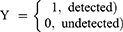Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 15
Predictors of Viral Load Status Over Time Among HIV Infected Adults Under HAART in Zewditu Memorial Hospital, Ethiopia: A Retrospective Study
Authors Agegn Gwadu A , Abebe Tegegne M, Belay Mihretu K, Tegegne AS
Received 10 November 2022
Accepted for publication 3 February 2023
Published 7 February 2023 Volume 2023:15 Pages 29—40
DOI https://doi.org/10.2147/HIV.S396030
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Ayitenew Agegn Gwadu,1 Mengistu Abebe Tegegne,1 Kidist Belay Mihretu,1 Awoke Seyoum Tegegne2
1Departments of Statistics, Dire Dawa University, Dire Dawa, Ethiopia; 2Department of Statistics, Bahir Dar University, Bahir Dar, Ethiopia
Correspondence: Awoke Seyoum Tegegne, Email [email protected]
Background: HIV attacks the CD4 cells which are responsible for the body’s immune response to infectious agents. The main objective of this study was to identify predictors of viral load status over time among HIV patients under HAART in Zewditu Memorial Hospital.
Methods: A retrospective institutional-based cohort study design was conducted on 161 HIV-infected adults under HAART whose follow-ups were from January 2014 up to December 2017. A generalized linear mixed-effects model was conducted to infer predictors of the status of viral load at 95% of CI).
Results: The descriptive statistics revealed that about 55.9% of the adults under treatment had a detected viral load status. Among the potential predictors, visiting time of patients (AOR = 0.731, 95%: (0.634,0.842) and p-value < 0.01), age of patients (AOR = 1.0666, 95% CI: (1.0527,1.0917) and p-value < 0.01), weight (AOR=. 0.904, 95% CI: (0.862, 0.946) and p-value < 0.01), baseline CD4 cell count (AOR = 0.996, 95% CI: (0.994, 0.998) and P-value < 0.01), educated patients (AOR = 0.030, 95% CI: (0.002, 0.385) and p-value=0.0053), rural patients (AOR = 6.30,95% CL: (1.78, 2.25) and p-value=0.0043), working status patients (AOR = 0.5905, 95% CI: (0.547,0.638), p-value < 0.01), poor adherent patients (AOR = 1.120, 95% CI; (1.035,1.391) and p-value = 0.016) and patients disclosed the disease status (AOR = 0.195, 95% CI: (0.023, 0.818) and p-value=0.0134) significantly affected the detection status of viral loads, keeping all other covariates constant.
Conclusion: The predictor variables; visiting times, the weight of patients, residence area, age of patients, educational level, clinical stages, functional status, baseline CD4 cell count, adherence status, and disclosure status of the disease statistically and significantly affected the status of viral load. Hence, health-related education should be given for patients to disclose their disease status, to be good adherents based on the prescription given to the health staff. Due attentions should be given for rural and uneducated patients. Attention should be forwarded to for non-adherent patients to follow the instruction given by the health staff.
Keywords: logistic regression, HAART, retrospective study, generalized linear mixed model, odds ratio
Introduction
Human Immune Deficiency Virus (HIV) is a virus that causes Acquired Immune Deficiency Syndrome (AIDS) by reducing a person’s ability to fight infection.1 HIV attacks an immune cell called the CD4 cell, which is responsible for the body’s immune response to infectious agents.1 An uninfected individual has around 1100 CD4 cells per milliliter of blood. These CD4 cells decrease in number while living with the HIV virus, so that HIV infected person’s CD4 cell count can be used to monitor the progression of the disease.2
According to the UNAIDS fact sheet updated report, about 36.7 million people were living with HIV globally in 2016.2 Among those infected people, adults account for 34.5 million. Since the start of the epidemic in 1981, 76.1 million people have become infected by the virus and 35 million people have died from AIDS-related illnesses respectively.3
East and Southern Africa is the hardest region hit by HIV.6 This region is the home of 6.2% of the world’s population and this indicates that about 19.4 million people are living with the virus and this further leads that over 50% of the total number of people living with the virus are living in this region.4
Ethiopia is one of the few African countries with the highest number of people living with HIV/AIDS.5 Based on a single-point estimate, about 1.2 million HIV-infected people are living in this country.6
Recent studies indicate that HIV infection has significantly decreased over the years in the country.7 Previous studies indicate that ART was introduced in 1996 for Ethiopian people.8 The effective use of antiretroviral drugs can control the virus and help to prevent transmission so that people with HIV and those at substantial risk can enjoy healthy, long, and productive lives.9 Even though the Ethiopian government launched free access to ART in different health sectors to improve the quality of life of PLWHA, lots of people die every year due to HIV/AIDS.10
A viral load is an indicator of how much Human Immunodeficiency Virus (HIV) is in the blood of an individual. Viral load gives an idea of how much of the HIV virus is in the patient’s body.11 The test measures the number of HIV copies in a milliliter of blood.12
Many studies on HIV patients related to CD4 cell count, adherence, body mass index, and related variables had been conducted but there is a scarcity of research conducted on factors affecting viral load status as it needs intensive laboratory work for viral load. The test results help the health staff to follow what is happening with the patient’s infection, how well the treatment is working, and guide treatment choices.13 Viral load also predicts how fast the disease progress.14 Therefore, the objective of the current study was to investigate factors affecting the viral load status over time among HIV adult HIV patients under treatment at Zewditu Memorial Hospital. The other objective of the current study was to assess whether the predictors of viral status happened in the developed countries also work in the study area.
