeutic goals for the ambulatory surgical patient with cardiac disease do not differ substantially from those recommended for patients undergoing more invasive procedures, higher-risk surgeries may warrant further testing.7,8 Patients with active cardiac conditions such as unstable or severe angina, acute or recent myocardial infarction (within 7 days), decompensated or acute heart failure, symptomatic dysrhythmias, and severe valvular disease are poor candidates for elective surgery, ambulatory or otherwise. For stable patients with cardiac risk factors, the American College of Cardiology/American Heart Association guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery combines evidence and multidisciplinary consensus to assist in determining what, if any, additional diagnostic testing is necessary before ambulatory surgery. Using the American College of Surgeons National Surgical Quality Improvement Program risk calculator (http://www.surgicalriskcalculator.com), the risk for MACE based on the combined clinical and surgical risk can be ascertained.9 According to the guidelines, patients with a risk for MACE lower than 1% require no further cardiac testing before surgery. Likewise, patients with a MACE risk higher than 1% and moderate to good functional capacity (>4 mets) can also proceed directly to surgery. Only patients at high risk for MACE who have poor functional capacity should undergo further testing, and only if the results will affect perioperative care.
Most practicing anesthesia providers would require a preoperative electrocardiogram (ECG) for patients with known coronary artery disease (CAD), significant arrhythmia, peripheral arterial disease, cerebrovascular disease, or other significant structural heart disease. Interestingly, the American College of Cardiology/American Heart Association guidelines do not include an ECG as a recommended component of the preoperative assessment in patients undergoing low-risk surgeries. Furthermore, they go on to state that a routine resting 12-lead ECG is not useful for asymptomatic patients undergoing low-risk procedures.10 Nevertheless, for patients with known CAD who may experience an intraoperative event such as sustained hypotension, the resting ECG remains a useful baseline standard against which to measure changes in the postoperative period.11
Although ischemic heart disease can contribute to adverse perioperative outcomes, routine preoperative pharmacological or exercise stress testing for patients with known CAD is not recommended before ambulatory surgical procedures, regardless of the patient’s functional capacity, unless it is indicated for other reasons.12
The patient with a history of prior coronary artery bypass surgery who remains asymptomatic requires no special diagnostic testing or documentation before ambulatory surgery. However, the perioperative management of patients with prior percutaneous coronary interventions has been a vexing source of controversy. This is especially true with regard to the timing of a surgical procedure in relationship to the percutaneous coronary interventions. Weighing the risk for stent thrombosis against the risk of bleeding has resulted in specific recommendations by the American College of Cardiology/American Heart Association; however, emerging data suggest that even the most recent guidelines may be debatable.13 The current recommendations include delay of an elective procedure for 365 days after the placement of a drug-eluting stent, and for 30 days after placement of a bare metal stent. Urgent procedures that are required in the less-than-recommended timeframe should be performed in a facility with cardiac catheterization capabilities to minimize the time from recognition of ischemia to revascularization. This would naturally exclude the majority of ASCs. The risk for discontinuation of dual antiplatelet therapy within 4–6 weeks of percutaneous coronary intervention with either drug-eluting or bare metal stents is significant with regard to the danger of in-stent thrombosis, and this threat decreases with increasing time. In patients with a bare metal stent placed more than 30 days before, and the drug-eluting stent placed more than 365 days before, and in whom the P2Y12 platelet receptor-inhibitor must be discontinued, aspirin should be continued if possible. The perioperative plan for dual antiplatelet therapy should be individualized to the patient, and the surgeon, cardiologist, and anesthesia provider should participate in the decision-making process, considering the risk of bleeding versus stent thrombosis.7,8
With regard to patients with a history of congestive heart failure, the importance of measures of left ventricular systolic function in predicting adverse perioperative cardiac events has been investigated in several studies.14,15 However, there are few data specifically addressing the patient undergoing an ambulatory surgical procedure. The American College of Cardiology/American Heart Association guidelines recommend preoperative echocardiography for patients with dyspnea of unknown origin or recently altered clinical status with known heart failure. Healy et al showed that patients with an ejection fraction of less than 30% were more likely to experience postoperative complications, including heart failure.16 Although this cohort of subjects underwent intermediate- to high-risk procedures, in the absence of evidence to support its safety, it would seem prudent to exclude patients with low ejection fractions from undergoing an anesthetic in a freestanding ambulatory venue.
