Back to Journals » Clinical Ophthalmology » Volume 15
Predictors of Visual Acuity After Treatment of Neovascular Age-Related Macular Degeneration – Current Perspectives
Authors Phan LT , Broadhead GK, Hong TH, Chang AA
Received 9 July 2021
Accepted for publication 28 July 2021
Published 11 August 2021 Volume 2021:15 Pages 3351—3367
DOI https://doi.org/10.2147/OPTH.S205147
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Long T Phan,1,2 Geoffrey K Broadhead,1,3 Thomas H Hong,1 Andrew A Chang1,3
1Sydney Retina, Sydney, New South Wales, Australia; 2Discipline of Orthoptics, University of Technology Sydney, Sydney, New South Wales, Australia; 3Save Sight Institute, The University of Sydney, Sydney, New South Wales, Australia
Correspondence: Andrew A Chang
Sydney Retina, Level 13, Park House, 187 Macquarie Street, Sydney, 2000, New South Wales, Australia
Tel +61 2 9221 3755
Fax +61 2 9221 1637
Email [email protected]
Abstract: Visual acuity is a key outcome measure in the treatment of neovascular age-related macular degeneration (nAMD) using anti-vascular endothelial growth factor agents. Large variations in visual responses between individuals within clinical trials and real-world studies may relate to underlying differences in patient and treatment factors. Most notably, a better baseline visual acuity, younger age and smaller choroidal neovascularization lesion size have been strongly associated with achieving better visual outcomes. In addition, there is emerging evidence for other roles including genetic factors and anatomical variables such as fluid status. Apart from patient-related factors, treatments that favor a higher number of injections tend to provide better visual outcomes. Overall, the identification of predictive factors does not currently play an essential role in the clinical management of patients with nAMD. However, they have allowed for the understanding that early detection, timely management and close monitoring of the disease are required to achieve optimal visual outcomes. Further investigation into predictive factors alongside the development of novel therapeutic agents may one day provide a means to accurately predict patient outcomes. Treatment regimens that offer flexible dosing patterns such as the treat-and-extend strategy currently provide a degree of personalization during treatment.
Keywords: age-related macular degeneration, anti-VEGF, visual acuity, demographic, genetic, anatomic
Introduction
Age-related macular degeneration (AMD) is a chronic disease of the eye which is the leading cause of irreversible vision impairment in developed countries.1 Prevalence rates of AMD for individuals aged between 45 and 85 years range between 7% and 18% across Asian and Western countries.2 Neovascular age-related macular degeneration (nAMD) or “wet” AMD, is an advanced form of AMD characterized by choroidal neovascularization (CNV), where newly formed blood vessels leak into the retina, causing distortion and rapid loss of vision. nAMD occurs in approximately 10% of individuals with AMD, however it is responsible for up to 90% of vision loss.2,3 The burden of AMD is expected to increase, as current prevalence rates are estimated to rise by approximately 50% over the next two decades.2
While the exact cause of CNV is unconfirmed, it is believed to be triggered by local retinal ischemia/hypoxia, caused by the buildup of abnormal extracellular deposits located between the retinal pigment epithelium (RPE) and Bruch’s membrane. The overexpression of vascular endothelial growth factor (VEGF) in response to retinal hypoxia has been identified as the main mediator behind the development of CNV.4–6 These findings have led to a paradigm shift in the treatment of nAMD through the introduction of anti-VEGF medication given intravitreally into the eye. Anti-VEGF agents primarily target and block VEGF-A isoforms, preventing further vision loss caused by angiogenesis, fluid leakage and subsequent scar formation. The improvement and stabilization of vision through fluid reduction is the primary goal of treatment, which also improves vision-related quality of life.7 As a result, visual acuity (VA) has been considered one of the primary markers for treatment success within several pivotal Phase 3 clinical trials; namely the MARINA8 and ANCHOR9 trials for ranibizumab (Lucentis; Genentech, South San Francisco, California), the VIEW1 & VIEW2 studies10,11 for aflibercept (Eylea; Regeneron, Tarrytown, New York) and more recently in the HAWK and HARRIER trials12 for brolucizumab (Beovu; Novartis, Basel, Switzerland). In addition to these agents which have been FDA approved for ocular use, bevacizumab (Avastin; Regeneron) has been used off-label, and its non-inferiority to ranibizumab has been demonstrated in the CATT studies.13,14 In each of their landmark studies, over 90% of patients maintained VA levels within 15 Early Treatment Diabetic Retinopathy Study (ETDRS) letters over the course of treatment. Remarkably also, 30–40% of patients demonstrated visual improvements beyond the 15 letter threshold, demonstrating superiority over previously used photodynamic therapy and laser photocoagulation treatment, which rarely saw VA improvement.15,16
However, while the introduction of anti-VEGF therapy has been revolutionary in reducing rates of legal blindness associated with AMD,17 not all patients respond positively, with a small remainder of patients (~5–10%) experiencing significant reductions in vision. These variations are also seen in the real world, with post-marketing trials and clinical studies finding larger proportions of patients who lose vision compared to the control trials.18–20 In addition to these early responses, further treatment variation occurs in the longer term past the first 1–2 years of treatment, with some patients experiencing gradual declines in visual acuity despite continuous intensive treatment and a good initial response.21–23
It currently remains unclear as to why such heterogeneity in treatment response exists. Though several retrospective analyses have identified several functional, demographic, genetic and anatomic factors associated with various visual outcomes. The identification of prognostic factors allows the provision of personalized medicine, as physicians can provide patients with a more accurate expectation for their visual prognosis. This review investigates factors which may have a predictive value in determining visual response after anti-VEGF treatment among patients with nAMD and assesses the current roles of predictive markers in treatment decision-making.
Literature Search Method
Articles up until January 2021 were initially searched using PubMed by 2 independent authors (LP & GB) using a combination of the terms “Macular degeneration”, “Neovascular”, “Predictive factors”, “Predictors”, “Visual acuity” and “Visual outcomes”. From 190 identified articles, 101 non-relevant articles and 9 non-English articles were excluded after screening through abstracts. The remaining articles were reviewed to generate a list of relevant predictors for further investigation. Sixty-two further studies were identified following manual searching of secondary analyses from major randomized clinical trials of anti-VEGF and separate searches that included additional terms specific for sub-categories of predictors including “smoking”, “pharmacogenetics”, “polymorphisms”, “CFH”, “ARMS2”, “HTRA1”, “VEGF-A”, “VEGFR-2”, “GWAS”, “Optical coherence tomography”, “Atrophy” and “Hemorrhage”. There was a focus on post-hoc analyses of clinical trials and large retrospective studies which used multivariate analysis. Smaller studies or those which used univariate analyses were also included if they demonstrated a significant novel finding. This excluded 48 articles, leaving 94 studies which were included in this review.
Variations in Outcome Reporting and Risk Factor Analyses
Different measures of efficacy have been used throughout the literature. Most studies report visual outcomes as a continuous variable either in terms of visual gain (either in ETDRS letters or using a logMAR equivalent), or as absolute levels of VA achieved by the end of the observational period. On the other hand, outcomes have also been evaluated categorically, through the grouping of participants via their visual response. Though the thresholds for these categories vary between studies, a loss of ≥15 letters for poor responders has been the most popular definition. Alongside these variations in outcome measures, there have been differences in study designs and statistical analyses and reporting of the various risk factors within the literature. As such meta-analyses have not been previously possible24,25 and will not be attempted in this exploration for the same reason.