Materials and Methods
Study Area and Design
A retrospective cohort study design was conducted at Zewditu Memorial Hospital, in Addis Ababa, Ethiopia.
Source of Data
The HIV-infected patients under HAART in the hospital were considered as a source of the population for this study. In this study, secondary data collected by health staff for treatment purposes were used for data analysis. The data was collected from the medical chart of each HIV/AIDS patient in the ART clinic in the hospital.
Study Population
The study population consists of all HIV-positive adult patients who started their HAART treatment in January 2014 – December 2017.
Inclusion Criteria
HIV/AIDS-infected patients who had at least two follow-ups in the ART clinic for refilling their prescriptions and who initiated their treatment from 1st January 2014 to 31st December 2017 at Zewditu Memorial Hospital were eligible for the current study.
Sample Size Determination
For the current investigation, there were 1304 HIV-positive patients whose follow-ups were from January 2014 to December 2017. Among these patients, 161 were adult patients and were considered as a sample. Hence, all patients under treatment whose follow-ups were in the study period were considered to be a sample.
Variables Under Current Investigation
The response variable under the current investigation was the viral load results of HIV-positive patients under treatment. The viral load result was measured every six months. From this response, the result may be detected (viral load result greater than or equal to 1000 cell/mm3) and undetected (viral load result which is less than 1000 cell/mm3) are classified under viral load test result of the HIV-infected patients.15 Thus, the response variable under study was dichotomous in nature and denoted as;
The predictor (independent) covariates under current investigation were sex (male, female), weight in kg, residence area (rural, urban), age in years, marital status (living with a partner, living without a partner), an education level (non-educated, educated), level of disclosure (yes, no), clinical stage (stage1, stage2, stage3, stage4), functional status (ambulatory, bedridden, working), level of adherence (adherent, non-adherent), TB co-infection status (no, yes).
Data Analysis
Descriptive statistics are used to describe the basic features of the data in a study. They provide simple summaries of the sample and the measures. The collected data had been cleaned, edited, and entered into data analysis. Frequency distributions, computing percentages, cross-tabulations, mean and standard deviation, graphs, and diagrams were used to describe whether there is any difference between two or more groups. Inferential statistics allow one to make inferences from the sample to the population.8 The data were analyzed using SAS and R statistical software packages. In data analysis, both fixed and random effects were included in the generalized linear mixed-effect model. The fixed effect accounted for the set of predictors that are fixed across the subjects and described the relationships between predictor and response variables. The random effect belongs to subject-specific effects.
Binary Logistic Regression
Logistic regression is a popular modeling approach when the dependent variable is dichotomous, ordinal, or multinomial. It allows us in predicting the log odds of outcomes of a dependent variable that may be affected by the set of variables namely continuous, discrete, categorical, or a mix of any of these. The error term in binary logistic regression is distributed binomially, not normally. Consequently, the response variable in logistic regression is not restricted to normality in the case of parameter estimation. Because of this, logistic regression was the most popular method for analyzing the current binary response data.