Valvular heart disease
The patient with a severely stenotic lesion of the aortic or mitral valve is also a poor candidate for surgery in an ambulatory setting. The diagnosis of aortic stenosis in a patient, who is either symptomatic or has either a valve area of less than 1 cm2 or a mean transvalvular pressure of greater than 40 mmHg, is at risk for perioperative complications, including myocardial infarction and death.17 Likewise, patients with critical mitral stenosis (valve area <1 cm2) or those who are symptomatic are at risk for perioperative pulmonary hypertension, pulmonary edema, dysrhythmias, and hypotension, and they may require resources not normally available in an ASC.
Cardiovascular implantable electronic devices
The preoperative evaluation of the patient with an implantable cardiac defibrillator or pacemaker requires effective communication between the surgical team and the electrophysiology team that regularly follows the patient. The Heart Rhythm Society and the American Society of Anesthesiologists issued a joint statement in 2011 to provide guidance to clinicians caring for patients with cardiovascular implantable electronic devices (CIEDs) in the perioperative period; the American Heart Association as well as the American Thoracic Society have endorsed this consensus document.18 Recommendations for care include ascertainment of the patient’s underlying cardiac condition for which the CIED was placed, in addition to the identification of the hardware, settings, and programming features. Because of the vast number of devices, there is no one singular recommendation that is appropriate for all patients with a CIED, and the plan of care should be individualized. The electrophysiology team may prescribe perioperative interrogation, reprogramming, application of a magnet during the procedure, or no intervention at all. The decision to perform surgery on the patient with a CIED in an ambulatory venue should take into consideration the availability of resources, including the electrophysiology team required to comply with the recommendations provided by those who have been managing the patient’s device before surgery.
Pulmonary disorders
According to the American College of Physicians, chronic obstructive pulmonary disease is the most commonly identified risk factor for postoperative pulmonary complications, with an odds ratio of 1.79.19 Although spirometry is useful in the initial diagnosis of chronic obstructive pulmonary disease or asthma, pulmonary function testing has not been shown to have any value in reducing postoperative pulmonary complications associated with ambulatory surgical procedures. Patients with active wheezing; changes in sputum including purulence, color, or amount; or those who report increased shortness of breath should have their procedure postponed. In addition, these patients should be referred back to the provider who manages their pulmonary disease for further management. Patients with chronic wheezing that does not respond to bronchodilators should have confirmatory documentation from their private physician indicating optimization of their disease. Furthermore, patients who have required oral or parenteral corticosteroids for the treatment of reactive airway disease in the 6 months before the planned procedure may require additional doses for support around the time of the procedure. Although the data are scarce, there is evidence to suggest that prophylactic steroids given in the preoperative interval 48–72 hours before the procedure may help reduce airway reactivity and decrease the chance of intraoperative bronchospasm.20–22
Patients who require daily supplemental oxygen are not good candidates for surgery in ASC. With little margin for decompensation, the management of a hypoxemic episode may require specialized respiratory services and a lower patient-to-nurse ratio in the recovery room.
