Back to Journals » Clinical Ophthalmology » Volume 11
Preoperative subconjunctival combined injection of bevacizumab and mitomycin C before the surgical excision of primary pterygium: clinical and histological results
Authors Alsmman AH , Radwan G , Abozaid MA , Mohammed UA , Abd Elhaliem NG
Received 15 November 2016
Accepted for publication 20 February 2017
Published 10 March 2017 Volume 2017:11 Pages 493—501
DOI https://doi.org/10.2147/OPTH.S127700
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Alahmady Hamad Alsmman,1 Gamal Radwan,1 Mortada Ahmed Abozaid,1 Usama Ali Mohammed,1 Nesreen Gamal Abd Elhaleim2
1Department of Ophthalmology, 2Department of Histology, Sohag Faculty of Medicine, Sohag University, Sohag, Egypt
Purpose: The aim of this study was to detect the clinical and histological effects of preoperative subconjunctival injection of both bevacizumab and mitomycin C (MMC) 1 month before the surgical excision of primary pterygium using a bare sclera technique.
Patients and methods: A total of 20 patients with primary pterygium underwent subconjunctival combined injection of 0.1 mL of MMC (0.1 mg/mL) and 0.1 mL of bevacizumab (1.25 mg/0.1 mL) 1 month before bare sclera excision of the pterygium. The excised pterygium tissues were examined histologically and immunohistologically by CD31 staining, and the patients were followed up clinically for at least 2 years. The excised pterygia of two patients without preoperative injection were used for histological comparison.
Results: Clinically, there were no intraoperative or postoperative complications. No recurrence was noted during the follow-up period. Histologically, the previously injected pterygia showed a decreased number of epithelial cells and stromal fibroblasts. The latter were rounded or oval and swollen rather than spindle shaped, and some were degenerating or apoptotic. Collagen and elastic fibers were degenerated, distorted, and decreased in density, while blood capillaries were obliterated. There was a significant decrease in CD31-positive cells in previously injected pterygia.
Conclusion: Preoperative subpterygium combined injection of bevacizumab and MMC is safe and effective in reducing the postoperative recurrence of primary pterygium. Histological and immunohistological changes in the form of decreased fibrovascular activity and degeneration of the extracellular matrix and nerve axons were noted.
Keywords: subconjunctival bevacizumab, subconjunctival mitomycin C, histological changes, primary pterygium, CD31
Introduction
Pterygium is a benign fibrovascular growth of the conjunctiva over the cornea. It is a common disease in tropical and subtropical regions with a worldwide prevalence of 2%–7%.1 Although historically described as a degenerative condition, it is more closely associated with inflammation and progressive fibrovascular proliferation.2
A pterygium commonly grows from the nasal side of the bulbar conjunctiva within the palpebral fissure and is usually associated with ultraviolet light exposure (eg, sunlight), dry weather, and dust. Symptoms of pterygium include persistent redness, foreign body sensation, tearing, and dry and itchy eyes. In advanced cases, the pterygium may affect vision through obscuring the optical center and inducing astigmatism and corneal scarring.3
The pathogenesis of pterygium includes inflammation, fibrovascular proliferation, and angiogenesis, which is responsible for pterygium formation and progression.4 Several studies confirmed that increased levels of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), transforming growth factor-beta (TGF-β), and platelet-derived growth factor secreted by fibroblasts and inflammatory cells are associated with the primary formation and recurrence of pterygia.5
Lee et al6 confirmed that VEGF is selectively elaborated by two cells, T-helper and type 2 lymphocytes.