Covariance Structures
Among the various covariance structures, compound symmetry (CS), unstructured (UN), and first-order autoregressive (AR (1)) were conducted and compared to each other to identify the one which fitted the data well. During the comparison of the three structures, AIC and BIC information criteria were considered assumed that the one with the smallest information criterion was the best for current data.
Methods of Parameter Estimation
Parameter estimation in the current investigation was conducted using maximum likelihood estimation (MLE). This was done by maximizing the joint probability (likelihood function) for values of the data. Maximum likelihood estimation includes both the regression coefficient and the variance components, that is, both fixed-effects and random-effects terms in the likelihood function and it treated parameters as fixed but unknown quantities when the variance component was estimated. Method of parameter estimation was conducted both for the subject‐specific and marginal residuals.
The Assessment of Goodness of Fit of Logistic Regression Model
In assessing the goodness of fit, first, the assumption (at least preliminarily satisfied with our efforts at the model-building stage) was conducted. Hence, the model contains those variables (main effects as well as interaction effects) that should be entered in the correct functional form. The goodness of fit measures how the model describes the response variable and it also investigates how close are predicted by the model with the observed values. Hence, for the current investigation, the Hosmer and Lemeshow Goodness of fit Test and the likelihood ratio tests were used to measure of goodness of fit for categorical data,16 and the Wald test statistic was used to test the significance of individual parameters (coefficients).
Missing Data Treatment
Missing data (or missing values) is defined as the data value that is not stored for a variable in the observation of interest. There were different imputing techniques for missing values treatment in the longitudinal study. For the current investigation, multiple imputation method was conducted for missing value treatment.17
Results
Table 1 displays patient characteristics for the HIV/AIDS data from Zewditu Memorial Hospital. Out of a sample of 161 patients, 67.7% were females and the remaining 32.3% were males. The majority of patients (67.7%) were from urban areas, about 56.52% of the participants were employed, and the remaining were not. Among the participants, about 68.32% were educated and the remaining were non-educated. The majority (50.17%) of the infected patients were at working functional status (ie, an individual able to perform usual work in and out of the house), followed by those with the ambulatory type of functional status, accounted for 30.21% of the total, and the rest were bedridden patients.
 |
Table 1 Baseline Characteristics of Potential Predictors for HIV/AIDS Patients |
Regarding the clinical stages, about 17.39% were at clinical stage I, 32.30% were at clinical stage II, 27.33% were at clinical stage III, and the rest were at clinical stage IV. Regarding the adherence status, about 60.25% of the patients were good adherent, 24.22% were fair adherent and the remaining patients were poor adherent.
As indicated in Table 2, the baseline characteristics of continuous variables were also summarized and the average baseline age in years, baseline CD4 cells/mm3, and baseline weight in kg were 31.47, 221, and 48.46, respectively.
 |
Table 2 Base Line Charachteristics of Continuous Variables |
Table 3 shows viral load status among HIV patients under treatment and the result indicates that about 90 of the patients were at detected status at baseline, 6 th, and 12th measurements, and 71 of them were not at detected status at baseline, 6 th, and 12th measurements. The result in Table 3 indicates that the status of viral progression decreased as visiting time increased.
 |
Table 3 Summaries of Descriptive Statistics for the Response Variables |
Selection of Covariance Structure in GIMM
For the Generalized Linear Mixed Effect Model (GLMM) to be valid, the covariance among repeated measures must be modeled properly. To identify the appropriate covariance structure, the commonly used covariance structures such as compound symmetry (CS), first-order autoregressive (AR (1)), heterogeneous compound symmetry (CSH), heterogeneous first-order autoregressive (HAR (1)), and unstructured (UN) were considered and compared to select the one with the smallest information criterion. In Table 4, the smallest values of AIC and BIC for the unstructured (UN) model suggest that it was the best fit to the current data as compared to the remaining covariance structures.
 |
Table 4 Comparison of Covariance Structure for Generalized Linear Mixed-Effects Model |
Selection of Random Effects in GLMM
Based on the unstructured (UN) covariance structure, the different generalized linear mixed models with the longitudinal outcomes were conducted by including the subject-specific random effects. Finally, the information criteria were used for the selection of random effects to be included in the generalized linear mixed-effects model (GLMM) as indicated in Table 5.
 |
Table 5 Selection of Random Effects to Be Included in the GLMM |
Table 5 indicates that the random intercept and slope model, which allows the intercept and slope to vary randomly among individuals, was selected. That means the individual status of viral load of HIV patients varies from visit to visit randomly. Therefore, the random intercept and slope model is a more parsimonious model for the generalized linear mixed-effects model because of its smallest AIC and BIC values.