Obstructive sleep apnea
Obstructive sleep apnea (OSA) is the complete or partial collapse of the upper airway during sleep, and it has been shown to be an independent risk factor for adverse outcome after surgery.23 There is evidence that between 80% and 90% of patients who suffer from OSA have not been formally diagnosed.24 Unfortunately, there is a lack of high-quality data to guide clinicians in the perioperative management of patients with known or suspected OSA. In 2005, the American Society of Anesthesiologists convened a task force to address the problem. Although the task force performed an extensive literature search, the resulting practice parameters were based predominately on the consensus of expert opinion and survey of practicing anesthesiologists.25,26 By assigning a point value to the severity of the patient’s diagnosed sleep apnea, the invasiveness of the surgical procedure, and the anticipated perioperative opioid requirements, the group developed an algorithm to assign a risk score. For patients with suspected sleep apnea who had not undergone polysomnography, the task force developed a checklist of signs and symptoms to assist in the identification of those at risk for OSA and in the designation of a presumptive diagnosis for patients who screened positive. Although consultants provided general recommendations for postoperative monitoring for the patient at risk for OSA, they acknowledged that the literature was insufficient to support guidelines supporting the view that patients at risk for OSA could be cared for in an outpatient setting. In addition, they were equivocal with regard to whether even superficial procedures could be performed safely on patients with known or suspected OSA in an outpatient setting. There are studies, however, that suggest that OSA is not a risk factor for unplanned admission, reintubation, or serious cardiovascular or cerebrovascular adverse outcomes after ambulatory surgery.27 Joshi et al and the Society for Ambulatory Anesthesia subsequently issued guidelines for the perioperative care of the ambulatory surgical patient who is at risk for OSA.28 The recommendations focus predominately on appropriate patient selection and take into account comorbid conditions that may have resulted from the repeated episodes of hypoxemia and hypercarbia experienced by patients with moderate to severe OSA. In 2012, the Joint Commission issued a sentinel event alert tasking all hospitals to develop a policy for screening patients for respiratory risk. Various societies and individual medical institutions have implemented algorithms that are aimed at the early identification and management of the patient with OSA (Figure 1). However, none have been validated to date, and large randomized controlled trials would be challenging. Multiple screening questionnaires exist with various levels of sensitivity and specificity. One of the most commonly employed tools is the STOP-BANG (snore, tired, observed apnea, arterial pressure, body mass index, age, neck circumference, and sex) questionnaire developed by Chung, an anesthesiologist in Toronto, Ontario, Canada.29 The Flemon’s Sleep Apnea Clinical Score, the Berlin Questionnaire, and the Maislin Prediction model have all been used to help identify the surgical patient at risk for OSA and respiratory depression.30–32 Inherent in the interpretation of the results of these questionnaires is the problem of determining what is considered high risk and assigning an acceptable cut point score that would indicate the need for increased postoperative surveillance for adverse outcome.
As noted earlier, the Society of Ambulatory Anesthesia recommendations for the perioperative care of the patient with known or suspected OSA who will be undergoing an outpatient procedure28 are written with the assumption that ASCs have and will be caring for patients with OSA. The recommendations focus on identifying patients at risk of OSA, using the STOP-BANG questionnaire and optimization of comorbidities. OSA has been associated with uncontrolled systemic hypertension, increased risk for cerebrovascular disease, atrial fibrillation, dysrhythmias, pulmonary hypertension, and derangements in glucose metabolism. The patient at risk for OSA with comorbid conditions that are not optimally medically managed is not an appropriate candidate for surgery in an ambulatory venue. The patient with OSA may be considered for an ambulatory procedure if they do not have conditions that would otherwise exclude them from an outpatient venue. Patients with a diagnosis of OSA for whom continuous positive airway pressure (CPAP) therapy has been prescribed should be encouraged to use their CPAP in the perioperative period. If, because of the nature of the procedure, CPAP cannot be used postoperatively in the patient with established use, the procedure should be moved to the inpatient venue. There is evidence that abrupt cessation of CPAP in patients with a history of regular use can result in return of pretreatment symptoms, including uncontrolled hypertension as well as new-onset congestive heart failure.
There is insufficient literature to provide guidance regarding the value of perioperative CPAP therapy for patients who have not been previously prescribed the treatment. Further research is needed to determine the effects of introducing and implementing empiric CPAP therapy in the immediate perioperative period on overall outcome.
