Back to Journals » Integrated Blood Pressure Control » Volume 10
Preeclampsia and cardiovascular disease: interconnected paths that enable detection of the subclinical stages of obstetric and cardiovascular diseases
Authors Valdés G
Received 19 April 2017
Accepted for publication 12 July 2017
Published 28 August 2017 Volume 2017:10 Pages 17—23
DOI https://doi.org/10.2147/IBPC.S138383
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Steven Atlas
Gloria Valdés
Department of Nephrology, Facultad de Medicina, Pontificia Universidad Católica, Santiago, Chile
Abstract: The potent and now longstanding evidence of the association between placentation-related disorders and cardiovascular disease should be translated into clinical practice in order to introduce a preventive approach to future obstetric and cardiovascular diseases. The purpose of this review is to integrate cardiovascular risk/disease and obstetric complications, which are linked by endothelial dysfunction, as windows of opportunity for improving women’s health. Questionnaires adaptable to local practices are proposed to incorporate cardiovascular and obstetrical indexes into two stages of a woman’s lifetime.
Keywords: pregnancy, preeclampsia, placentation-related disorders, cardiovascular disease, cardiovascular stress test, prevention, endothelial dysfunction, women’s health
Plain language summary
This review concentrates the evidence accumulated since the mid-1990s showing that women who suffer hypertensive pregnancies or have premature and/or low-weight babies are at an increased risk of presenting later in life with hypertension and cardiovascular events, such as coronary artery disease, stroke, obstruction of the carotid and lower limb arteries, cardiac failure, and thromboembolism. I intend to reinforce the need for obstetricians to recognize this association to ensure that cardiovascular risks (CVRs) are identified after a complicated pregnancy, to introduce early prevention of cardiovascular disease. It should also stimulate cardiologists to incorporate the outcomes of previous pregnancies when gauging the risk of women consulting for symptoms of myocardial ischemia or infarction. This summary focuses on making women aware that a hypertensive pregnancy is not a one-in-a-lifetime event, but a marker of future gestational complications and cardiovascular events after they lose the protection of estrogen. Though much more research is needed, the triad of women, their obstetricians/gynecologists, and cardiologists can do much to improve health in women and their offspring, who tend to have increased CVR when exposed to an unfavorable intrauterine environment.
Introduction
Since the first associations of preeclampsia with cardiovascular disease (CVD) in the mid-1990s and the start of the 21st century, numerous publications have corroborated this finding and added associations with other placentation-related disorders, including recurrent abortions, intrauterine growth retardation, preterm labor, abruptio placenta, and stillbirths.1–16 The risk of CVD increases according to the clinical severity of the maternal and fetal manifestations, as demonstrated by a prospective registry follow-up of 506,350 women in Norway in whom the presentation of major coronary events increased 2.1-fold with previous preeclampsia, while adding intrauterine growth retardation or preterm birth augmented this risk to 3.3- and 5.4-fold risk, respectively.16 Coronary calcification, a precedent of coronary artery disease, is increased three decades after a preeclamptic pregnancy.17 Women submitted to angiography for suspected coronary lesions who had previous hypertensive pregnancies presented earlier with clinical manifestations and an increased number of stenotic arteries compared to women with normotensive pregnancies.18
Systematic reviews and meta-analyses represent potent tools for integrating information and contribute to its rational application and are especially relevant to this issue. In the last decade, four such papers have addressed and proven the association of preeclampsia with CVD with remarkable consistency (Table 1).19–22 However, CVR screening has not been incorporated into the preconception or early pregnancy clinical and laboratory evaluations to permit the correction of modifiable factors in order to reduce the risk of gestational hypertension. The short-term and medium-term follow-up of women with hypertension in pregnancy is only performed by a few post-preeclampsia clinics.23,24
Moreover, no retrospective evaluation of the obstetric history is routinely obtained in women presenting with symptoms of CVD several decades after the reproductive period, though the American Heart and Stroke Associations have stressed the remote risk of a complication of pregnancy.25,26 This is especially important for improving the screening for ischemic heart disease, the leading cause of death in women, which remains underdiagnosed and undermanaged for a number of reasons. The main confounding factors are the surreptitious clinical presentation of coronary artery disease in females, the maintenance of the current biochemical and electrocardiographic criteria applied to men, the lesser frequency of coronary arterial lesions, and the presence of microvascular dysfunction, which in the absence of obstructive lesions presents major adverse outcomes in 30% of patients during follow-up.27–29
This review represents a call to action to translate epidemiological, clinical, and molecular biology research into patient care, stressing the recent appeal of Arabin and Baschat.14 The future diffusion of preventive programs will benefit mothers who failed an early stress test, as well as the offspring exposed to an unfavorable intrauterine environment, thus perpetuating the cycle generated by an unfavorable pregnancy and CVD.30,31