The Longitudinal Analysis for the Viral Load Status
In this section, the analysis started by selecting important variables that should be included in the multivariable analysis. From the result of the univariate analysis of generalized linear mixed effect models; observation time, weight, residence area, age of patients, educational levels, clinical stages, functional status, baseline CD4 cell count, and disclosure status of patients were important factors that can affect the status of viral load result of patients and included in the multivariable analysis.
In this study, the generalized linear mixed model (both fixed and random effect) and different longitudinal sub-models were considered to study the status of viral load. In model comparisons, the subject-specific random effects and random intercept, and a random slope were considered to fit the appropriate model.
Table 6 indicates that among the variables considered in multivariate data analysis, observational time/visiting times, age of patients, the weight of patients, baseline CD4 cell count, level of education, residence area, HIV clinical stages, functional status of patients, adherence status and disclosure status of the disease statistically and significantly affected the variable of interest.
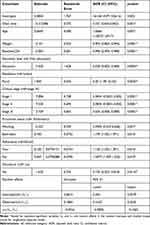 |
Table 6 Parameter Estimates Under the Generalized Linear-Mixed Effects Model Analysis |
As visiting time of patients increased by one unit, the expected odds of having a detected viral load decreased by 26.9%, keeping the other covariates constant (AOR = 0.731, 95%: (0.634,0.842) and p-value <0.01).
The age of patients significantly affected the variable of interest. As the age of patients increased by one year, the expected odds of having a detected viral load was increased by 6.7% keeping the other variables constant (AOR = 1.0666, 95% CI: (1.0527,1.0917) and p-value <0.01).
Weight also significantly affected the variable of interest. Hence, as the weight of patients increased by one kg, the expected odds of having a detected viral load decreased by 9.6%, keeping the other covariates constant (AOR = 0.904, 95% CI: (0.862, 0.946) and p-value <0.01). In a similar argument, as the number of baseline CD4 cell count increased by one cell/mm3 of blood, the expected odds of having a detected viral load was decreased by 0.4%, keeping the other covariates constant (AOR = 0.996, 95% CI: (0.994, 0.998) and P-value <0.01).
The expected odds of having a detected viral load for educated HIV patients was decreased by 97% as compared to non-educated patients, keeping the other covariates constant (AOR = 0.030, 95% CI; (0.002, 0.385) and p-value= 0.0053).
The expected odds of having a detected viral load for rural patients was found to be 6.3 times that of urban patients, controlling for the other variables in the model (AOR=6.30,95% CL: (1.78, 2.25) and p-value= 0.0043).
The WHO clinical stages had a significant effect on the variable of interest. Hence, the expected odds of having a detected viral load for patients with WHO Stage I was decreased by 99.96% as compared to those HIV patients at stage IV, keeping the other covariates constant (AOR = 0.0004, 95% CI: (0.0001,0.002) and p-value <0.01). Similarly, the expected odds of having a detected viral load for HIV patients with WHO stage II was decreased by 99.95% as compared to HIV patients at stage IV, keeping the other covariates constant (AOR = 0.0005, 95% CI; (0.0001,0.002) and p-value <0.01).
The functional status of HIV patients also played a significant role in the variation of the status of viral load. Hence, the expected odds of having a detected viral load for patients with working status was decreased by 41% as compared to patients with ambulatory functional status, keeping the other factors constant (AOR = 0.5905, 95% CI: (0.547,0.638), p-value <0.01). However, the expected odds of having a detected viral load for patients with bedridden functional status was increased by 17% as compared to ambulatory functional status, keeping the other covariates constant (AOR = 1.199, 95% CI: (1.035, 1.391), p-value = 0.016).
Adherence status also played a significant role in the variation of the status of viral load. Hence, the expected odds of having a detected viral load for poor adherent patients was increased by 12% as compared to good adherent patients, keeping the other factors constant (AOR = 1.120, 95% CI; (1.035,1.391) and p-value= 0.016). Similarly, the expected odds of having a detected viral load for fair adherent patients was increased by 4.8% as compared to good adherent patients, keeping the other covariates constant (AOR = 1.0477, 95% CI: (1.097,1.225) and p-value=0.018).