Functional Variables
Visual Acuity
Baseline VA has been the most thoroughly investigated variable and its relationship with visual response following treatment has been well established as the most significant predictor of visual outcome in both clinical trials (Table 1) and real-world studies (Table 2).
 |
Table 1 Summary of Clinical Trials Investigating Predictors of Visual Outcomes in Anti-VEGF Treated Patients |
 |
Table 2 Summary from Major Real-World Studies Investigating Predictors of Visual Outcomes in Anti-VEGF Treated Patients |
VA changes following treatment are heavily influenced by ceiling effects, where patients with better VA at presentation have a reduced capacity or “ceiling” for VA gains compared to those who present with lower baseline VA levels. Post-hoc analysis of the MARINA study found a 1.2–1.6 letter reduction in VA gains for every 5-letter increase in baseline VA.26 Meanwhile in the VIEW studies, VA gains were +0.65 letters higher for every 1 letter reduction in baseline VA.27 However, the presence of intraretinal fluid (IRF) at baseline reduced the expected letter gain to +0.22.27
While it appears that anti-VEGF treatment is more effective in eyes with poorer VA, patients presenting with better initial VA are more likely to have better final VA. A large retrospective analysis from the Moorfields Eye Hospital (MEH) database reported a 43% increase in likelihood for achieving and maintaining a VA of 20/40 for every 5-letter increase in VA at baseline.28 Meanwhile, data from the Swedish Macular Register revealed that eyes with initial VA >60 letters (20/63) had only a 20% risk of having low VA (≤60 letters) after 1 or 2 years of treatment, compared to 60% in eyes with low initial VA.29 This relationship has also been observed in several long-term studies,30–35 suggesting that the larger visual gains in those with worse initial VA are not enough to overcome a good starting VA despite continuous treatment. As those with worse initial VA are also more likely to respond negatively to treatment, low baseline VA may be an indicator for worse disease severity as there may be underlying pathology not treatable by anti-VEGF, such as atrophy, scarring or other anatomical changes not controlled for in multivariate analysis. van Asten et al,36 found that those with worse baseline VA were more likely to be non-responders (defined by loss of more than 30% initial letters) after the first 3 months of treatment (OR: 3.3, VA 20/63-20/200 vs >20/63). Similarly, analysis of data from the Fight Retinal Blindness! (FRB) registry found that eyes with VA better than 20/40 were 39% less likely to experience a ≥30 letter loss than those with worse baseline VA after 5 years of treatment.37
Although baseline VA is consistently associated with visual outcomes, one’s early response may be a better predictor of their visual trajectory.38–40 The CATT studies found that an individual’s VA gain at week 12 of treatment was a stronger predictor of their long-term visual gains than the combination of all their significant baseline predictors including initial VA (R2 for 2 year VA gains: 0.30 vs 0.13).40 Similar findings have also been found from the FRB registry,39 where those who achieved good vision (≥70 letters) by their 4th injection were more likely to maintain good vision after 3 years of treatment than those who did not (OR: 9.8, VA ≥70 vs <70 letters).
As anti-VEGF therapy does not cure nAMD, the nature of the relationship between presenting VA and its response to treatment suggests that individuals should be treated earlier in the disease course. Studies which find that a shorter duration between symptom occurrence and treatment initiation is also associated with better visual outcomes support this notion.41–44
Patient Characteristics
Age
Similar to VA, strong relationships between age and visual outcomes were identified in the early clinical studies, with less VA gain seen in older patients (Table 1).26,40,45–47 In the MARINA study,26 a 13.6 year difference in age at disease diagnosis was associated a 5-letter reduction in VA gains in the older patient. Meanwhile in the ANCHOR study,45 an 18.8 year difference in age was associated with a 5-letter reduction in VA gain. In the HARBOR study,48 patients aged ≤73 years at baseline gained 4.5 letters more than those aged >73 after ranibizumab treatment. The VIEW studies found that older age was also associated with negative treatment outcomes, with older patients more likely to lose VA over their first year of aflibercept treatment (OR for >1 letter loss: 2.1, ages 80–89 vs 46–69 years).47 Over the first 2 years of CATT,40,46 older age was associated with less VA gains, worse final VA levels and a decreased likelihood for a ≥15 letter VA increases, however this was no longer significant at the 5-year follow-up49 suggesting that age does not influence long-term outcomes. In real world studies, the relationship between age and visual outcome is not as consistent (Table 2). Though associations are found in larger observational cohorts,28,29,50 suggesting this is due to smaller sample sizes and larger patient variations in combination with its relatively small effect size.
The effect of age may be influenced by other factors, with Yamashiro et al.51 finding that age was associated with 12-month VA changes in typical nAMD patients, but not for those presenting with the polypoidal choroidal vasculopathy (PCV) variant of AMD. As age is a major risk factor for advanced AMD, its relationship with visual outcomes likely represents part of the natural history of the disease. These individuals should be considered more carefully during treatment.
Gender
There have been some associations between prevalent AMD and gender which may suggest that the course of treatment may differ between men and women.52 However, despite gender being regularly included in risk factor analyses in clinical trials and retrospective studies, no significant associations have been found between gender and the visual response to anti-VEGF treatment in AMD, except in the 5 year follow-up of the CATT study,49 where females were more likely to ≥15 letter VA gains than males (OR: 1.79).
Ethnicity
The influence of ethnicity is inconclusive as few studies have been performed in diverse populations, however most large studies have found no direct relationship between ethnicity and visual outcome.28,48 Outcomes related to ethnic background may be tied to CNV lesion sub-type due to the higher prevalence of PCV seen within Black and Asian populations compared to Caucasian populations.53–55 PCV has been found to be associated with poor anatomic responses to ranibizumab treatment54,56 and is likely to result in worse visual outcomes in the longer term. Differences in genetic susceptibilities may underlie ethnic differences in treatment outcomes.
Systemic Disease and Social Habits
There are several well-known systemic diseases and behavioral risk factors for AMD such as cardiovascular disease, smoking and nutrition.57 van Asten et al36 found that patients with a history of diabetes mellitus were 2.1x more likely to have a non-response to treatment, however no associations were found for cardiovascular disease, smoking status or body mass index. Piermarocchi et al.58 reported that those with hypertension as well as current and former smokers gained less VA (−3.86 and −4 letters respectively) over 1 year of ranibizumab treatment. Similarly, Lee et al.59 found that current smokers were more likely have poor VA improvement (VA gain below group median) after ranibizumab treatment (OR: 7.5). Meanwhile, the 5-year follow-up of CATT found that those who were current smokers at baseline were more likely to have worse final VA (OR for VA <20/200: 2.61),49 suggesting that smoking may exert long-term detrimental effects on VA. In contrast to these findings, a larger majority of studies have failed to find associations.48,60–66 However, while their role in determining treatment response is unclear, these risk factors remain as strong modifiable risk factors for disease prevention and the improvement of general health.
Genetics
Like other patient factors, genetic polymorphisms that have been strongly associated with the development of nAMD have also been investigated for their role in determining treatment response. Initial investigations were done into AMD risk alleles such as single nucleotide polymorphisms (SNPs) involving the CFH & ARMS2 genes. Analysis of data from the CATT clinical trials was unable to find any associations between SNPs of CFH, ARMS2, HTRA1 and C3 with treatment response across drugs or dosing regimens.67 Similar results were obtained from analyzing data from the IVAN trials,68 which also could not find associations in SNPs of CFH, FZD4, ARMS2 and HTRA1. However for the CFH gene, two meta-analyses which have included the CATT and IVAN studies,69,70 have confirmed that the Y402H polymorphism of CFH was in fact associated with treatment response, with those carrying the minor allele having reduced VA gains. This may be linked to ethnic variations, with subgroup analyses in both papers finding the relationship occurring in Caucasian populations and not East Asians, however it may be due to the significantly lower incidence rates of CFH polymorphisms in Asians and the limited number of Asian studies included. On the other hand, two meta-analyses of studies investigating polymorphisms of ARMS2 have found that the minor allele of A69S was associated better treatment responses to anti-VEGF among East Asians;71,72 though not all studies included used visual acuity to define treatment response. For HTRA1 gene, a meta-analysis of five studies found no associations between its polymorphisms and treatment response.73
Attention has also turned to investigate SNPS involving VEGF, such as VEGF-A & VEGFR2/KDR polymorphisms. However, there have been many conflicting results with large studies failing to find associations.74,75 For VEGF-A, Lazzeri et al76 found that SNP rs699947 was related to an early visual response following 3 months of RBZ treatment, with patients carrying the minor allele experiencing positive VA gains (+6.3–7.4 letters) compared to those without, who lost VA following treatment (−1.8 letters). However, Park et al77 and Cruz-Gonzalez et al78 have both found that the minor allele of rs699947 to be associated with worse visual outcomes after 5 and 12 months respectively. Individuals carrying the minor allele of rs833061 were also more likely to gain VA (≥5 letters) after 1 year of RBZ treatment (OR: 1.62).78 For VEGFR-2, Hermann et al64 found that SNPs rs4576072 and rs6828477 were independent predictors for VA gains, with carriers of three minor alleles experiencing positive VA gains (~13 letters) compared to those without any minor alleles after 1 year of RBZ treatment. However, the larger CATT and IVAN studies failed to find associations between SNPS of VEGF-A and VEGFR-2 and VA response.74,79
In 2017, 8 polymorphisms of VEGF-A (rs699947, rs699946, rs833069, rs833061, rs2146323, rs1413711, rs2010963 and rs1570360) and 1 polymorphism of VEGFR-2 (rs2071559) were investigated by Wu et al,80 in a meta-analysis of 8 studies, which found anti-VEGF treatment to be more effective in patients homozygous for the minor allele of VEGF-A rs833061. While this meta-analysis also included studies which assessed anatomic outcomes, sub-analysis of studies describing purely visual outcomes found stronger associations, with OR’s for a positive visual response ranging from 2.6 to 3.8 across the genotypic models.