Formation of new blood vessels from preexisting vasculature is called angiogenesis, and it is involved in a large number of physiological processes, such as growth and differentiation, ovulation, and would healing. It may also be involved in pathological conditions, such as neoplasia and proliferative eye diseases, such as proliferative diabetic retinopathy and neovascular glaucoma, which cause severe visual loss.7 New vascularization is associated with the activation of cell-derived angiogenic factors with synthesis of extracellular matrix responsible for anchorage of migrating endothelium.8
The surgical treatment of pterygium focuses on the excision and prevention of recurrence.9 Recurrent pterygia are more aggressive and dangerous than primary ones because the underlying cornea may be thinner, the extensive proliferation adversely affects visual acuity, and further recurrence after the second surgery is common.10
The recurrence is mainly due to accelerated fibroblastic proliferation produced by the trauma of operation much in the same way as the production of keloid tissue. Fibroblastic proliferation and invasion adequately explain the clinical appearance and behavior of a pterygium with some histological support.2
Mitomycin C (MMC) is an alkylating agent with cytotoxic effects, which inhibits DNA synthesis and is widely used in ophthalmology. MMC leads to the death of cells caused by the inability to repair the genotoxic injury caused by alkylation. It acts against all cells regardless of the cell cycle and even acts in cells that are not synthesizing DNA. Inhibition of DNA synthesis leads to inhibition of mitoses, especially when MMC comes into contact with cells that are in the late G1 and early S phases of the cell cycle.11–13 The topical application of MMC following the excision of pterygium can reduce the rate of recurrence. However, its apoptotic effects can cause serious complications such as scleral thinning and ischemia.14 With subconjunctival injection of MMC, the epithelial and scleral toxicities can be diminished. The subconjunctival route also allows exact dose delivery, which is equivalent to approximately one drop of 0.2 mg/mL of MMC, rather than the inexact and substantially higher dosing with sponge delivery during ocular surgery.15 Bevacizumab (Avastin®; Genentech, Inc, San Francisco, CA, USA) is a humanized monoclonal antibody to VEGF used by intravenous route and approved mainly for the treatment of colorectal cancer.16 Various clinical studies across the world applied bevacizumab for intravitreal administration and confirmed its safety and efficacy in wet age-related macular degeneration and macularedema.17
In patients with impending recurrent pterygium, marked regression of limbal-conjunctival neovessels and long-standing delayed recurrence have been reported with the use of topical bevacizumab.18
We thought of coupling the antifibrotic effect of MMC with the antivascular effect of bevacizumab in order to obtain a lower recurrence rate than that obtained by either of the two drugs alone with reduction of MMC concentration to decrease the complications. Hence, this study aimed to determine the safety, efficacy, and histological effects of subconjunctival combined injection of both bevacizumab and MMC 1 month before the excision of the primary pterygium using the bare sclera technique.
Patients and methods
This prospective study was conducted at the Department of Ophthalmology of Sohag University Hospital, Egypt, in the period between January and June 2013. Twenty eyes of 20 patients with primary pterygia measuring ≥4.5 mm2 in the surface area were included in this study. The exclusion criteria included patients with other ocular diseases, eg, iritis, herpetic keratitis, glaucoma, and cataract or previous ocular surgery, including previous pterygium surgery, as well as any predisposing condition to ulceration or poor wound healing, such as dry eye or atopic keratoconjunctivitis. Pregnant or lactating women and patients with a history of strokes or thrombus elsewhere in the body were also excluded.
This study followed the tenets of the Declaration of Helsinki, and an approval was obtained from the ethics committee of Sohag Faculty of Medicine. A written informed consent was signed by every patient after full explanation of the technique and the possible complications of the procedure.
A complete ocular examination, including measurement of the uncorrected and best corrected visual acuity and refractive state, and a photographic documentation of the pterygia were performed for each patient before surgery. The surface area of the pterygium in square millimeters was calculated by multiplying the height of the pterygium by half its base, with the height measured as the distance from the apex to the limbus and the base measured at the limbus.
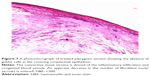
One month before the surgery, a subpterygium injection of 0.1 mL of MMC (0.1 mg/mL) with 0.1 mL of Avastin (1.25 mg/0.1 mL) using an insulin syringe under topical anesthesia was done (Figure 1). The MMC was freshly prepared on the day of injection using sterile distilled water as a diluent to achieve a concentration of 0.1 mg/mL.
  | Figure 1 Subpterygium injection of MMC and bevacizumab by insulin syringe under topical anesthesia. |
All patients were operated by the first author. Each pterygium was excised, and the bare sclera was minimally cauterized if needed.