On the other hand, the expected odds of having a detected viral load for patients who disclosed the disease status to people living together was decreased by 80.5% as compared to patients not disclosed the disease status, keeping the other covariates constant (AOR = 0.195, 95% CI: (0.023, 0.818) and p-value= 0.0134).
Assessment of Goodness of Fit of the Model (Hosmer and Lemeshow Test)
Hosmer and Lemeshow’s goodness-of-fit statistic measures the correspondence between the actual and predicted values of the dependent variable. In Table 7, Hosmer and Lemeshow Test with p-value = 0.0854 are greater than 0.05 and this indicates that there was no evidence to reject the null hypothesis which states that the model is good. Therefore, the model is a good fit. Table 7 indicates that the binary logistic regression model used for the status of viral load result fits the data very well.
 |
Table 7 Hosmer and Lemeshow Model Adequacy Test |
Discussion
As visiting time of patients increased, the number of viral loads decreases. The potential reason may be the fact that as patients follow their appointment with the health staff, they can get proper treatment. In addition, patients can take proper diagnoses for other opportunistic diseases and take immediate corrective measures for reducing additional diseases. The proper treatment and non-existence of other additional diseases lead to the recovery of CD4 count and reduction of viral loads. This result is aligned with previously conducted research.12
Age also affected the variable of interest. Naturally, as the age of an individual increases, their white blood cells decrease which implies that CD4 cell count also decreases.18 This result is consistent with previously conducted study.19 However, this result is contradicted by the result obtained in another research conducted at Western Cape in South Africa.20 The research conducted in Western Cape states that individuals aged ≥35 years had significantly lower odds of having a detectable HIV viral load compared to those aged <35 years. The potential reason for the difference might be the difference in study time, study area, and sample size used for data analysis. Hence, further research is recommended for this result.
Weight also significantly affects the variable of interest. Hence, as the weight of patients increases, the probability of getting detectable viral load decrease. The result of this regard is supported by another study.21 A study conducted previously states that patients with HIV infection who are losing weight may have unsuppressed virus loads.21
Baseline CD4 cell count is another covariate highly associated with the detectable status of viral load for HIV patients. Hence, a higher baseline CD4 cell count leads to a lower possibility of having detectable viral load status. Hence, as indicated by many other types of research, the CD4 cell count and viral load status are negatively associated.22,23
Level of education is also highly associated with the viral load status of HIV patients. Hence, the possibility of getting a detectable viral load for educated patients is less likely as compared to non-educated patients. This result is similar to one of the previous studies24 which state that education would predict both CD4 and viral load status in a diverse sample assessed entirely during the era of HAART and accounting for the adherence effect. More educated patients may have better care of their health and they may have enough understanding about HAART and further leads to the status of viral load not being detectable.25 However, this result is contradicted by another result obtained in the previous study states that there is no relationship between viral load progress with a level of education.26 Hence, this needs further investigation for consistency of results.
The residence area in this study has a significant role in the variation of viral load status (whether it can be detectable or not). Rural patients have more probability of getting a detectable viral load as compared to urban patients. The potential reason for this might be the fact that most of the time rural HIV patients came to health institutions for HIV diagnosis after destruction of CD4 cell counts as they give more emphasis for day to day activities rather than checking their health status. Hence, unless they get a severe case, they did not go to health institutions as compared to urban patients. This result is supported by another study.27 The previous study states that residence and the viral load measurements are superimposed on a geographic representation of the study area.