Pathophysiology
Apart from epidemiological studies that support the association of placentation-derived disorders (hereafter represented by preeclampsia) with CVD, many pathophysiological conditions are common to both: arterial hypertension, obesity, insulin resistance, diabetes, hyperlipidemia, oxidative stress, inflammation (autoimmune or infectious), snoring, familial premature CVD and CVD-related genotypes.11,32–34 Additional common features are the mild to moderate preventive effect of aspirin in pregnancy and coronary artery disease late in life and the atherosclerotic plaques and atherosis.35–38 In contrast, deficient endometrial preconditioning in adolescence, increased placental mass in multiple pregnancies, and smoking, which benefits from the reduction of sFLT1, differ between preeclampsia and CVD.39,40
Healthy endothelial cells throughout life protect from atherosclerosis and its clinical cardiovascular complications through nitric oxide and related vasoactive factors by modulating blood flow and preventing platelet aggregation, fibrosis, proliferation of vascular smooth muscle, monocyte infiltration, and lipid acummulation.41 During the reproductive stage, endothelial function is primordial in the uteroplacental and systemic adaptations of pregnancy. In the presence of a responsive endothelium, the remodeling of the spiral arteries is primed by the extravillous trophoblast approaching and transforming its nearby spiral artery before invading its wall, subsequently replacing its muscle layer to finally lodge in its luminal border.42,43 On the other hand, defective maternal endothelial function has been posited to impair the paracrine response of the spiral arteries to nitric oxide generated by the extravillous trophoblast, impairing their remodeling into saccular vessels that provide appropriate placental perfusion.44 Shallow remodeling causes placental ischemia and increased deportation of placental debris into the maternal circulation, which accentuates the endothelial dysfunction secondary to preexisting CVR and the imbalance between vasoconstrictive and vasodilatory factors.45,46 Glitches in the processes of adaptation, added to the expanded maternal blood volume and challenges to cardiac function, expose a failure in the systemic and local adaptations of pregnancy, presenting decades later as CVD.49 The predictive capacity of future cardiovascular resilience or susceptibility has the advantage of being comprised of the relatively long duration of pregnancy, the extended area of maternal and placental endothelium (syncytiotrophoblast), and the gestational “excursions into the metabolic syndrome”.5 However, the validity of this prolonged “stress test”, and the “windows of opportunities” it provides, is not currently heeded.
Endothelial dysfunction is characterized by flow-mediated vasodilation and has been found to precede preeclampsia, persisting up to 3 years postpartum.47 Of four studies with a longer follow-up, one including 47% of subjects with severe preeclampsia found persistence of endothelial dysfunction after 10–20 years.48 Another study with a mean follow-up of 6 years detected decreased flow-mediated vasodilation, microalbuminuria, increased uric acid, and a 32% prevalence of chronic hypertension in previous preeclamptics compared to no hypertension in control subjects.49 The remaining two had biochemical indexes indicative of vascular activation 5–11 years after the index pregnancy.50,51 The value of biochemical markers of endothelial dysfunction was validated by a review and meta-analysis of 65 studies that tested vascular dysfunction by an array of images and/or biochemical markers in 3,356 cases of pregnancy-induced hypertension and 5,346 controls, as women with prior hypertensive pregnancies had vascular functional and structural abnormalities that derive from endothelial dysfunction.52 Though the endothelial dysfunction is usually considered a remnant of the injury provoked by the factors deported by the placenta to the maternal circulation, the fact that endothelial dysfunction is present after pregnancy in women with recurrent abortions supports its causal role because they were exposed intermittently to minimal placental factors.44 This postulate is reinforced by the presence of preconceptional CVRs linked to endothelial dysfunction in women presenting with a hypertensive pregnancy or preeclampsia (familial, pre- and gestational diabetes mellitus, familial premature myocardial infarction, overweight, obesity, elevated blood pressure, leukocyte count, and elevated triglycerides).53–56
Endothelial dysfunction became central to preeclampsia when Roberts et al57 and Redman et al58 presented the novel concept that preeclampsia is a two-stage syndrome that begins with defective spiral artery remodeling leading to placental underperfusion and the clinical syndrome provoked by endothelial injury due to cytotoxic, inflammatory, oxidative, and immunological cytotoxic factors. Later, an intermediate stage was added to include placental ischemia/reperfusion and probable mechanic damage of the blood jet entering the intervillous space.59,60 Finally, a recent study has attributed a determinant role of decidualization, stretching the concept of a multistage syndrome to five phases while substantiating the potential effect of deficient endometrial preconditioning in teenage pregnancy.39,61
Therefore, I propose integrating the pathophysiology and preconceptional and postgestational management to emphasize a clinical continuum that provides several stages for screening and preventing complications of subsequent pregnancies, as well as CVR and CVD (Figure 1). Thus, introducing CV prevention during the reproductive phase will translate current evidence into routine obstetric practice.