VEGF isoform and receptor polymorphisms have the potential to result in differences in treatment responses between anti-VEGF medications, as aflibercept has additional binding capabilities to PGF and VEGF-B compared to bevacizumab and ranibizumab which only target isoforms of VEGF-A. A Phase 4 trial of aflibercept81 found strong associations with polymorphisms of VEGF-B (rs12366035) and C5 (rs25681), with those homozygous for their minor alleles more likely to have ≥15 letter gains (OR: 217 and 19.7, respectively). Smaller associations were also found for polymorphisms within CX3CR1, CETP, IL6 and CCL2. These results are promising as it suggests that responses to different anti-VEGF agents may be tied to separate gene polymorphisms.
Apart from selected targeted studies, broader approaches using genome-wide association studies have the allowed identification of other candidate genes associated with treatment response such as CTGF,82 OR52B4,83 and CCT3,84 however a lack of association with previously investigated genes have also raised further uncertainty.
While the role of pharmacogenomics is promising, the prevalence of predictive genes must be common enough and their effects must be strong enough to warrant routine genetic testing in a clinical setting. Despite the availability of several meta-analyses, more individual studies are required in order to further investigate the effects of less commonly assessed SNPs, treatment-related effects and ethnic contributions. Furthermore, external clinical validation of the effects of identified SNPs are required through prospective trials to confirm their roles.
Anatomic Factors
Given the expanded role of imaging in the diagnosis and management of nAMD, considerable efforts have been made to identify potential anatomic characteristics that may predict visual outcomes. Although initially predominantly examination or angiographically based, the expanded role of OCT has meant that many of these factors are now predominantly assessed via OCT imaging. Broadly speaking, factors can be predictive from baseline or during treatment, and both are discussed below.
Lesion Type and Lesion Size
In terms of VA gains, no significant difference has been found between the responsiveness of classic or occult lesions to anti-VEGF agents in large RCTs (Table 1). However, CATT did show that those with classic lesions had lower final VA at 1 year compared to occult lesions (64.2 vs 70.4 letters) yet were more likely to gain ≥15 letters on univariate analysis,46 and VIEW 1/2 showed that those with classic lesions were more likely to have a final VA worse than 20/200 at 1 year but were more likely to lose ≥15 letters instead.47 Since those with classic lesions more commonly present with worse VA in these studies, we would expect this to translate into better overall VA gains due to the effects of baseline VA. However, the lack of differences suggests that apart from a small group of good responders, those with classic lesions perform relatively worse compared to other subtypes.
Retinal Angiomatous Proliferation (RAP) lesions have also been associated with increased VA gains after anti-VEGF therapy compared to other lesion types in both the CATT and VIEW trials.79 These benefits are most pronounced early in therapy (during the 1st year), with differences in visual outcomes between RAP lesions and other angiographic lesion types becoming non-significant after 2 years of therapy.85 However, RAP lesions have also been linked to higher rates of geographic atrophy (GA), notably in the CATT study,85 and it remains to be seen if this has any effect on RAP lesions as a predictor of vision with even longer follow-up times, given the role of atrophy in long-term visual decline, as discussed below.
Larger baseline lesion size has been consistently associated with worse VA gain in multiple large RCT’s, including the MARINA,26 ANCHOR,86 CATT46,87 and VIEW studies.88 In the CATT, compared to those with a lesion size ≤2.54 mm2, patients with a lesion size >10.2 mm2 experienced less VA gain (+4.2 vs +8.7 letters), had a lower proportion of ≥15 letter gainers (23.8% vs 30.1%) and had worse final VA (64.5 vs 69.9 letters) after 1 year of treatment.46
Retinal Thickness
OCT measured retinal thickness (RT) is a commonly assessed clinical trial outcome and has been used as a criteria for treatment in some trials including HAWK/HARRIER,89 and it is important to determine in each instance what is meant by retinal RT. Frequently used terms such as central retinal thickness (CRT) or central macular thickness (CMT) in some publications may also include subretinal fluid (SRF) in this measurement, and in some case also include pigment epithelial detachment (PED) height, although here we refer to thickness of the retina alone, excluding SRF or PED. In the CATT, thinner (<120µm, 57.7 letters) or thicker (>212, 64.0 letters) retinal thickness (not including SRF or PED) had worse final VA than those between those two ranges (12–212µm, 72.0 letters) after 2 years of therapy.90 Similarly, the PrONTO study also found a correlation between change in RT and VA change at 3 and 12 months,91 suggesting improved retinal thickness is a predictor of greater VA gain.
There is also recent evidence that fluctuations in RT may be a poor prognostic factor. Retrospective analysis of pooled data from the CATT and IVAN trials showed that greater fluctuations in RT were associated with worse VA gains after 2 years, with individuals in the highest quartile for RT variations experiencing and average of 6.27 less letter gain than those who had the least variation in RT (95% CI: −8.45 to −4.0). Individuals with higher variations in RT were also more likely to develop fibrosis and/or GA.92
Retinal Exudation – Intraretinal Fluid (IRF), Subretinal Fluid (SRF) and Subretinal Hyperreflective Material (SHRM)
Both IRF and SRF have been studied extensively as markers of disease activity. The presence of IRF has been demonstrated to be associated with worse vision both at baseline and during treatment in large clinical trials including both CATT and VIEW,27,90 as well as at baseline in the EXCITE study.93 In VIEW,44 those with IRF gained 3.85 less letters after 1 year of aflibercept treatment. Recent analysis has also suggested that the volume of IRF is of importance, with increased IRF volume associated with progressively worse BCVA change in post-hoc analysis of the HARBOR trial,94 as well as in post-hoc analysis of the FLUID trial.95 Location of IRF was also important in the FLUID analysis, with IRF in the central 1mm significantly associated with reduced VA gain, but IRF in the surrounding 1–6mm not associated with VA change.95
The role of SRF, in contrast, is less clear. Analysis of CATT, VIEW and HARBOR has shown that SRF at baseline may be predictive of better visual outcomes,48,90,94,96,97 and that residual SRF may be associated with larger VA improvement at 24 months in the HARBOR trial. Both the EXCITE and FLUID trials have shown that individuals with SRF could tolerate extended treatment intervals without adversely affecting visual outcomes.98,99 However, post-hoc analysis of the FLUID trial has shown that increasing SRF volume within the central 1–6mm (but not the central 1mm) of the retina is associated with increasingly reduced VA (−0.2 letters per 100nL).95 Similarly, post-hoc analysis of the HAWK and HARRIER trials showed that eyes with greater SRF volume at the end of dose loading (12 weeks) had lower VA gain from weeks 12 to 96 than those with lower SRF volume, suggesting that the effect of SRF as a prognostic factor may in part be dependent on the volume of SRF present.100
SHRM is an OCT-detectable form of exudation that manifests as hyperreflectivity between the RPE and the retina. The presence of SHRM, particularly at the foveal center, has been associated with significantly worse VA in the CATT study at year 2 (73.5 vs 63.9 letters),101 as well as being a predictor of poor final VA at year 5.87 Decreased SHRM volume correlated with improved vision in post-hoc sub-analysis of the OSPREY trial,102 suggesting that SHRM is an important marker of outcomes in neovascular AMD.