The WHO clinical stages have a significant effect on the variable of interest. Hence, the expected odds of having a detected viral load for patients with WHO Stage I was decreased as compared to those HIV patients at stage IV. It is known that the number of viral loads for HIV patients with WHO stage IV is far greater than those of the patients with stage I. Hence, patients at Stage IV are at a severe stage and are associated with a high possibility of getting a detectable viral load.25,28 Hence, patients who started their treatment at an earlier stage can survive for a long period of time because of good recovery or reduction of the viral load result.29 This might make them able to take the treatment properly due to less replication of the virus in their body.30
The functional status of HIV disease also has a significant role in the variation of viral load for HIV patients. Hence, there is less likelihood of the possibility of getting a detectable status of viral load for patients with working status as compared to ambulatory or bedridden status. This result is supported by previous studies.31,32 The potential reason for this might be the fact that patients who are in working functional status can take prescribed medication by themselves on the time given by the health staff which leads to good recovery of viral load results.33 Such patients can express their feeling for the health staff and this helps in the right direction/decision of the health staff for the ART program.34
Medication adherence has a significant effect on the status of viral load in the study area. Hence, There is a high possibility of getting a detectable viral load for poor or fair adherent patients as compared to good adherent patients given other health conditions constant. This result is supported by another study.35,36 Hence, patients who adhered to the prescribed medication can reduce their viral load and they may have a long life with the virus, given the other external conditions constantly. This result is supported by previously conducted research.37
Disclosing the HIV disease status has its own significant effect on the variable of interest. Hence, patients who disclosed the disease status may have good adherence and follow-up status and this leads to the destruction of the number of viruses and recovery of CD4 cell counts.38 This result is similar to another study conducted in.39,40
Conclusion
The descriptive result indicates about 55.9% the participants in the current study had a detectable viral load. The study examined the socio-demographic and clinical factors of viral load results of HIV/AIDS patients in Zewditu memorial hospital. The variables like visiting time of patients/observational time of patients, age of patients, the weight of patients, baseline CD4 cell count, level of education, residence area, WHO clinical stages, functional status of patients, adherence level of patients, and disclosure status of the disease significantly affected the viral load status of patients. The model adequacy for the current investigation was tested using Hosmer and Lemeshow goodness-of-fit statistics. The results indicate that the model was a good fit for the current data.
As a recommendation, more attention should be given to rural patients, patients with HIV WHO stage IV, aged patients, non-adherent patients, patients with low baseline CD4 cell count, and for patients who did not disclose their HIV disease status. Hence, health-related education should be given to patients with poor adherence, and for patients not disclosed the disease status.
This study was not without limitations. One of the limitations of the current study is that some important potential predictor variables such as opportunistic infections, income status of patients, etc are not included in the current investigation. Including such data may give additional information about the predictors of viral load status. Therefore, it is recommended for future research to consider such covariates.
Abbreviations
HAART, highly active anti-retroviral therapy; PLWHA, people living with HIV; FMOH, Federal Ministry of Health; HIV, human immunodeficiency virus; CI, confidence interval; GLMM, generalized linear mixed effect model; CD4, Cluster Differentiation 4.
Data Sharing Statement
The data used for the current investigation are available at the hands of the corresponding author.
Ethical Approval and Consent to Participate
Dire Dawa University Ethical Review Board approved the study as per the Declaration of Helsinki. Hence, Ethical clearance was obtained from Dire Dawa University Ethical Review Committee with Ref≠ DDU/017/2021. Informed consent was waived by the review committee as all the data source of patients’ medical record numbers was anonymously registered using codes without personal identifiers such as the names of patients. The individual patients were not subject to any harm as far as confidentiality was kept.
Acknowledgment
We would like to express our profound gratitude to the management of Zewditu Memorial Hospital, for allowing us to have access to the pertinent medical registers from which we extracted the data used in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Duggal S, Chugh TD, Duggal AK. HIV and malnutrition: effects on immune system. Clin Dev Immunol. 2012;2012. doi:10.1155/2012/784740
2. Soghoian DZ, Jessen H, Flanders M, et al. HIV-specific cytolytic CD4 T cell responses during acute HIV infection predict disease outcome. Sci Transl Med. 2012;4(123):123ra25–123ra25. doi:10.1126/scitranslmed.3003165
3. Birhanu H, Alle A, Birhanu MY. Rate and predictors of mortality among adults on antiretroviral therapy at debre markos referral hospital, North West Ethiopia. HIV/AIDS. 2021;13:251.