All the excised tissues were sent for histological and immunohistological analyses. The findings were compared with the histological features of pterygia of two patients who did not have preoperative injection of Avastin or MMC and rather had conjunctival autografts to prevent recurrence. These two patients used as control for histological comparison had the same exclusion criteria as the preinjected patients. The excised pterygia were washed with saline and immersed in 10% neutral formalin. Fixed tissues were processed for preparation of serial paraffin sections of 5 μm thickness that were subjected to19
- Hematoxylin and eosin staining for general histological examination.
- Light green staining for collagen fibers
- Immunohistochemical staining for CD31 antibody to assess the microvessel density by avidin–biotin–peroxidase complex method. The tissues were deparaffinized in xylene and then rehydrated. They were immersed in citrate buffer (0.1 mL, pH 0.6) and put in the microwave for 12 min. After that, all sections were treated for 10 min with 0.3% hydrogen peroxide. CD31 monoclonal antibody (dilution 1/50; Dako) was applied overnight at 4°C, and then, the process was completed with the addition of secondary antibody. Positive control slides were included in all cases, as well as negative control (omitting the step of primary antibody). The reaction appeared as brownish membranous reaction.
Morphometric and statistical analyses
All measurements were taken using the image analyzer (Leica Q 500 MC program; Leica, Wetzlar, Germany) in the Department of Histology, Sohag Faculty of Medicine, Sohag University. Examinations were performed under five high-power fields per five different sections in each specimen. Microvessel density was evaluated by CD31 staining of vascular endothelial cells as described by Aspiotis et al.20
Histological statistical analyses were performed using a paired t-test (SPSS program, Version 17; IBM Corporation, Somers, NY, USA), and the results are represented as mean ± standard error.
Postoperative topical antibiotic (moxifloxacin 0.5%) was administered four times daily for 1 week, and steroid (prednisolone acetate 0.5%) was administered four times daily with gradual withdrawal for 6 weeks. All patients were examined after 1 day, 1 week, 1 month, 3 months, and then every 6 months for 2 years. The postoperative recurrence was classified into the following four grades:9
Grade 1: no vessels at the surgical site with healthy normal conjunctiva.
Grade 2: fine episcleral vessels at the surgical site l with healthy cojunctiva.
Grade 3: fibrovascular tissue localized to the area of excision not extending to the cornea; however, the fibrovascular proliferation impending actual recurrence.
Grade 4: a true recurrence with fibrovascular tissue covering the excision area and invading the cornea.
In this study, we considered the first and second grades as no recurrence, while the third grade was considered as an early recurrence and the fourth grade as recurrent pterygium.
Results
This study included 20 eyes of 20 patients with primary pterygia, including 13 males (65% of 20 patients) and 7 females (35% of 20 patients) with the age ranging from 44 to 68 years (mean of 58±2.8 years).
The affected eye was right in 12 eyes (60% of 20 patients) and left in 8 eyes (40% of 20 patients), and the follow-up period ranged from 25 to 36 months with a mean of 30±3.4 months.
The preoperative spherical refractive error ranged between −4.75 and +3.25 D (mean −1.2±0.6 D), while cylindrical error ranged between −4.00 and +5.50 D (mean +3.3±1.3 D). The surface area of the pterygium ranged from 5 to 12.5 mm2 with a mean of 8±2.1 mm2. The follow-up period ranged from 26 to 36 months with a mean of 30.4±3.6 months. Table 1 shows the preoperative data of 20 patients involved in our study, while Table 2 shows the data of the two cases used as control for histological comparison.
  | Table 1 Preoperative data of 20 patients involved in our study |
  | Table 2 The data of the two cases used as control |
On the first postoperative day, all patients had corneal epithelial defects, which healed completely within 1 week with no corneal fluorescein staining. Diffuse punctate epithelial erosions with excessive photophobia was seen in one eye (5%) that resolved with topical lubricants within 1 month postoperatively.
Subconjunctival hemorrhage during sublesional injection occurred in one case (5%), persisted for 2 weeks, and resolved spontaneously.
As regards the postoperative recurrence (Figure 2),
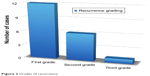  | Figure 2 Grades of recurrence. |
- Of the 19 eyes, 12 eyes (60% of 20 patients) with no recurrence were classified as first grade and 7 eyes (35% of 20 patients) were classified as second grade.