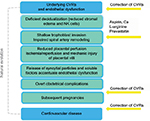  | Figure 1 Diagram depicting the stages of preeclampsia integrated to subsequent pregnancies and cardiovascular risk and disease in order to install preventive measures and impact women’s health. Notes: The natural evolution of CVRs to disease is purposefully depicted in gray, as an active preventive strategy should reduce the spontaneous transition. The different gestational stages are framed in blue to highlight the fact that underlying maternal factors enhance impaired vascular remodeling and its alterations. Yellow arrows point to the four windows that permit preventive interventions. In women with a high risk of severe preeclampsia, the addition of L-arginine to the early use of low dose aspirin and calcium supplementation in women with low intake has proven safe and effective, whereas pravastatin is emerging as a potential intervention.62–65 This flowchart complements the one recently proposed by Arabin and Baschat regarding the different levels of clinical care that should be provided at the different stages.14 Abbreviations: CVR, cardiovascular risk; NK, natural killer. |
Conclusion
As the evidence derived from retrospective and prospective epidemiological registries, partial pathophysiological insight, clinical studies, systematic reviews, and meta-analyses substantiating the association of preeclampsia and CVD has achieved level 1, the time is ripe for implementing a safe, integral obstetric, and cardiological approach.66 The fact that the subjects of these studies are derived from different ethnic and geographic backgrounds indicates that the association is not limited to confined groups.
From a practical standpoint, questionnaires and basic laboratory screenings have to be developed and consensuated among the leading scientific societies in the field, to be applied in centers of excellence as well as underdeveloped nations. The Global Pregnancy Collaboration (CoLab) provides a very solid starting point for clinical registries and biobanks that would contribute to elucidate the missing links of the association of gestational diseases and CVDs.67 Based on this and our clinical research experience, I have drafted questionnaires for obstetric and cardiology attentions that may be adapted to local clinical practices (Tables 2 and 3). These questionnaires should make a proviso for aspects missed in past studies, such as the need to include women with impeccable pregnancies in the control group (no preterm delivery or intrauterine growth restriction in normotensive pregnancies, spontaneous abortions), the characterization of the different types of pregnancy hypertension, the parity of the index pregnancy, and the precise use and timing of hormonal replacement, data that have not been considered in studies including women over 60 years of age.12,13,68 The weight of the evidence is such that waiting for prospective controlled cohort studies, full understanding of the molecular pathways involved in this association, identification of the most cost-effective markers, and an evaluation of the effect of CVR modification on pregnancy constitute an ethical conundrum.
  | Table 2 Questionnaire to evaluate of cardiovascular risks in pregnant women Abbreviations: BMI, body mass index; col, cholesterol; HDL, high-density lipoprotein. |
Acknowledgments
I thank Alfredo Germain, MD, for including me in the first studies of endothelial function performed in Chile. Watching in real time the normal or reduced brachial flow-mediated vasodilation and waveforms of the uterine arteries underscored the gatekeeper role of the spiral arteries to accept or oppose the invading trophoblast. The English usage was edited by San Francisco Edit.
Disclosure
The author reports no conflicts of interest in this work.