Regardless of whether the patient has known or suspected OSA, heightened observation and surveillance for hypoxemia and hypoventilation are warranted.
End-stage renal disease
End-stage renal failure is associated with a number of physiologic derangements and comorbid conditions that have the potential to carry great significance in the perioperative period.33 Hypertension, diabetes mellitus, and immune-mediated disorders have been implicated in the etiology of end-stage renal disease (ESRD), and anemia and accelerated CAD are frequently associated with chronic renal failure. In addition, patients with ESRD are commonly prescribed multiple medications and may have complicated treatment regimens. Preoperative assessment of patients with ESRD should include a detailed history of the etiology of their renal disease and identification and optimization of associated comorbidities, as well as information regarding dialysis method and schedule. The majority of ambulatory surgical patients with ESRD present to an ASC for either ophthalmologic or vascular access procedures. It is not unusual for these patients to have poor peripheral intravenous access sites, and scarring from prior dialysis access can make central venous access difficult. Preoperative collaboration with the surgeon is required to ensure that the site of the arteriovenous fistula or graft is not violated, and close communication with the nephrologist facilitates appropriate timing of perioperative dialysis. Hypotension, seizure, cardiac arrhythmia, and sudden cardiac death have all been reported during dialysis. Although most providers prefer to avoid performing surgery in the patient who is due for or has missed a dialysis session, proceeding in the period immediately after dialysis may be associated with risk as well. Peridialysis hypoxemia and hypoventilation, nausea, headache, and itching are relatively common in patients who undergo routine dialysis; therefore, it would seem prudent to schedule dialysis 12–24 hours before the planned ambulatory surgical procedure.34
It is mandatory to obtain postdialysis electrolyte values, and the most frequently seen derangements occur with regard to serum potassium. Whether the results reflect hyperkalemia secondary to inadequate elimination of potassium or hypokalemia as a result of excess removal of potassium during dialysis, the literature does not support specific guidelines for the limits of acceptable values. Although these patients tolerate substantial variations in potassium levels relatively well, those known to experience extremely high or low values may not be appropriate candidates for ambulatory surgery. Electrocardiographic manifestations of critically high levels of potassium levels constitute a clinical emergency and include widening of the QRS complex, increased QT intervals, and inverted, flattened, or peaked T waves. Atrial and ventricular arrhythmias can be seen in patients with hypokalemia and, likewise, render these patients inappropriate candidates for surgery in an ambulatory venue.
Obesity
The increasing prevalence of obesity in the United States has become a major public health problem. Although at risk for cardiovascular disease, diabetes, OSA, and hypoventilation syndrome, the obese patient should not be excluded from ambulatory surgery at an ASC. With careful patient selection, specialized equipment, and appropriate staff training, these patients can be cared for safely and successfully in an outpatient venue.
It is important to realize that there are multiple categories of obesity based on body mass index (BMI) and described by the World Health Organization.35 Although a patient with a BMI greater or equal to 30 kg/m2 is classified as obese, obesity has not been associated with adverse perioperative outcomes in the ambulatory surgical population.36 This is perhaps related to the fact that BMI cannot distinguish whether the patient has excess adipose tissue or muscle. In fact, there are a number of highly trained professional athletes who would fit into the category of morbid obesity if BMI alone is taken into account. The American Academy of Family Physicians as well the National Heart, Lung, and Blood Institute classify obesity by stages. Stage I corresponds to a BMI of 30.0–34.9 kg/m2, Stage II is 35–39.9 kg/m2, and Stage III is greater than 40 kg/m2.37,38 Additional nomenclature includes super morbid obesity (>50 kg/m2) and ultra obesity (>70 kg/m2). Other important measurements used to determine risk in this population are neck and waist circumferences. Although not historically performed during routine preoperative screening, measurement of waist circumference carries great importance in predicting predisposition to metabolic syndrome and cardiovascular disease. Individuals with a waist circumference of greater than 35 inches for a female and 40 inches for a male are at more than 5 times the risk for multiple cardiometabolic conditions than individuals with normal waist circumference, even after adjusting for BMI.39
The obese patient has reduced functional capacity and may show signs of restrictive lung disease on pulmonary function tests. Those with visceral adipose deposition have an increased load on their diaphragm, as the abdominal contents encroach on the chest during breathing. In addition, it may be difficult to determine their true functional capacity, as their exercise tolerance may be limited by joint pain and testing options may be limited by the patient’s size. Although there is no evidence that preoperative pulmonary function testing will improve outcome in obese population, patients in whom obesity hypoventilation syndrome is suspected may warrant investigation of the presence or severity of pulmonary hypertension. In addition, elevation of CO2 on simple serum chemistry may provide a clue to potential hypoventilation in the obese patient.