- One eye (5% of 20 patients) was classified as third stage (early recurrence): this case with early recurrence was the same one that had subconjunctival hemorrhage during preoperative injection. This patient was followed up for 33 months without progression from the third to the fourth stage.
- No cases were classified as fourth stage of recurrence.
- The two patients used as control for histological comparison were followed up for 24 months, with no recurrence detected and classified as grade 1.
There were no serious complications, such as scleral thinning or melting, corneal decompensation, postoperative iritis, cataract, and glaucoma, detected in any patient during the follow-up period.
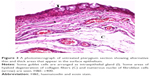
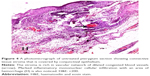
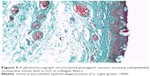
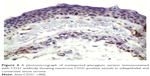
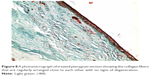
Histological comparison was conducted between the noninjected and preinjected pterygia. The noninjected pterygia sections showed alternative thin and thick areas in the surface epithelium. Some goblet cells were arranged as intraepithelial glands. Some areas of hyaloid degeneration of collagen fibers and numerous nuclei of fibroblast cells were seen (Figures 3–5). Using CD31 staining, more intense angiogenic activity was observed in the conjunctival tissue, especially at the subepithelial area (Figure 6). The preinjected pterygia sections had an apparent decrease in the number of goblet and fibroblast cells with less inflammatory cellular infiltration and absence of neovascularization (Figures 7 and 8). CD31 staining also revealed an apparent decrease in the positive immunostained cells (Figure 9).
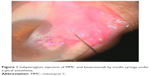
  | Figure 8 A photomicrograph of treated pterygium section showing the collagen fibers that are regularly arranged close to each other with no signs of degeneration. |
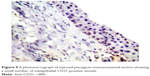  | Figure 9 A photomicrograph of injected pterygium-immunostained section showing a small number of subepithelial CD31-positive vessels. |
There was also a significant increase in the mean of area percentage of collagen fibers in the treated pterygia (30.51±0.9) compared to that in the untreated group (16.07±1.2), as well as a significant decrease in microvessel density in the injected pterygia (10.57±1.4) compared to that in the noninjected ones (21.83±1.8) (P≤0.05) (Figure 10).
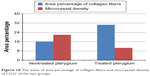  | Figure 10 The mean of area percentage of collagen fibers and microvessel density of CD31 of the two groups. |
Discussion
Pterygium is a benign fibrovascular growth of the conjunctiva over the cornea. Due to high prevalence in the world, especially in our tropical sunny and dusty countries with high recurrence rate, pterygium has always been a major problem for ophthalmologists.21 In this study, we tried to overcome this problem by the use of two famous drugs widely used in ophthalmology. The first drug is MMC with well-known antifibroblastic activity, which has been used intraoperatively since 1988 as an adjunctive therapy during pterygium excision to prevent recurrence.22 The second drug is bevacizumab with anti-VEGF activity to reduce the vascularization, which is the main cause of recurrence.23 The strength of the current study is using a combination of bevacizumab and MMC subconjunctivally before the bare sclera excision of primary pterygium. One eye (4%) was complicated by subconjunctival hemorrhage that resolved within 2 weeks. Another eye (4%) was complicated by punctate epithelial erosions that resolved within 1 month with lubricants and steroids. As regards the recurrence rate, there was only one case (4%) with fibrovascular proliferation at the site of excision without crossing the limbus into the cornea. This is considered as no recurrence that appeared after 13 months, increased slightly within 33 months, and occurred in the same eye that was complicated by intraoperative subconjunctival hemorrhage, which may suggest that the coagulated blood enhanced the fibroblastic activity. In this study, we decreased the concentration of MMC to avoid serious ophthalmological complications as we used 0.1/mL, which is lower than the concentration used in most of the previous studies, ranging from 0.2 to 0.15/mL. We also applied both drugs through the subconjunctival route to control the dose and decrease the effect on the ocular surface. No serious complications were reported. The lack of recurrence is probably due to the use of combination of two drugs with synergistic effect, while the low concentration of MMC (0.1 mg/mL) decreased the incidence of complications.