4. Dukhi N, Mokhele T, Parker W-A, et al. Compliance with lockdown regulations during the COVID-19 pandemic in South Africa: findings from an online survey. Open Public Health J. 2021;14(1):45–55. doi:10.2174/1874944502114010045
5. Girum T, Wasie A, Worku A. Trend of HIV/AIDS for the last 26 years and predicting achievement of the 90-90-90 HIV prevention targets by 2020 in Ethiopia: a time series analysis. BMC Infect Dis. 2018;18(1):1–10. doi:10.1186/s12879-018-3214-6
6. Tsegaw M, Andargie G, Alem G, et al. Screening HIV-associated neurocognitive disorders (HAND) among HIV positive patients attending antiretroviral therapy in South Wollo, Ethiopia. J Psychiatr Res. 2017;85:37–41. doi:10.1016/j.jpsychires.2016.10.016
7. Wudineh F, Damtew B. Mother-to-child transmission of HIV infection and its determinants among exposed infants on care and follow-up in Dire Dawa City, Eastern Ethiopia. AIDS Res Treat. 2016;2016:1–6. doi:10.1155/2016/3262746
8. Amberbir A, Woldemichael K, Getachew S, et al. Predictors of adherence to antiretroviral therapy among HIV-infected persons: a prospective study in Southwest Ethiopia. BMC Public Health. 2008;8(1):1–9. doi:10.1186/1471-2458-8-265
9. World Health Organization. Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. World Health Organization; 2015.
10. Lula A, Tsegaye D, Yoseph H. Under nutrition and associated factors among adult on highly active antiretroviral therapy in Wolaita Sodo teaching and referral hospital, southern nation’ s nationalities people’ s region, Ethiopia. Int J Nutr Metab. 2017;9(2):10–19. doi:10.5897/IJNAM2016.0208
11. Jarzebowski W, Caumes E, Dupin N, et al. Effect of early syphilis infection on plasma viral load and CD4 cell count in human immunodeficiency virus–infected men: results from the FHDH-ANRS CO4 cohort. Arch Intern Med. 2012;172(16):1237–1243. doi:10.1001/archinternmed.2012.2706
12. Arredondo M, Garrido C, Parkin N, et al. Comparison of HIV-1 RNA measurements obtained by using plasma and dried blood spots in the automated Abbott real-time viral load assay. J Clin Microbiol. 2012;50(3):569–572. doi:10.1128/JCM.00418-11
13. Bhangu A, Lawani I, Ng-Kamstra JS. Global guidance for surgical care during the COVID-19 pandemic. J Br Surg. 2020;107(9):1097–1103. doi:10.1002/bjs.11646
14. Ironson G, Balbin E, Stuetzle R, et al. Dispositional optimism and the mechanisms by which it predicts slower disease progression in HIV: proactive behavior, avoidant coping, and depression. In: Positive Psychology in Behavioral Medicine. Psychology Press; 2014:86–97.
15. Mishra S, Khadka S, Dhital S, et al. Biomarkers in Immune Reconstitution Inflammatory Syndrome (IRIS) among people living with Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome (HIV/AIDS). J AIDS Clin Res. 2017;8(728):2. doi:10.4172/2155-6113.1000728
16. Perera A, Sooriyarachchi M, Wickramasuriya SL. A goodness of fit test for the multilevel logistic model. Commun Stat Simul Comput. 2016;45(2):643–659. doi:10.1080/03610918.2013.868906
17. Sullivan TR, White IR, Salter AB, et al. Should multiple imputation be the method of choice for handling missing data in randomized trials? Stat Methods Med Res. 2018;27(9):2610–2626. doi:10.1177/0962280216683570
18. Heiser P, Dickhaus B, Schreiber W, et al. White blood cells and cortisol after sleep deprivation and recovery sleep in humans. Eur Arch Psychiatry Clin Neurosci. 2000;250(1):16–23. doi:10.1007/PL00007534
19. Daskalopoulou M, Lampe FC, Sherr L, et al. Non-disclosure of HIV status and associations with psychological factors, ART non-adherence, and viral load non-suppression among people living with HIV in the UK. AIDS Behav. 2017;21(1):184–195.