The effect of changes in retinal exudation volume highlights the importance of ongoing monitoring and comparison of retinal imaging across the course of nAMD treatment, as worsening of exudation volumes may result in worse visual outcomes. This may require alterations to management to more effectively control.
Pigment Epithelial Detachments
The presence of PED has been associated with worse baseline vision in nAMD, as well as reduced VA gain in some series such as the CATT study,46 although this was not seen in the HARBOR study.103 Response of a PED to therapy has not been associated with visual outcomes in multiple studies, including retrospective analysis of the HARBOR and VIEW trials,97,103,104 although post-hoc analysis of the VIEW study showed that patients with a PED at baseline who developed IRF during follow up had the lowest VA gains of any combination of anatomic parameters.27
Based on these findings, treatment aimed at eliminating or reducing the size of a PED is currently not recommended,105 although ongoing monitoring and treatment of any signs of retinal exudation, particularly IRF, is encouraged, given the poorer prognosis of IRF in combination with PED.
RPE Atrophy
Long-term follow-up of a number of clinical trial cohorts has shown that atrophy development is a major cause of long-term visual decline. Five-year outcomes of the CATT cohort showed that the development of atrophy was a significant reason for visual decline in this cohort (mean final VA 62 letters for no foveal pathology vs 53 for GA),87 and foveal GA at year 2 was associated with worse vision at year 5. The presence of nonfoveal GA at baseline was a risk factor for visual acuity loss at 2 years in the CATT,106 suggesting that GA progression is an important reason for vision loss even during the first few years of anti-VEGF therapy. Similarly, post-hoc analysis of the subset of the Age-Related Eye Diseases Study 2 (AREDS2) cohort who had neovascular AMD identified atrophy as being the cause of 60% of cases of poor vision (<20/200).106 Pooled analysis of the ANCHOR, MARINA and HORZION studies also showed that macular atrophy progression was the major cause of visual decline 7 years after commencing treatment,107 implying that increasing central atrophy is a poor prognostic factor.
Hemorrhage and Subretinal Fibrosis
The presence of clinical hemorrhage by itself has not been associated with worse visual outcomes, with the CATT study showing that lesions composed of >50% hemorrhage had similar VA gains at 2 years compared to those that were not.108 Hemorrhage, however, needs to be clearly defined, as the presence of sub-retinal hemorrhage can significantly impair vision, particularly those of larger sizes (>1DD) and those located directly below the fovea, and large, foveal sub-macular hemorrhage is associated with poor visual outcomes, particularly if left untreated.109
The presence of scar has also been associated with worse visual outcomes in trials, notably the CATT.87,90 Interestingly, larger hemorrhage (>1DD) was a risk factor for scar development in post-hoc analysis of the CATT, suggesting that part of the poor visual prognosis of these large hemorrhagic lesions may relate to the risk of scarring.110 Post-hoc analysis of the AREDS2 cohort treated for neovascular AMD also identified fibrosis as being responsible for 40% of the cases of poor vision (<20/200),111 implying that preventing scar formation remains an important goal in preserving vision.
Treatment Regime and Visual Outcomes
In combination with patient-related factors, decisions made upon and throughout the course of treatment may also influence visual outcomes. Initially, anti-VEGF was approved for fixed dosing every 4 weeks, and this was later extended to 8 weeks as new anti-VEGF molecules with higher binding affinity were discovered.10,11 In combination with the CATT14 and IVAN112 studies, which demonstrated that dosing via a pro re nata (PRN) regimen provided similar visual outcomes, more flexible dosing regimens have been adopted by treating practitioners which has also included the treat and extend (TREX) regime. Under a PRN regimen, patients typically are followed on a monthly basis however at each interval, the decision to treat is guided by disease activity, determined by the presence or absence of exudation. Meanwhile, the TREX regime is considered a proactive approach whereby patients who achieve an exudative-free status on monthly dosing, have their review and treatment interval extended, typically in either 1- or 2-week increments. Upon the presence of exudation, treatment intervals are then reduced, with the goal of maintaining an exudative-free status under the longest possible dosing interval. By design, TREX offers patients with better anatomical outcomes (as there is less recurrence of exudation) and a higher level of individualization, whilst reducing the burden associated with frequent clinical visits. Both the TREX-AMD113 and CANTREAT114 studies demonstrated that the TREX regime provided similar visual outcomes to fixed monthly dosing while requiring less injections.
Between PRN and TREX dosing, a systematic review of 70 studies found TREX to provide larger VA gains compared to PRN over a 12-month period (+10.4 vs +5.4 letters respectively), though they received a higher number of injections (8.1 vs 5.6 injections).115 In the third year of the TREX-AMD randomized trial, those who spent the first 2 years on TREX and switched to PRN for the final year, had significantly worse visual outcomes compared to those who remained on a TREX regime for the remainder of the study.116 In a 4-year study, Spooner et al compared progression rates of macular atrophy among 264 eyes treated with anti-VEGF using either PRN or TREX regimes.117 They found that VA gains among the TREX group were higher compared to the PRN group after 1 year of treatment (+2.7 vs +0.3 letters respectively), however these gains were lost after 4 years (+0.9 vs −0.5 letters, respectively). More long-term prospective data is needed between these two regimes. As data from real-world studies suggest that patients receive fewer injections than those studied in clinical trials, the benefit seen from a TREX regime likely comes from its proactive nature, as a higher number of injections are also associated with better visual outcomes.28,37,50,118
Multivariate Predictive Modelling
Using a combination of OCT biomarkers and VA over the first 3 months of treatment from HARBOR, Schmidt-Erfurth et al119 used machine learning algorithms to predict 1 year VA outcomes with an accuracy of 71% and an error margin of 8.6 letters. A similar attempt using both VA and OCT data from electronic medical records by Rohm et al,120 provided comparable levels of accuracy, with errors of 5.5 and 8 letters for predicting 3 and 12 month VA respectively. The incorporation of more predictive variables such as genetic data as well as the examination of larger datasets may provide more precise models in the future. However, because preserving vision is the primary goal of anti-VEGF therapy, rather than quantifying vision, it may be more valuable to develop models which identify non-responders, as this could trigger the earlier consideration of alternative treatment routes such as the switching of anti-VEGF drugs or additional therapy.
Conclusion
Several factors have been found to influence a patient’s visual outcome during nAMD treatment (Table 3). However, they play a limited role in the current scope of practice as they do not have the precision in determining whether an individual will respond favorably or not to treatment, nor is there sufficient evidence to guide treatment choices based on individual factors, as the effects of these factors are not associated with certain treatment agents or regimens.
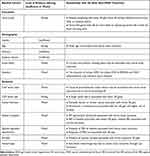 |
Table 3 Summary of Predictive Factors, Their Effects on Visual Outcomes Following Anti-VEGF Treatment and the Level of Supporting Evidence Within the Literature |
Nevertheless, there are several clinical aspects that can be drawn from these findings. Considering that AMD is a disease of senescence, the strong associations seen for VA, age and lesion size suggests that early detection and timely management is required to achieve optimal visual outcomes. Alongside treatment, exacerbating factors; notably smoking; should also be reduced or ceased if possible, given their possible association with worse visual outcomes, and with AMD progression in general.
Currently, individualized treatment is achieved by using flexible dosing strategies such as PRN or TREX. While these strategies do not necessarily offer superior visual outcomes, they may indirectly improve patient’s quality of life and reduce their disease burden through economic relief. These OCT-guided approaches may be further optimized from knowledge of anatomical predictors, as it is suggested that the presence of IRF should be more aggressively controlled in comparison to SRF. While this provides room for further flexibility and individualization during treatment, it is essential that patients remain closely monitored for anatomical changes which may subsequently affect their visual trajectory. Proactive approaches such as TREX appear to be an effective middle-ground.