In order to increase its efficacy and decrease its epithelial toxicity, Donnenfeld et al15 injected MMC in the head of pterygium 1 month before pterygium excision. They delivered MMC subconjunctivally to minimize the exposure to the ocular surface and reduce the epithelial stem cell toxicity associated with topical application. They used 0.1 mL of 0.15 mg/mL concentration because this concentration is slightly higher than the therapeutic window but well below the toxic level associated with cell death. They included 36 eyes in their study and reported recurrence in two patients (6%) after a mean follow-up period of 24.4 months. The route of administration of the drug matches with our study; however, the concentration of MMC in our study was reduced by 50% to avoid complications. The recurrence rate is less in the current study in comparison to theirs; however, the number of cases is less in our study.
Ghoneim et al24 compared the efficacy of a preoperative subconjunctival injection of 0.15 mg/mL of MMC 1 day before the bare sclera excision with an intraoperative application of MMC. In their study, the rates of recurrence were 5.7% in the previously injected pterygia and 8.57% in topical application after 1 year of follow-up. The complications in their study were scleral thinning that occurred in one eye in each group and resolved by 5 months postoperatively. In contrast to our study, no recurrence or scleral thinning occurred with longer follow-up period due to the synergistic effect of both drugs and the decreased concentration of MMC.
VEGF has been shown to be increased in the pterygium tissue and may be strongly involved in its pathogenesis,25,26 and hence an anti-VEGF therapy may be used for inducing regression of blood vessels and size of pterygium and prevention of recurrence.27
Teng et al28 were the first to report a case of subconjunctival injection of bevacizumab in primary pterygium. They injected 1.25/0.05 mL of bevacizumab subconjunctivally into an inflamed primary pterygium at the limbus. Although the signs and symptoms of inflammation and neovascularization regressed rapidly, the effect was short term, lasting ~2 weeks. The transient effect was likely related to the limited bioavailability of the drug in the setting of continued VEGF expression. In the current study, we surgically removed the pterygia after injection using the bare scleral technique as we thought that it was a radical solution to the problem.
Shenasi et al29 in their study after subconjunctival bevacizumab injection immediately after the excision of primary pterygium found that the recurrence rate was lower in these patients. In the current study, we thought that 1 month before surgery allowed good time for drug application and tissue preparation than intraoperative use. In their randomized clinical trial, Razeghinejad and Banifatemi30 performed pterygium excision with a rotational conjunctival flap for 44 patients with primary pterygium. The patients were randomized into the following two groups: group 1 received a total of 7.5 mg subconjunctival bevacizumab (5 mg/0.2 mL on the day of surgery and 2.5 mg/0.1 mL on the fourth day after surgery) and group 2 received subconjunctival balanced salt solution in the same manner. After 6 months, four patients in group 1 versus eight patients in group 2 (P=0.17) had fibrovascular tissue crossing the limbus. They concluded that the frequency of fibrovascular tissue crossing the limbus in the bevacizumab group was half that in the BSS group; however, the difference failed to reach a statistically significant level. The concentration of bivacizumab used was higher than that used in our study, with higher recurrence rate, which may be due to the use of MMC in our study.
In contrast to our study and most of the previous studies on the use of bivacizumab in pterygium surgery, Maha et al31 and Mohamed et al32 found that a subconjunctival injection of 1.25 mg/0.05 mL of bevacizumab at the end of the surgery did not result in a statistically significant difference in the recurrence rates or postoperative conjunctival erythema with discomfort, lacrimation, photophobia, or healing of corneal epithelial defects after pterygium excision.
The histological examination of noninjected patients showed that the covering epithelium containing numerous goblet cells collected in the form of intraepithelial glands. This might be due to the exposure to ultraviolet rays and dust particles in the air, which are the main predisposing factors for pterygium. The stroma showed inflammatory mononuclear cellular infiltration with dilated congested blood vessels and areas of degeneration of collagenous fibers. Similar findings were confirmed ultrastructurally by Chang et al33 after subconjunctival injection of 0.1 mL of 0.15 mg/mL of MMC 1 month before the excision of pterygium.