References
Jonsdottir LS, Arngrimsson R, Geirsson RT, Sivaldason H, Sigfusson N. Death rates for ischemic heart disease in women with a history of hypertension in pregnancy. Acta Obstet Gynecol Scand. 1995;74(10):772–776. | ||
Hannaford P, Ferry S, Hirsh S. Cardiovascular sequelae of toxaemia of pregnancy. Heart. 1997;77(2):154–158. | ||
Smith GC, Pell JP, Walsh D. Pregnancy complications and maternal risk of ischaemic heart disease: a retrospective cohort study on 129,290 births. Lancet. 2001;357(9273):2002–2006. | ||
Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after preeclampsia; population based cohort study. BMJ. 2001;323(7323):1213–1217. | ||
Sattar N, Greer IA. Pregnancy complications and maternal cardiovascular risk: opportunities for intervention and screening? BMJ. 2002;325(7356):157–160. | ||
Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: results from cohort study. BMJ. 2003;326(7394):845–849. | ||
Pell JP, Smith GC, Walsh D. Pregnancy complications and subsequent maternal cerebrovascular events: a retrospective cohort study of 119,668 births. Am J Epidemiol. 2004;159(4):336–342. | ||
Arnadottir GA, Geirsson RT, Arngrimsson R, Jonsdottir LS, Olafsson O. Cardiovascular death in women who had hypertension in pregnancy: a case-control study. BJOG. 2005;112(3):286–292. | ||
Catov JM, Newman AB, Roberts JM, et al; Health ABC Study. Preterm delivery and later maternal cardiovascular disease risk. Epidemiology. 2007;18(6):733–739. | ||
Craici IM, Wagner SJ, Hayman SR, Garovic VD. Pre-eclamptic pregnancies: an opportunity to identify women at risk for future cardiovascular disease. Womens Health (Lond). 2008;4(2):133–135. | ||
Berends AL, de Groot CJ, Sijbrands EJ, et al. Shared constitutional risks for maternal vascular-related pregnancy complications and future cardiovascular disease. Hypertension. 2008;51(4):1034–1041. | ||
Cirillo PM, Cohn BA. Pregnancy complications and cardiovascular disease death: fifty year follow-up of the Child Health and Development Studies Pregnancy Cohort. Circulation. 2015;132(13):1234–1242. | ||
DeRoo L, Skjærven R, Wilcox A, et al. Placental abruption and long-term maternal cardiovascular disease mortality: a population-based registry study in Norway and Sweden. Eur J Epidemiol. 2016;31(5):501–511. | ||
Arabin B, Baschat AA. Pregnancy: an underutilized window of opportunity to improve long-term maternal and infant health – an appeal for continuous family care and interdisciplinary communication. Front Pediatr. 2017;5:69. | ||
Ananth CV, Hansen AV, Williams MA, Nybo Andersen AM. Cardiovascular disease in relation to placental abruption: a population-based cohort study from Denmark. Paediatr Perinat Epidemiol. 2017;31(3):209–218. | ||
Riise HK, Sulo G, Tell GS, et al. Incident coronary heart disease after preeclampsia: role of reduced fetal growth, preterm delivery, and parity. J Am Heart Assoc. 2017;6(3):e004158. | ||
White WM, Mielke MM, Araoz PA, et al. A history of preeclampsia is associated with a risk for coronary calcification 3 years later. Am J Obstet Gynecol. 2016;214(4):519.e1–8. | ||
Valdés G, Quezada F, Marchant E, et al. Association of remote hypertension in pregnancy with coronary artery disease: a case-control study. Hypertension. 2009;53(4):733–738. | ||
Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335(7627):974–986. | ||
McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008;156(5):918–930. | ||
Brown MC, Best KE, Pearse MS, Waugh J, Robson SC, Bell R. Cardiovascular disease risk in women with pre-eclampsia; systematic review and meta-analysis. Eur J Epidemiol. 2013;28(1):1–19. | ||
Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and future cardiovascular health. A systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10(2):e003497. | ||
Cusimano MC, Pudwell J, Roddy M, Cho CK, Smith GN. The maternal health clinic: an initiative for cardiovascular risk identification in women with pregnancy-related complications. Am J Obstet Gynecol. 2014;210(5):438.e1–9. | ||