In the current treatment landscape, currently available agents have been compared based on non-inferiority of visual outcomes. With emerging anti-VEGF agents such as brolucizumab offering longer treatment intervals and greater anatomic outcomes,121 the consideration of additional markers of efficacy may also be required during treatment decision making. The release of newer therapeutics in combination with further knowledge into predictive factors one day may allow the personalization of more effective treatments for individuals with specific baseline characteristics, disease subtypes or genetic susceptibilities.
Disclosure
A. Chang is a consultant for Allergan, Bayer, Novartis, and Roche. The authors report no other conflicts of interest in this work.
References
1. Flaxman SR, Bourne RR, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Global Health. 2017;5(12):e1221.
2. Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health. 2014;2(2):e106. doi:10.1016/S2214-109X(13)70145-1
3. Ferris FL, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102(11):1640–1642. doi:10.1001/archopht.1984.01040031330019
4. Kvanta A, Algvere P, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996;37(9):1929–1934.
5. Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–845. doi:10.1038/359843a0
6. Spilsbury K, Garrett KL, Shen W-Y, Constable IJ, Rakoczy PE. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am J Pathol. 2000;157(1):135–144. doi:10.1016/S0002-9440(10)64525-7
7. Finger RP, Guymer RH, Gillies MC, Keeffe JE. The impact of anti–vascular endothelial growth factor treatment on quality of life in neovascular age-related macular degeneration. Ophthalmology. 2014;121(6):1246–1251. doi:10.1016/j.ophtha.2013.12.032
8. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Eng J Med. 2006;355(14):1419–1431. doi:10.1056/NEJMoa054481
9. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Eng J Med. 2006;355(14):1432–1444. doi:10.1056/NEJMoa062655
10. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. doi:10.1016/j.ophtha.2012.09.006
11. Schmidt-Erfurth U, Kaiser PK, Korobelnik J-F, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six–week results of the VIEW studies. Ophthalmology. 2014;121(1):193–201. doi:10.1016/j.ophtha.2013.08.011
12. Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84. doi:10.1016/j.ophtha.2019.04.017
13. Martin DF, Maguire MG, Fine SL, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. doi:10.1016/j.ophtha.2012.03.053
14. Group CR. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Eng j Med. 2011;364(20):1897–1908. doi:10.1056/NEJMoa1102673
15. Group ToA-RMDwPTS. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin. One-year results of 2 randomized clinical trials-TAP report 1. Arch Ophthalmol. 1999;117:1329–1345. doi:10.1001/archopht.117.10.1329
16. Group MPS. Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Arch Ophthalmol. 1993;111:1200–1209. doi:10.1001/archopht.1993.01090090052019
17. Bloch SB, Larsen M, Munch IC. Incidence of legal blindness from age-related macular degeneration in Denmark: year 2000 to 2010. Am J Ophthalmol. 2012;153(2):209–213. doi:10.1016/j.ajo.2011.10.016
18. Zarranz-Ventura J, Liew G, Johnston RL, et al. The neovascular age-related macular degeneration database: report 2: incidence, management, and visual outcomes of second treated eyes. Ophthalmology. 2014;121(10):1966–1975. doi:10.1016/j.ophtha.2014.04.026
19. Chong V. Ranibizumab for the treatment of wet AMD: a summary of real-world studies. Eye. 2016;30(2):270–286. doi:10.1038/eye.2015.217
20. Holz FG, Figueroa MS, Bandello F, et al. Ranibizumab treatment in treatment-naive neovascular age-related macular degeneration: results from LUMINOUS, a global real-world study. Retina. 2020;40(9):1673. doi:10.1097/IAE.0000000000002670
21. Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K, Group S-US. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120(11):2292–2299. doi:10.1016/j.ophtha.2013.03.046
22. Maguire MG, Martin DF, Ying G-S, et al. Five-year outcomes with anti–vascular endothelial growth factor treatment of neovascular age-related macular degeneration: the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123(8):1751–1761. doi:10.1016/j.ophtha.2016.03.045
23. Chandra S, Arpa C, Menon D, et al. Ten-year outcomes of antivascular endothelial growth factor therapy in neovascular age-related macular degeneration. Eye. 2020;34(10):1888–1896. doi:10.1038/s41433-020-0764-9
24. Finger RP, Wickremasinghe SS, Baird PN, Guymer RH. Predictors of anti-VEGF treatment response in neovascular age-related macular degeneration. Surv Ophthalmol. 2014;59(1):1–18. doi:10.1016/j.survophthal.2013.03.009
25. Gill CR, Hewitt CE, Lightfoot T, Gale RP. Demographic and clinical factors that influence the visual response to anti-vascular endothelial growth factor therapy in patients with neovascular age-related macular degeneration: a systematic review. Ophthalmol Ther. 2020;9(4):725–737. doi:10.1007/s40123-020-00288-0
26. Boyer DS, Antoszyk AN, Awh CC, et al. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114(2):246–252. doi:10.1016/j.ophtha.2006.10.045
27. Schmidt-Erfurth U, Waldstein SM, Deak -G-G, Kundi M, Simader C. Pigment epithelial detachment followed by retinal cystoid degeneration leads to vision loss in treatment of neovascular age-related macular degeneration. Ophthalmology. 2015;122(4):822–832. doi:10.1016/j.ophtha.2014.11.017
28. Fu DJ, Keenan TD, Faes L, et al. Insights from survival analyses during 12 years of anti-vascular endothelial growth factor therapy for neovascular age-related macular degeneration. JAMA Ophthalmol. 2020;139:57.
29. Westborg I, Albrecht S, Rosso A. Risk for low visual acuity after 1 and 2 years of treatment with ranibizumab or bevacizumab for patients with neovascular age-related macular degeneration. Retina. 2017;37(11):2035–2046. doi:10.1097/IAE.0000000000001431
30. Gillies M, Arnold J, Bhandari S, et al. Ten-year treatment outcomes of neovascular age-related macular degeneration from two regions. Am J Ophthalmol. 2020;210:116–124. doi:10.1016/j.ajo.2019.10.007
31. Pedrosa AC, Sousa T, Pinheiro-Costa J, et al. Treatment of neovascular age-related macular degeneration with anti-VEGF agents: predictive factors of long-term visual outcomes. J Ophthalmol. 2017;2017:4263017. doi:10.1155/2017/4263017
32. Ho AC, Kleinman DM, Lum FC, et al. Baseline visual acuity at wet AMD diagnosis predicts long-term vision outcomes: an analysis of the iris registry. Ophthalmic Surg Lasers Imaging Retina. 2020;51(11):633–639. doi:10.3928/23258160-20201104-05
33. Brynskov T, Munch IC, Larsen TM, Erngaard L, Sørensen TL. Real‐world 10‐year experiences with intravitreal treatment with ranibizumab and aflibercept for neovascular age‐related macular degeneration. Acta Ophthalmol. 2020;98(2):132–138. doi:10.1111/aos.14183
34. Arpa C, Khalid H, Chandra S. et al. Ten-year survival trends of neovascular age-related macular degeneration at first presentation. Br J Ophthalmol;2020.
35. Spooner K, Fraser-Bell S, Hong T, Phan L, Wong JG, Chang A. Long-term anti–vascular endothelial growth factor treatment for neovascular age-related macular degeneration: the LATAR study: report 1: ten-year, real-world outcomes. Ophthalmol Retina. 2020;5:511.