When scheduled for an ambulatory venue, care must be taken to ensure the presence of specialized equipment that may be required in the care of the morbidly obese patient. Upper weight limits of stretchers, wheelchairs, and operating room tables should be ascertained. Difficulty with mask ventilation and tracheal intubation should be anticipated, and specialized regional anesthesia equipment may be required. Last, extra personnel should be available to help with turning and transport of the patient.
Diabetes
The primary aim of the preoperative assessment of the patient with diabetes who is scheduled for ambulatory surgery is to evaluate the status of comorbidities associated with diabetes and to ensure appropriate perioperative blood glucose levels. Patients who have a poor understanding of their therapeutic diabetes regimens, who have uncontrolled blood sugars, or who are unable to monitor their own blood glucose levels are not appropriate candidates for surgery in an ambulatory setting. Measurement of the patient’s hemoglobin A1c levels can provide clues to their average glycemic control in the 3–4 months before their evaluation.
During the preoperative visit, the patient should be queried with regard to glycemic-related medications, timing and dosing, episodes of hyper- or hypoglycemia, and hospital admissions for issues stemming from glycemic control.
Although there is little evidence to support an acceptable upper limit of blood glucose in which it is safe to proceed with surgery, those patients with manifestations of hyperglycemia including dehydration, acidosis, or hyperosmolar states should have elective surgery postponed regardless of venue. Many facilities have developed their own guidelines for acceptable preoperative blood glucose levels. These guidelines may be based on ease and availability of intraoperative point of care testing not only for blood glucose but also for blood gas analysis and serum electrolytes, which are subject to alterations based on glycemic status.
Instructions for preoperative oral hypoglycemic and insulin administration for the day before surgery, as well on the day of surgery, should be tailored to the individual patient. The timing of the discontinuation of metformin before a surgical procedure is controversial. Current guidelines recommend the last dose of metformin be administered 8 hours before the planned procedure, secondary to concerns about risk of lactic acidosis.40 However, there is no evidence to support this practice before ambulatory surgery.41 The Society for Ambulatory Anesthesia, in their 2010 Consensus Statement on Perioperative Blood Glucose Management in Diabetic Patients Undergoing Ambulatory Surgery, recommends holding oral and noninsulin injectable hypoglycemics on the day of surgery.41 Although there are several societies that have issued recommendations for perioperative insulin therapy, most concur that long-acting insulins should be continued at full dose the night before surgery and that the morning dose be decreased to 50%–75% of the usual dose. Similarly, insulin pumps should require no change in dose the day before surgery and may be continued at a basal rate during the procedure. Intermediate-acting insulins expected to peak at 4–10 hours may require a reduced evening dose in addition to a reduced morning dose to avoid hypoglycemia, and short-acting insulin should be held on the day of the procedure. Mixed insulins can be problematic, as they may have a relatively rapid onset with a long duration of action that can be unpredictable in the setting of surgical stress.
Response to insulin varies by individual patient, and frequently, the patient or family member responsible for their care is superior at managing preoperative insulin doses if they are given a target to achieve.