Sia WW, Montgomery-Fajic E, Germaine D, et al. OS106. The postpartum preeclampsia clinic (PPPEC) – an interdisciplinary clinic for cardiovascular risk reduction for women with preeclampsia. Pregnancy Hypertens. 2012;2(3):237. | ||
Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women – 2011 update. A guideline from the American Heart Association. J Am Coll Cardiol. 2011;57(12):1404–1423. | ||
Bushnell C, McCullough LD, Awad IA, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(5):1545–1548. | ||
Bairey Mertz CN. Sex, death and the diagnosis gap. Circulation. 2014;130(9):740–742. | ||
Pepine CJ, Ferdinand KC, Shaw LJ, et al; ACC CVD in Women Committee. Emergence of nonobstructive coronary artery disease: a woman’s problem and need for change in definition on angiography. J Am Coll Cardiol. 2015;66(17):1918–1933. | ||
Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia: results from the NHLBI Women’s Ischemia Syndrome Evaluation (WISE). J Am Coll Cardiol. 2010;55(25):2825–2832. | ||
Davis EF, Lazdam M, Lewandowski AJ, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129(6):e1552–e1561. | ||
Lazdam M, Davis EF, Lewandovski AJ, et al. Prevention of vascular dysfunction after preeclampsia: a potential long-term outcome measure and emerging goal of treatment. J Pregnancy. 2012;2012:704146. | ||
Loke YK, Brown JW, Kwok CS, Niruban A, Myint PK. Association of obstructive sleep apnea with risk of serious cardiovascular events. A systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2012;5(5):720–728. | ||
Bin YS, Cistulli PA, Ford JB. Population-based sleep apnea in pregnancy and maternal and infant outcomes. J Clin Sleep Med. 2016;12(6):871–877. | ||
Johnson MP, Brennecke SP, East CE, et al; FINNPEC Study Group. Genetic dissection of the pre-eclampsia susceptibility locus on chromosome 2q22 reveals shared novel risk factors for cardiovascular disease. Mol Hum Reprod. 2013;19(7):423–437. | ||
Collaborative Low-dose Aspirin Study in Pregnancy Collaborative Group. CLASP: a randomised trial of low-dose aspirin for the prevention and treatment of pre-eclampsia among 9364 pregnant women. Lancet. 1994;343(8898):619–629. | ||
Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 pt 1):402–414. | ||
Antithrombotic Trailists (ATT) Collaboration. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trial. Lancet. 2009;373(9678):1849–1860. | ||
Staff AC, Dechend R, Pijnenborg R. Learning from the placenta acute atherosis and vascular remodeling in preeclampsia – novel aspects for atherosclerosis and future cardiovascular health. Hypertension. 2010;56(6):1026–1034. | ||
Brosens I, Benagiano G, Brosens JJ. The potential perinatal origin of placentation disorders in the young primigravida. Am J Obstet Gynecol. 2015;212(5):580–585. | ||
Jeyabalan A, Powers RW, Durica AR, Harger GF, Roberts JM, Ness RB. Cigarette smoke exposure and angiogenic factors in pregnancy and preeclampsia. Am J Hypertens. 2008;21(8):943–947. | ||
Celermajer D. Endothelial dysfunction: does it matter? Is it reversible? J Am Coll Cardiol. 1997;30(2):325–333. | ||
Craven CM, Morgan T, Ward K. Decidual spiral artery remodelling begins before cellular interaction with cytotrophoblasts. Placenta. 1988;19(4):241–252. | ||
Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine retardation and preeclampsia. Biol Reprod. 2003;69(1):1–7. | ||
Germain AM, Romanik MC, Guerra I, et al. Endothelial dysfunction: a link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension. 2007;49(1):90–95. | ||
Valdés G, Kaufmann P, Corthorn J, Erices R, Brosnihan KB, Joyner-Grantham J. Vasodilator factors in the systemic and local adaptations to pregnancy. Reprod Biol Endocrinol. 2009;7:79. | ||
Valdés G, Corthorn J. Challenges posed to the maternal circulation by pregnancy. Integr Blood Press Control. 2011;4:45–53. | ||
Weissgerber TL, Milic NM, Milin-Lazovic JS, Garovic V. Impaired flow-mediated dilation before, during and after preeclampsia: a systematic review and meta-analysis. Hypertension. 2016;67(2):415–423. | ||
Henriques AC, Carvalho FH, Feitosa HN, Macena RH, Mota RM, Alencar JC. Endothelial dysfunction after pregnancy-induced hypertension. Int J Gynaecol Obstet. 2014;124(3):230–234. | ||