36. van Asten F, Rovers MM, Lechanteur YT, et al. Predicting non-response to ranibizumab in patients with neovascular age-related macular degeneration. Ophthalmic Epidemiol. 2014;21(6):347–355. doi:10.3109/09286586.2014.949010
37. Nguyen CL, Gillies MC, Nguyen V, et al. Characterization of poor visual outcomes of neovascular age-related macular degeneration treated with anti–vascular endothelial growth factor agents. Ophthalmology. 2019;126(5):735–742. doi:10.1016/j.ophtha.2018.11.036
38. Fulcher C, Hazel CA, Pacey I, Ali H, Ghanchi FD. Predicting visual outcomes in patients treated with aflibercept for neovascular age-related macular degeneration: data from a real-world clinical setting. Eur J Ophthalmol. 2020;30(3):543–549. doi:10.1177/1120672119839299
39. Nguyen V, Daien V, Guymer R, et al. Projection of long-term visual acuity outcomes based on initial treatment response in neovascular age-related macular degeneration. Ophthalmology. 2019;126(1):64–74. doi:10.1016/j.ophtha.2018.08.023
40. Ying GS, Maguire MG, Daniel E, et al. Association of baseline characteristics and early vision response with 2-year vision outcomes in the comparison of AMD treatments trials (CATT). Ophthalmology. 2015;122(12):2523–2531.e2521. doi:10.1016/j.ophtha.2015.08.015
41. Canan H, Sızmaz S, Altan-Yaycıoğlu R, Sarıtürk C, Yılmaz G. Visual outcome of intravitreal ranibizumab for exudative age-related macular degeneration: timing and prognosis. Clin Interv Aging. 2014;9:141–145. doi:10.2147/CIA.S56863
42. Lim JH, Wickremasinghe SS, Xie J, et al. Delay to treatment and visual outcomes in patients treated with anti-vascular endothelial growth factor for age-related macular degeneration. Am J Ophthalmol. 2012;153(4):678–686. doi:10.1016/j.ajo.2011.09.013
43. Fang K, Tian J, Qing X, et al. Predictors of visual response to intravitreal bevacizumab for treatment of neovascular age-related macular degeneration. J Ophthalmol. 2013;2013:1–9. doi:10.1155/2013/676049
44. Kim JH, Chang YS, Kim JW, Kim CG, Yoo SJ, Cho HJ. Intravitreal anti-vascular endothelial growth factor for submacular hemorrhage from choroidal neovascularization. Ophthalmology. 2014;121(4):926–935. doi:10.1016/j.ophtha.2013.11.004
45. Kaiser PK, Brown DM, Zhang K, et al. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007;144(6):850–857. doi:10.1016/j.ajo.2007.08.012
46. Ying G-S, Huang J, Maguire MG, et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120(1):122–129. doi:10.1016/j.ophtha.2012.07.042
47. Lanzetta P, Cruess AF, Cohen SY, et al. Predictors of visual outcomes in patients with neovascular age‐related macular degeneration treated with anti‐vascular endothelial growth factor therapy: post hoc analysis of the VIEW studies. Acta Ophthalmol. 2018;96(8):e911. doi:10.1111/aos.13751
48. Regillo CD, Busbee BG, Ho AC, Ding B, Haskova Z. Baseline predictors of 12-month treatment response to ranibizumab in patients with wet age-related macular degeneration. Am J Ophthalmol. 2015;160(5):1014–1023.e1012. doi:10.1016/j.ajo.2015.07.034
49. Ying G-S, Maguire MG, Pan W, et al. Baseline predictors for five-year visual acuity outcomes in the comparison of AMD treatment trials. Ophthalmol Retina. 2018;2(6):525–530. doi:10.1016/j.oret.2017.10.003
50. Holz FG, Tadayoni R, Beatty S, et al. Key drivers of visual acuity gains in neovascular age-related macular degeneration in real life: findings from the AURA study. Br J Ophthalmol. 2016;100(12):1623–1628. doi:10.1136/bjophthalmol-2015-308166
51. Yamashiro K, Tomita K, Tsujikawa A, et al. Factors associated with the response of age-related macular degeneration to intravitreal ranibizumab treatment. Am J Ophthalmol. 2012;154(1):125–136. doi:10.1016/j.ajo.2012.01.010
52. Kawasaki R, Yasuda M, Song SJ, et al. The prevalence of age-related macular degeneration in Asians: a systematic review and meta-analysis. Ophthalmology. 2010;117(5):921–927. doi:10.1016/j.ophtha.2009.10.007
53. Kwok A, Lai T, Chan C, Neoh E, Lam D. Polypoidal choroidal vasculopathy in Chinese patients. Br J Ophthalmol. 2002;86(8):892–897. doi:10.1136/bjo.86.8.892
54. Kokame GT, deCarlo TE, Kaneko KN, Omizo JN, Lian R. Anti–vascular endothelial growth factor resistance in exudative macular degeneration and polypoidal choroidal vasculopathy. Ophthalmol Retina. 2019;3(9):744–752. doi:10.1016/j.oret.2019.04.018
55. Lorentzen TD, Subhi Y, Sørensen TL. Prevalence of polypoidal choroidal vasculopathy in white patients with exudative age-related macular degeneration: systematic review and meta-analysis. Retina. 2018;38(12):2363–2371. doi:10.1097/IAE.0000000000001872
56. Hatz K, Prünte C. Polypoidal choroidal vasculopathy in Caucasian patients with presumed neovascular age-related macular degeneration and poor ranibizumab response. Br J Ophthalmol. 2014;98(2):188–194. doi:10.1136/bjophthalmol-2013-303444
57. Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728–1738. doi:10.1016/S0140-6736(12)60282-7
58. Piermarocchi S, Miotto S, Colavito D, Leon A, Segato T. Combined effects of genetic and non-genetic risk factors affect response to ranibizumab in exudative age-related macular degeneration. Acta Ophthalmol. 2015;93(6):e451. doi:10.1111/aos.12587
59. Lee S, Song SJ, Yu HG. Current smoking is associated with a poor visual acuity improvement after intravitreal ranibizumab therapy in patients with exudative age-related macular degeneration. J Korean Med Sci. 2013;28(5):769. doi:10.3346/jkms.2013.28.5.769
60. Kang S, Roh YJ. One-year results of intravitreal ranibizumab for neovascular age-related macular degeneration and clinical responses of various subgroups. Jpn J Ophthalmol. 2009;53(4):389–395. doi:10.1007/s10384-009-0670-y
61. Singh RP, Fu EX, Smith SD, Williams DR, Kaiser PK. Predictive factors of visual and anatomical outcome after intravitreal bevacizumab treatment of neovascular age-related macular degeneration: an optical coherence tomography study. Br J Ophthalmol. 2009;93(10):1353–1358. doi:10.1136/bjo.2008.141879
62. Abedi F, Wickremasinghe S, Richardson AJ, et al. Variants in the VEGFA gene and treatment outcome after anti-VEGF treatment for neovascular age-related macular degeneration. Ophthalmology. 2013;120(1):115–121. doi:10.1016/j.ophtha.2012.10.006
63. Byun YJ, Lee SJ, Koh HJ. Predictors of response after intravitreal bevacizumab injection for neovascular age-related macular degeneration. Jpn J Ophthalmol. 2010;54(6):571–577. doi:10.1007/s10384-010-0866-1
64. Hermann MM, van Asten F, Muether PS, et al. Polymorphisms in vascular endothelial growth factor receptor 2 are associated with better response rates to ranibizumab treatment in age-related macular degeneration. Ophthalmology. 2014;121(4):905–910. doi:10.1016/j.ophtha.2013.10.047
65. Jakobsen DB, Torp TL, Stefansson E, Peto T, Grauslund J. Retinal metabolic and structural alterations in response to aflibercept treatment in neovascular age-related macular degeneration. Acta Ophthalmol. 2019;97(5):525–531. doi:10.1111/aos.13996
66. Lee H, Ji B, Chung H, Kim HC. Correlation between optical coherence tomographic hyperreflective foci and visual outcomes after anti-vegf treatment in neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Retina. 2016;36(3):465–475. doi:10.1097/IAE.0000000000000645
67. Hagstrom SA, Ying GS, Pauer GJT, et al. Pharmacogenetics for genes associated with age-related macular degeneration in the Comparison of AMD Treatments Trials (CATT). Ophthalmology. 2013;120(3):593–599. doi:10.1016/j.ophtha.2012.11.037
68. Lotery AJ, Gibson J, Cree AJ, et al. Pharmacogenetic associations with vascular endothelial growth factor inhibition in participants with neovascular age-related macular degeneration in the IVAN Study. Ophthalmology. 2013;120(12):2637–2643. doi:10.1016/j.ophtha.2013.07.046
69. Chen G, Tzekov R, Li W, Jiang F, Mao S, Tong Y. Pharmacogenetics of complement factor H Y402H polymorphism and treatment of neovascular AMD with anti-VEGF agents: a meta-analysis. Sci Rep. 2015;5(1):1–9.