20. George S, McGrath N, Oni T. The association between a detectable HIV viral load and non-communicable diseases comorbidity in HIV positive adults on antiretroviral therapy in Western Cape, South Africa. BMC Infect Dis. 2019;19(1):1–11. doi:10.1186/s12879-019-3956-9
21. Mwamburi DM, Wilson IB, Jacobson DL, et al. Understanding the role of HIV load in determining weight change in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40(1):167–173. doi:10.1086/426591
22. Phillips AN, Staszewski S, Weber R, et al. HIV viral load response to antiretroviral therapy according to the baseline CD4 cell count and viral load. JAMA. 2001;286(20):2560–2567. doi:10.1001/jama.286.20.2560
23. Skowron G, Street JC, Obee EM. Baseline CD4 (+) cell count, not viral load, correlates with virologic suppression induced by potent antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28(4):313–319. doi:10.1097/00126334-200112010-00002
24. Ironson G, O’Cleirigh C, Fletcher MA, et al. Psychosocial factors predict CD4 and viral load change in men and women with human immunodeficiency virus in the era of highly active antiretroviral treatment. Psychosom Med. 2005;67(6):1013. doi:10.1097/01.psy.0000188569.58998.c8
25. Chang L, Ernst T, Witt MD, et al. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naı̈ve HIV patients. Neuroimage. 2002;17(3):1638–1648. doi:10.1006/nimg.2002.1254
26. Coker M, Etiebet M-A, Chang H, et al. Socio-demographic and adherence factors associated with viral load suppression in HIV-infected adults initiating therapy in northern Nigeria: a randomized controlled trial of a peer support intervention. Curr HIV Res. 2015;13(4):279–285. doi:10.2174/1570162X13666150407143838
27. Tanser F, Vandormael A, Cuadros D, et al. Effect of population viral load on prospective HIV incidence in a hyperendemic rural African community. Sci Transl Med. 2017;9(420):eaam8012. doi:10.1126/scitranslmed.aam8012
28. Kalichman SC, Di Berto G, Eaton L. Human immunodeficiency virus viral load in blood plasma and semen: review and implications of empirical findings. Sex Transm Dis. 2008;35:55–60.
29. von Bahr L, Sundberg B, Lönnies L, et al. Long-term complications, immunologic effects, and role of passage for outcome in mesenchymal stromal cell therapy. Biol Blood Marrow Transplant. 2012;18(4):557–564. doi:10.1016/j.bbmt.2011.07.023
30. Atlantis T. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768–774.
31. Gill CJ, Griffith JL, Jacobson D, et al. Relationship of HIV viral loads, CD4 counts, and HAART use to health-related quality of life. J Acquir Immune Defic Syndr. 2002;30(5):485–492. doi:10.1097/00126334-200208150-00004
32. O’Cleirigh C, Ironson G, Weiss A, et al. Conscientiousness predicts disease progression (CD4 number and viral load) in people living with HIV. Health Psychol. 2007;26(4):473. doi:10.1037/0278-6133.26.4.473
33. Agegn A, Abebe M, Belay K. Predictors of viral load test result over time among HIV adult patients on Highly Active Antiretroviral Therapy (HAART): a retrospective study in Zewditu Memorial Hospital, Ethiopia; 2022
34. Erlandson KM, Allshouse AA, Jankowski CM, et al. Comparison of functional status instruments in HIV-infected adults on effective antiretroviral therapy. HIV Clin Trials. 2012;13(6):324–334. doi:10.1310/hct1306-324
35. Martin M, Del Cacho E, Codina C, et al. Relationship between adherence level, type of the antiretroviral regimen, and plasma HIV type 1 RNA viral load: a prospective cohort study. AIDS Res Hum Retroviruses. 2008;24(10):1263–1268. doi:10.1089/aid.2008.0141
36. Friedman MR, Stall R, Silvestre AJ, et al. Effects of syndemics on HIV viral load and medication adherence in the multicentre AIDS cohort study. AIDS. 2015;29(9):1087–1096. doi:10.1097/QAD.0000000000000657
37. Gross R, Bilker WB, Friedman HM, et al. Effect of adherence to newly initiated antiretroviral therapy on plasma viral load. AIDS. 2001;15(16):2109–2117. doi:10.1097/00002030-200111090-00006
38. Simon Rosser B, Horvath KJ, Hatfield LA, et al. Predictors of HIV disclosure to secondary partners and sexual risk behavior among a high-risk sample of HIV-positive MSM: results from six epicenters in the US. AIDS Care. 2008;20(8):925–930. doi:10.1080/09540120701767265
39. Jasseron C, Mandelbrot L, Dollfus C, et al. Non-disclosure of a pregnant woman’s HIV status to her partner is associated with non-optimal prevention of mother-to-child transmission. AIDS Behav. 2013;17(2):488–497. doi:10.1007/s10461-011-0084-y
40. Liu CM, Osborne BJW, Hungate BA, et al. The semen microbiome and its relationship with local immunology and viral load in HIV infection. PLoS Pathog. 2014;10(7):e1004262. doi:10.1371/journal.ppat.1004262
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.