Aykas F, Solak Y, Erden A, et al. Persistence of cardiovascular risk factors in women with previous preeclampsia: a long-term follow-up study. J Investig Med. 2015;63(4):641–645. | ||
Östlund E, Al-Nashi M, Hamad RR, et al. Normalized endothelial function but sustained cardiovascular risk profile 11 years following a pregnancy complicated by preeclampsia. Hypertens Res. 2013;36(12):1081–1087. | ||
Sandvik MK, Leirgul E, Nygård O, et al. Preeclampsia in healthy women and endothelial dysfunction 10 years later. Am J Obstet Gynecol. 2013;209(6):569.e1–569.e10. | ||
Grand’Maison S, Pilote L, Okano M, Landry T, Dayan N. Markers of vascular dysfunction after hypertensive disorders of pregnancy. A systematic review and meta-analysis. Hypertension. 2016;68(6):1447–1458. | ||
Egeland GM, Klungsoyr K, Oyen N, Tell GS, Naess O, Skjaerven R. Preconceptional cardiovascular risk factor differences between gestational hypertension and preeclampsia. Hypertension. 2016;67(6):1173–1180. | ||
Harville EW, Viikari JSA, Raitakkkari OL. Preconceptional cardiovascular risk factors and pregnancy outcome. Epidemiology. 2011;22(5):724–730. | ||
Hedderson MM, Darbinian JA, Sridhar SB, Quesenberry CP. Prepregnacy risk cardiometabolic and inflammatory risk factors and subsequent risk of hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2012;207(1):68.e1–68.e9. | ||
Magnussen EB, Vatten LJ, Lund-Nielsen TI, Salvesen KA, Smith GD, Romundstad PR. Prepregnancy cardiovascular risk factors as predictive of pre-eclampsia: population based cohort. BMJ. 2007;335(7627):978. | ||
Roberts JM, Taylor RN, Musci TJ, Rodgers GM, Hubel CA, McLaughlin MK. Preeclampsia: an endothelial cell disorder. Am J Obstet Gynecol. 1998;161(5):1200–1204. | ||
Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999; 180(2 pt 1):499–506. | ||
Hung T-H, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxia/reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res. 2002;90(12):1274–1281. | ||
Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30(6):473–482. | ||
Rabaglino MB, Post Uiterweer ED, Jeyabalan A, Hogge WA, Conrad KP. Bioinformatics approach reveals evidence for impaired endometrial maturation before and during early pregnancy in women who developed preeclampsia. Hypertension. 2015;65(2):421–429. | ||
Villar J, Abdel-Aleem H, Merialdi M, et al; World Health Organization Calcium Supplementation for the Prevention of Preeclampsia Trial Group. World Health Organization randomized trial of calcium supplementation among low calcium intake pregnant women. Am J Obstet Gynecol. 2006;194(3):639–649. | ||
Vadillo-Ortega F, Perichart-Perera O, Espino S, et al. Effect of supplementation during pregnancy with L-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: randomised controlled trial. BMJ. 2011;342:d2901. | ||
Germain AM, Valdés G, Romanik MC, Reyes MS. Evidence supporting a beneficial role for long-term L-arginine supplementation in high-risk pregnancies. Hypertension. 2004;44(1):e1. | ||
Costantine MM, Cleary K, Hebert MF, et al; Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric-Fetal Pharmacology Research Units Network. Safety and pharmacokinetics of pravastatin used for the prevention of preeclampsia in high-risk pregnant women: a pilot randomized controlled trial. Am J Obstet Gynecol. 2016;214(6):720.e1–720.e17. | ||
Centre for Evidence-Based Medicine. Oxford Centre for Evidence-Based Medicine – Levels of Evidence. 2009. Available from: http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/. Accessed April 1, 2017. | ||
Staff AC, Redman CW, Williams D, et al; Global Pregnancy Collaboration (CoLab). Pregnancy and long-term maternal cardiovascular health: progress through harmonization of research cohorts and biobanks. Hypertension. 2016;67(2):251–261. | ||
Bairey Merz CN, Shufelt C, Johnson BD, Azziz R, Braunstein GD. Reproductive hormone exposure timing and ischemic heart disease: complicated answers to a simple question. Maturitas. 2010;65(4):297–298. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