70. Hong N, Shen Y, Yu CY, Wang SQ, Tong JP. Association of the polymorphism Y402H in the CFH gene with response to anti-VEGF treatment in age‐related macular degeneration: a systematic review and meta‐analysis. Acta Ophthalmol. 2016;94(4):334–345. doi:10.1111/aos.13049
71. Hu Z, Xie P, Ding Y, Yuan D, Liu Q. Association between variants A69S in ARMS2 gene and response to treatment of exudative AMD: a meta-analysis. Br J Ophthalmol. 2015;99(5):593–598. doi:10.1136/bjophthalmol-2014-305488
72. Zhang J, Liu Z, Hu S, Qi J. Meta-analysis of the pharmacogenetics of ARMS2 A69S polymorphism and the response to advanced age-related macular degeneration. Ophthalmic Res. 2021;64(2):192–204.
73. Zhou Y-L, Chen C-L, Wang Y-X, et al. Association between polymorphism rs11200638 in the HTRA1 gene and the response to anti-VEGF treatment of exudative AMD: a meta-analysis. BMC Ophthalmol. 2017;17(1):1–9. doi:10.1186/s12886-017-0487-2
74. Hagstrom SA, Ying G-S, Pauer GJ, et al. Vegfa and vegfr2 gene polymorphisms and response to anti–vascular endothelial growth factor therapy: comparison of age-related macular degeneration treatments trials (CATT). JAMA Ophthalmol. 2014;132(5):521–527. doi:10.1001/jamaophthalmol.2014.109
75. Boltz A, Ruiß M, Jonas JB, et al. Role of vascular endothelial growth factor polymorphisms in the treatment success in patients with wet age-related macular degeneration. Ophthalmology. 2012;119(8):1615–1620. doi:10.1016/j.ophtha.2012.02.001
76. Lazzeri S, Figus M, Orlandi P, et al. VEGF-A polymorphisms predict short-term functional response to intravitreal ranibizumab in exudative age-related macular degeneration. Pharmacogenomics. 2013;14(6):623–630. doi:10.2217/pgs.13.43
77. Park UC, Shin JY, Kim SJ, et al. Genetic factors associated with response to intravitreal ranibizumab in Korean patients with neovascular age-related macular degeneration. Retina. 2014;34(2):288–297. doi:10.1097/IAE.0b013e3182979e1e
78. Cruz-Gonzalez F, Cabrillo-Estévez L, López-Valverde G, Cieza-Borrella C, Hernández-Galilea E, González-Sarmiento R. Predictive value of VEGF A and VEGFR2 polymorphisms in the response to intravitreal ranibizumab treatment for wet AMD. Graefes Arch Clin Exp Ophthalmol. 2014;252(3):469–475. doi:10.1007/s00417-014-2585-7
79. Hagstrom SA, Ying G-S, Maguire MG, et al. VEGFR2 gene polymorphisms and response to anti–vascular endothelial growth factor therapy in age-related macular degeneration. Ophthalmology. 2015;122(8):1563–1568. doi:10.1016/j.ophtha.2015.04.024
80. Wu M, Xiong H, Xu Y, et al. Association between VEGF-A and VEGFR-2 polymorphisms and response to treatment of neovascular AMD with anti-VEGF agents: a meta-analysis. Br J Ophthalmol. 2017;101(7):976–984. doi:10.1136/bjophthalmol-2016-309418
81. Jelstrup AB, Pomares E, Navarro R, Group BS. Relationship between aflibercept efficacy and genetic variants of genes associated with neovascular age-related macular degeneration: the BIOIMAGE trial. Ophthalmologica. 2020;243(6):461–470. doi:10.1159/000508902
82. Francis PJ. The influence of genetics on response to treatment with ranibizumab (Lucentis) for age-related macular degeneration: the Lucentis Genotype Study (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2011;109:115.
83. Riaz M, Lorés-Motta L, Richardson AJ, et al. GWAS study using DNA pooling strategy identifies association of variant rs4910623 in OR52B4 gene with anti-VEGF treatment response in age-related macular degeneration. Sci Rep. 2016;6(1):1–10. doi:10.1038/srep37924
84. Lorés-Motta L, Riaz M, Grunin M, et al. Association of genetic variants with response to anti–vascular endothelial growth factor therapy in age-related macular degeneration. JAMA Ophthalmol. 2018;136(8):875–884. doi:10.1001/jamaophthalmol.2018.2019
85. Daniel E, Shaffer J, Ying G-S, et al. Outcomes in eyes with retinal angiomatous proliferation in the comparison of age-related macular degeneration treatments trials (CATT). Ophthalmology. 2016;123(3):609–616. doi:10.1016/j.ophtha.2015.10.034
86. Rosenfeld PJ, Shapiro H, Tuomi L, Webster M, Elledge J, Blodi B. Characteristics of patients losing vision after 2 years of monthly dosing in the Phase III ranibizumab clinical trials. Ophthalmology. 2011;118(3):523–530. doi:10.1016/j.ophtha.2010.07.011
87. Jaffe GJ, Ying G-S, Toth CA, et al. Macular morphology and visual acuity in year five of the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2019;126(2):252–260. doi:10.1016/j.ophtha.2018.08.035
88. Steinle NC, Du W, Gibson A, Saroj N. Outcomes by baseline choroidal neovascularization features in age-related macular degeneration: a post hoc analysis of the VIEW Studies. Ophthalmol Retina. 2021;5(2):141–150. doi:10.1016/j.oret.2020.07.003
89. Dugel PU, Singh RP, Koh A, et al. HAWK and HARRIER: ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2021;128(1):89–99. doi:10.1016/j.ophtha.2020.06.028
90. Sharma S, Toth CA, Daniel E, et al. Macular morphology and visual acuity in the second year of the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2016;123(4):865–875. doi:10.1016/j.ophtha.2015.12.002
91. Fung AE, Lalwani GA, Rosenfeld PJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143(4):566–583. doi:10.1016/j.ajo.2007.01.028
92. Evans RN, Reeves BC, Maguire MG, et al. Associations of variation in retinal thickness with visual acuity and anatomic outcomes in eyes with neovascular age-related macular degeneration lesions treated with anti–vascular endothelial growth factor agents. JAMA Ophthalmol. 2020;138(10):1043–1051. doi:10.1001/jamaophthalmol.2020.3001
93. Simader C, Ritter M, Bolz M, et al. Morphologic parameters relevant for visual outcome during anti-angiogenic therapy of neovascular age-related macular degeneration. Ophthalmology. 2014;121(6):1237–1245. doi:10.1016/j.ophtha.2013.12.029
94. Schmidt-Erfurth U, Vogl W-D, Jampol LM, Bogunović H. Application of automated quantification of fluid volumes to Anti–VEGF therapy of neovascular age-related macular degeneration. Ophthalmology. 2020;127(9):1211–1219. doi:10.1016/j.ophtha.2020.03.010
95. Reiter GS, Grechenig C, Vogl W-D, et al. Analysis of fluid volume and its impact on visual acuity in the fluid study as quantified with deep learning. Retina. 2021;41(6):1318–1328.