In this study, the presence of abundant CD31-positive microvessels in noninjected pterygium was consistent with the previous reports of Aspiotis et al.20
They considered that neoangiogenesis is an important pathogenic factor of pterygium. Previous studies added that inflammatory cells in the stroma of pterygium secrete proinflammatory cytokines that lead to extracellular matrix remodeling and angiogenesis. Growth factors with potent angiogenic activity have been found to be secreted from fibroblasts and inflammatory pterygium cells.5 In the current study, the usage of MMC and bevacizumab led to a significant decrease in CD31-positive cells and an apparent decrease in the inflammatory cellular infiltration and the number of fibroblasts and goblet cells. There was a significant increase in the area percentage of collagen fibers. This could be secondary to the anti-VEGF action of bevacizumab and the antifibroblastic activity of MMC.22,34
All the previous studies focused on the use of only one of these drugs to decrease the recurrence of pterygium. To the best of our knowledge, this study is the first to inject a combination of bevacizumab and MMC subconjunctivally before bare sclera excision of the primary pterygium.
Conclusion
The combined subconjunctival injection of bevacizumab and MMC 1 month before bare sclera excision of the primary pterygium is a safe and effective procedure that can further decrease the rate of recurrence without increasing the rate of complications. However, larger scale studies with longer follow-up periods are needed to prove this.
Disclosure
The authors report no conflicts of interest in this work.
References
Hill JC, Maske R. Pathogenesis of pterygium. Eye (Lond). 1989;3(pt 2):218–226. | ||
Cameron ME. Histology of pterygium: an electron microscopic study. Br J Ophthalmol. 1983;67(9):604–608. | ||
Kunimoto D, Kanitkar K, Makar M. The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2004:50–51. | ||
Mauro J, Foster CS. Pterygia: pathogenesis and the role of subconjunctival bevacizumab in treatment. Semin Ophthalmol. 2009;24(3):130–134. | ||
Kria L, Ohira A, Amemiya T. Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-beta and tumor necrosis factor-alpha in the pterygium. Acta Histochem. 1996;98(2):195–201. | ||
Lee CG, Link H, Baluk P, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10(10):1095–1103. | ||
Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235(4787):442–447. | ||
Tsanou E, Ioachim E, Stefaniotou M, et al. Immunohistochemical study of angiogenesis and proliferative activity in epiretinal membranes. Int J Clin Pract. 2005;59(10):1157–1161. | ||
Tan DT, Chee SP, Dear KB, Lim AS. Effect of pterygium morphology on pterygium recurrence in a controlled trial comparing conjunctival autografting with bare sclera excision. Arch Ophthalmol. 1997;115(10):1235–1240. Erratum in: Arch Ophthalmol. 1998;116(4):552. | ||
Busin M, Halliday BL, Arffa RC, McDonald MB, Kaufman HE. Precarved lyophilized tissue for lamellar keratoplasty in recurrent pterygium. Am J Ophthalmol. 1986;102(2):222–227. | ||
Kumari R, Sharma A, Ajay AK, Bhat MK. Mitomycin C induces bystander killing in homogeneous and heterogeneous hepatoma cellular models. Mol Cancer. 2009;8:87. | ||
Wang XY, Crowston JG, White AJ, Zoellner H, Healey PR. Interferon-alpha and interferon-gamma modulate Fas-mediated apoptosis in mitomycin-C-resistant human Tenon’s fibroblasts. Clin Experiment Ophthalmol. 2014;42(6):529–538. | ||
Nassiri N, Farahangiz S, Rahnavardi M, Rahmani L, Nassiri N. Corneal endothelial cell injury induced by mitomycin-C in photorefractive keratectomy: nonrandomized controlled trial. J Cataract Refract Surg. 2008;34(6):902–908. | ||