Ideally, patients with diabetes should be scheduled as the first case of the day to avoid fluctuating blood sugars while fasting, and they should be instructed to identify themselves as diabetic on presentation to the facility, in case they experience hypoglycemia while awaiting preoperative preparation.
Summary
The preoperative evaluation of the ambulatory surgical patient provides the opportunity to identify and optimize potential perioperative risk factors, answer patient questions about their planned procedure, and provide them with instruction to facilitate the optimum outcome. In addition, with information obtained during the visit, the anesthesia provider can better collaborate with the surgeon to develop a sound perioperative plan of care.
Disclosure
The authors report no conflicts of interest in this work.
References
Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Report. 2009;(11):1–25. | |
Warner MA, Shields SE, Chute CG. Major morbidity and mortality within 1 month of ambulatory surgery and anesthesia. JAMA. 1993;270(12):1437–1441. | |
Keyes GR, Singer R, Iverson RE, et al. Analysis of outpatient surgery center safety using an internet-based quality improvement and peer review program. Plast Reconstr Surg. 2004;113(6):1760–1770. | |
Engbaek J, Bartholdy J, Hjortsø NC. Return hospital visits and morbidity within 60 days after day surgery: a retrospective study of 18,736 day surgical procedures. Acta Anaesthesiol Scand. 2006;50(8):911–919. | |
Apfelbaum JL, Connis RT, Nickinovich DG, et al; American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology. 2012;116(3):522–538. | |
American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Practice advisory for preanesthesia evaluation: A report by the American Society of Anesthesiologists Task Force on Preanesthesia evaluation. Anesthesiology. 2002;96(2):485–496. | |
Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;64(22):e77–e137. | |
Roberts JD, Sweitzer B. Perioperative evaluation and management of cardiac disease in the ambulatory surgery setting. Anesthesiol Clin. 2014;32(2):309–320. | |
Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–42.e1, 3. | |
Jeger RV, Probst C, Arsenic R, et al. Long-term prognostic value of the preoperative 12-lead electrocardiogram before major noncardiac surgery in coronary artery disease. Am Heart J. 2006;151(2):508–513. | |
Payne CJ, Payne AR, Gibson SC, Jardine AG, Berry C, Kingsmore DB. Is there still a role for preoperative 12-lead electrocardiography? World J Surg. 2011;35(12):2611–2616. | |
Mangano DT, London MJ, Tubau JF, et al; Study of Perioperative Ischemia Research Group. Dipyridamole thallium-201 scintigraphy as a preoperative screening test. A reexamination of its predictive potential. Circulation. 1991;84(2):493–502. | |
Devereaux PJ, Mrkobrada M, Sessler DI, et al; POISE-2 Investigators. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1494–1503. | |
Kazmers A, Cerqueira MD, Zierler RE. Perioperative and late outcome in patients with left ventricular ejection fraction of 35% or less who require major vascular surgery. J Vasc Surg. 1988;8(3):307–315. | |
Flu WJ, van Kuijk JP, Hoeks SE, et al. Prognostic implications of asymptomatic left ventricular dysfunction in patients undergoing vascular surgery. Anesthesiology. 2010;112(6):1316–1324. | |
Healy KO, Waksmonski CA, Altman RK, Stetson PD, Reyentovich A, Maurer MS. Perioperative outcome and long-term mortality for heart failure patients undergoing intermediate- and high-risk noncardiac surgery: impact of left ventricular ejection fraction. Congest Heart Fail. 2010;16(2):45–49. | |
Agarwal S, Rajamanickam A, Bajaj NS, et al. Impact of aortic stenosis on postoperative outcomes after noncardiac surgeries. Circ Cardiovasc Qual Outcomes. 2013;6(2):193–200. | |
Crossley GH, Poole JE, Rozner MA, et al. The Heart Rhythm Society (HRS)/American Society of Anesthesiologists (ASA) Expert Consensus Statement on the perioperative management of patients with implantable defibrillators, pacemakers and arrhythmia monitors: facilities and patient management this document was developed as a joint project with the American Society of Anesthesiologists (ASA), and in collaboration with the American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Heart Rhythm. 2011;8(7):1114–1154. | |