96. Busbee BG, Ho AC, Brown DM, et al. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120(5):1046–1056. doi:10.1016/j.ophtha.2012.10.014
97. Waldstein SM, Simader C, Staurenghi G, et al. Morphology and visual acuity in aflibercept and ranibizumab therapy for neovascular age-related macular degeneration in the VIEW trials. Ophthalmology. 2016;123(7):1521–1529. doi:10.1016/j.ophtha.2016.03.037
98. Guymer RH, Markey CM, McAllister IL, et al. Tolerating subretinal fluid in neovascular age-related macular degeneration treated with ranibizumab using a treat-and-extend regimen: FLUID study 24-month results. Ophthalmology. 2019;126(5):723–734. doi:10.1016/j.ophtha.2018.11.025
99. Waldstein SM, Wright J, Warburton J, Margaron P, Simader C, Predictive S-EU. Value of retinal morphology for visual acuity outcomes of different ranibizumab treatment regimens for neovascular AMD. Ophthalmology. 2016;123(1):60–69. doi:10.1016/j.ophtha.2015.09.013
100. Jhaveri CD Visual and anatomical outcomes for brolucizumab and aflibercept in patients with nAMD: 96-week data from HAWK and HARRIER.
101. Willoughby AS, Ying G-S, Toth CA, et al. Subretinal hyperreflective material in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2015;122(9):1846–1853.
102. Ehlers JP, Zahid R, Kaiser PK, et al. Longitudinal assessment of ellipsoid zone integrity, subretinal hyperreflective material, and subretinal pigment epithelium disease in neovascular age-related macular degeneration. Ophthalmol Retina. 2021. doi:10.1016/j.oret.2021.02.012
103. Sarraf D, London NJ, Khurana RN, et al. Ranibizumab treatment for pigment epithelial detachment secondary to neovascular age-related macular degeneration: post hoc analysis of the HARBOR study. Ophthalmology. 2016;123(10):2213–2224. doi:10.1016/j.ophtha.2016.07.007
104. Javaheri M, Hill L, Ghanekar A, Stoilov I. Changes in treatment-naive pigment epithelial detachments associated with the initial anti–vascular endothelial growth factor injection: a post hoc analysis from the HARBOR Trial. JAMA Ophthalmol. 2021;139(2):219–223. doi:10.1001/jamaophthalmol.2020.5130
105. Khanani AM, Eichenbaum D, Schlottmann PG, Tuomi L, Sarraf D. Optimal management of pigment epithelial detachments in eyes with neovascular age-related macular degeneration. Retina. 2018;38(11):2103. doi:10.1097/IAE.0000000000002195
106. Ying G-S, Kim BJ, Maguire MG, et al. Sustained visual acuity loss in the comparison of age-related macular degeneration treatments trials. JAMA Ophthalmol. 2014;132(8):915–921. doi:10.1001/jamaophthalmol.2014.1019
107. Bhisitkul RB, Mendes TS, Rofagha S, et al. Macular atrophy progression and 7-year vision outcomes in subjects from the ANCHOR, MARINA, and HORIZON studies: the SEVEN-UP study. Am J Ophthalmol. 2015;159(5):915–924. doi:10.1016/j.ajo.2015.01.032
108. Altaweel MM, Daniel E, Martin DF, et al. Outcomes of eyes with lesions composed of> 50% blood in the comparison of age-related macular degeneration treatments trials (CATT). Ophthalmology. 2015;122(2):391–398.
109. Stanescu-Segall D, Balta F, Jackson TL. Submacular hemorrhage in neovascular age-related macular degeneration: a synthesis of the literature. Surv Ophthalmol. 2016;61(1):18–32. doi:10.1016/j.survophthal.2015.04.004
110. Daniel E, Pan W, Ying G-S, et al. Development and course of scars in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2018;125(7):1037–1046. doi:10.1016/j.ophtha.2018.01.004
111. Okeagu CU, Agrón E, Vitale S, et al. Principal cause of poor visual acuity after neovascular age-related macular degeneration: age-related eye disease study 2 report number 23. Ophthalmol Retina. 2021;5(1):23–31. doi:10.1016/j.oret.2020.09.025
112. Chakravarthy U, Harding SP, Rogers CA, et al. A randomised controlled trial to assess the clinical effectiveness and cost-effectiveness of alternative treatments to Inhibit VEGF in Age-related choroidal Neovascularisation (IVAN). Health Tech Assessment. 2015;19(78):1. doi:10.3310/hta19780
113. Wykoff CC, Ou WC, Brown DM, et al. Randomized trial of treat-and-extend versus monthly dosing for neovascular age-related macular degeneration: 2-year results of the TREX-AMD study. Ophthalmol Retina. 2017;1(4):314–321. doi:10.1016/j.oret.2016.12.004
114. Kertes PJ, Galic IJ, Greve M, et al. Efficacy of a treat-and-extend regimen with ranibizumab in patients with neovascular age-related macular disease: a randomized clinical trial. JAMA Ophthalmol. 2020;138(3):244–250. doi:10.1001/jamaophthalmol.2019.5540
115. Chin-Yee D, Eck T, Fowler S, Hardi A, Apte RS. A systematic review of as needed versus treat and extend ranibizumab or bevacizumab treatment regimens for neovascular age-related macular degeneration. Br J Ophthalmol. 2016;100(7):914–917. doi:10.1136/bjophthalmol-2015-306987
116. Wykoff CC, Ou WC, Croft DE, et al. Neovascular age-related macular degeneration management in the third year: final results from the TREX-AMD randomised trial. Br J Ophthalmol. 2018;102(4):460–464. doi:10.1136/bjophthalmol-2017-310822
117. Spooner KL, Fraser-Bell S, Cozzi M, et al. Macular atrophy incidence and progression in eyes with neovascular age-related macular degeneration treated with vascular endothelial growth factor inhibitors using a treat-and-extend or a pro re nata regimen: four-year results of the MANEX Study. Ophthalmology. 2020;127(12):1663–1673. doi:10.1016/j.ophtha.2020.06.019
118. Fasler K, Moraes G, Wagner S, et al. One- and two-year visual outcomes from the Moorfields age-related macular degeneration database: a retrospective cohort study and an open science resource. BMJ Open. 2019;9(6):e027441. doi:10.1136/bmjopen-2018-027441
119. Schmidt-Erfurth U, Bogunovic H, Sadeghipour A, et al. Machine learning to analyze the prognostic value of current imaging biomarkers in neovascular age-related macular degeneration. Ophthalmol Retina. 2018;2(1):24–30. doi:10.1016/j.oret.2017.03.015
120. Rohm M, Tresp V, Müller M, et al. Predicting visual acuity by using machine learning in patients treated for neovascular age-related macular degeneration. Ophthalmology. 2018;125(7):1028–1036. doi:10.1016/j.ophtha.2017.12.034
121. Singh RP, Wykoff CC, Tadayoni R, et al. Visual and expanded anatomical outcomes for brolucizumab versus aflibercept in patients with neovascular AMD: 96-week data from HAWK and HARRIER. Invest Ophthalmol Vis Sci. 2019;60(9):5194.
122. Zweifel SA, Saroj N, Shapiro H, Freund KB. The effect of fellow eye visual acuity on visual acuity of study eyes receiving ranibizumab for age-related macular degeneration. Retina. 2012;32(7):1243–1249. doi:10.1097/IAE.0b013e3182469064
123. Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148(1):43–58. doi:10.1016/j.ajo.2009.01.024
124. Brown DM, Tuomi L, Shapiro H, Group PS. Anatomical measures as predictors of visual outcomes in ranibizumab-treated eyes with neovascular age-related macular degeneration. Retina. 2013;33(1):23–34. doi:10.1097/IAE.0b013e318263cedf
125. Frenkel RE, Shapiro H, Stoilov I. Predicting vision gains with anti-VEGF therapy in neovascular age-related macular degeneration patients by using low-luminance vision. Br J Ophthalmol. 2016;100(8):1052–1057. doi:10.1136/bjophthalmol-2015-307575
126. Khurana RN, Chang L, Day BM, Ghanekar A, Stoilov I. Timing of peak vision gains in patients with neovascular age-related macular degeneration treated with ranibizumab. Ophthalmol Retina. 2020;4(8):760–766. doi:10.1016/j.oret.2020.02.011
127. Schroeder M, Westborg I, Lövestam Adrian M. Twelve per cent of 6142 eyes treated for neovascular age-related macular degeneration (nAMD) presented with low visual outcome within 2 years. Analysis from the Swedish macula registry (SMR). Acta Ophthalmol. 2020;98(3):274–278. doi:10.1111/aos.14239
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