Katircioglu YA, Altiparmak U, Engur Goktas S, Cakir B, Singar E, Ornek F. Comparison of two techniques for the treatment of recurrent pterygium: amniotic membrane vs conjunctival autograft combined with mitomycin C. Semin Ophthalmol. 2015;30(5–6):321–327. | ||
Donnenfeld ED, Perry HD, Fromer S, Doshi S, Solomon R, Biser S. Subconjunctival mitomycin C as adjunctive therapy before pterygium excision. Ophthalmology. 2003;110(5):1012–1026. | ||
Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. | ||
Rosenfeld PJ, Moshfeghi AA, Puliafito CA. Optical coherence tomography findings after an intravitreal injection of bevacizumab (Avastin) for neovascular age related macular degeneration. Ophthalmic Surg Laser Imaging. 2005;36(4):331–335. | ||
Wu PC, Kuo HK, Tai MH, Shin SJ. Topical bevacizumab eye drops for limbal-conjunctival neovascularization in impending recurrent pterygium. Cornea. 2009;28(1):103–104. | ||
Bancroft JD, Gamble M, editors. Theory and Practice of Histological Techniques. 2nd ed. London: Churchill Livingstone; 2002. | ||
Aspiotis M, Tsanou E, Gorezis S, et al. Angiogenesis in pterygium: study of microvessel density, vascular endothelial growth factor, and thrombospondin-1. Eye (Lond). 2007;21(8):1095–1101. | ||
Liang QF, Xu L, Jin XY, You QS, Yang XH, Cui TT. Epidemiology of pterygium in aged rural population of Beijing, China. Chin Med J (Engl). 2010;123(13):1699–1701. | ||
Tomasz M. Mitomycin C: small, fast and deadly (but very selective). Chem Biol. 1995;2(9):575–579. | ||
Hosseini H, Nejabat M, Khalili MR. Bevacizumab (Avastin) as a potential novel adjunct in the management of pterygia. Med Hypotheses. 2007;69(4):925–927. | ||
Ghoneim EM, Abd-El Ghny AA, Gab-Allah AA, Kamal MZ. Preoperative subconjunctival injection of mitomycin C versus intraoperative topical application as an adjunctive treatment for surgical removal of primary pterygium. Middle East Afr J Ophthalmol. 2011;18(1):37–41. | ||
Marcovich AL, Morad Y, Sandbank J, et al. Angiogenesis in pterygium: morphometric and immunohistochemical study. Curr Eye Res. 2002;25(1):17–22. | ||
Lee DH, Cho HJ, Kim JT, Choi JS, Joo CK. Expression of vascular endothelial growth factor and inducible nitric oxide synthase in pterygia. Cornea. 2001;20(7):738–742. | ||
Gebhardt M, Mentlein R, Schaudig U, et al. Differential expression of vascular endothelial growth factor implies limbal origin of pterygia. Ophthalmology. 2005;112(6):1023–1030. | ||
Teng CC, Patel NN, Jacobson L. Effect of subconjunctival bevacizumab on primary pterygium. Cornea. 2009;28(4):468–470. | ||
Shenasi A, Mousavi F, Shoa-Ahari S, Rahimi-Ardabili B, Fouladi RF. Subconjunctival bevacizumab immediately after excision of primary pterygium: the first clinical trial. Cornea. 2011;30(11):1219–1222. | ||
Razeghinejad M, Banifatemi M. Subconjunctival bevacizumab for primary pterygium excision; a randomized clinical trial. J Ophthalmic Vis Res. 2014;9(1):22–30. | ||
Maha M, Amal M, Mohamed M. Intraoperative subconjunctival bevacizumab as an adjunctive treatment in primary pterygium: a preliminary report. Ophthalmic Surg Lasers Imaging. 2012;43(6):459–466. | ||
Mohammad R, Hamid H, Farzin A, et al. Preliminary results of subconjunctival bevacizumab in primary pterygium excision. Ophthalmic Res. 2010;43:134–138. | ||
Chang YS, Chen WC, Tseng SH, Sze CI, Wu CL. Subconjunctival mitomycin C before pterygium excision an ultrastructural study. Cornea. 2008;27(4):471–475. | ||
Pasqualetti G, Danesi R, Del Tacca M, Bocci G. Vascular endothelial growth factor pharmacogenetics: a new perspective for anti-angiogenic therapy. Pharmacogenomics. 2007;8(1):49–66. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.