Qaseem A, Snow V, Fitterman N, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med. 2006;144(8):575–580. | |
Su FW, Beckman DB, Yarnold PA, Grammer LC. Low incidence of complications in asthmatic patients treated with preoperative corticosteroids. Allergy Asthma Proc. 2004;25(5):327–333. | |
Kabalin CS, Yarnold PR, Grammer LC. Low complication rate of corticosteroid-treated asthmatics undergoing surgical procedures. Arch Intern Med. 1995;155(13):1379–1384. | |
Pien LC, Grammer LC, Patterson R. Minimal complications in a surgical population with severe asthma receiving prophylactic corticosteroids. J Allergy Clin Immunol. 1988;82(4):696–700. | |
Memtsoudis S, Liu SS, Ma Y, et al. Perioperative pulmonary outcomes in patients with sleep apnea after noncardiac surgery. Anesth Analg. 2011;112(1):113–121. | |
Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep. 1997;20(9):705–706. | |
Gross JB, Bachenberg KL, Benumof JL, et al; American Society of Anesthesiologists Task Force on Perioperative Management. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: a report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2006;104(5):1081–1093. | |
American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Practice guidelines for the perioperative management of patients with obstructive sleep apnea: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Management of patients with obstructive sleep apnea. Anesthesiology. 2014;120(2):268–286. | |
Stierer TL, Wright C, George A, Thompson RE, Wu CL, Collop N. Risk assessment of obstructive sleep apnea in a population of patients undergoing ambulatory surgery. J Clin Sleep Med. 2010;6(5):467–472. | |
Joshi GP, Ankichetty SP, Gan TJ, Chung F. Society for Ambulatory Anesthesia consensus statement on preoperative selection of adult patients with obstructive sleep apnea scheduled for ambulatory surgery. Anesth Analg. 2012;115(5):1060–1068. | |
Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, Sun Y. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth. 2012;108(5):768–775. | |
Flemons WW, Whitelaw WA, Brant R, Remmers JE. Likelihood ratios for a sleep apnea clinical prediction rule. Am J Respir Crit Care Med. 1994;150(5 Pt 1):1279–1285. | |
Chung F, Yegneswaran B, Liao P, et al. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108(5):822–830. | |
Maislin G, Pack AI, Kribbs NB, et al. A survey screen for prediction of apnea. Sleep. 1995;18(3):158–166. | |
Cloyd JM, Ma Y, Morton JM, Kurella Tamura M, Poultsides GA, Visser BC. Does chronic kidney disease affect outcomes after major abdominal surgery? Results from the National Surgical Quality Improvement Program. J Gastrointest Surg. 2014;18(3):605–612. | |
Bansal VK, Vertuno LL. Surgery. In: Daugirdas JT, Ing TS, editors. Handbook of Dialysis. 2nd ed. Boston: Little, Brown; 1994:545–552. | |
World Health Organization. BMI Classification. Available from: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html. Accessed June 2, 2015. | |
Thomas H, Agrawal S. Systematic review of same-day laparoscopic adjustable gastric band surgery. Obes Surg. 2011;21(6):805–810. | |
Rao G. Office-based strategies for the management of obesity. Am Fam Physician. 2010;81(12):1449–1456. | |
National Heart, Lung, and Blood Institute, National Institutes of Health. Department of Health and Human Services. Available from: http://www.nhlbi.nih.gov/files/docs/guidelines/prctgd_c.pdf. Accessed June 2, 2015. | |
Shen W, Punyanitya M, Chen J, et al. Waist circumference correlates with metabolic syndrome indicators better than percentage fat. Obesity (Silver Spring). 2006;14(4):727–736. | |
Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334(9):574–579. | |
Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378–1387. |
Supplementary material
  | Figure S1 Preoperative screening tool. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.