Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 11
Predictors of major lower limb amputation in type 2 diabetic patients referred for hospital care with diabetic foot syndrome
Authors Shatnawi NJ, Al-Zoubi NA , Hawamdeh HM, Khader YS , Gharaibeh K , Heis HA
Received 23 February 2018
Accepted for publication 27 April 2018
Published 22 June 2018 Volume 2018:11 Pages 313—319
DOI https://doi.org/10.2147/DMSO.S165967
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ming-Hui Zou
Nawaf J Shatnawi,1 Nabil A Al-Zoubi,1 Hassan M Hawamdeh,2 Yousef S Khader,3 Khaled Garaibeh,4 Hussein A Heis1
1Department of Surgery, Jordan University of Science and Technology, Irbid, Jordan; 2Department of Basic Medical Science, Jordan University of Science and Technology, Irbid, Jordan; 3Department of Community Medicine, Public Health and Family Medicine, Jordan University of Science and Technology, Irbid, Jordan; 4Department of Orthopedic Surgery, Jordan University of Science and Technology, Irbid, Jordan
Purpose: This study was conducted to determine the risk factors of major lower extremity amputations in type 2 diabetic patients referred for hospital care with diabetic foot syndrome.
Patients and methods: This retrospective study involved 225 type 2 diabetic patients referred for management of diabetic foot syndrome at King Abdullah University Hospital in the period between January 2014 and December 2015. A structured customized diabetic foot data collection form with diabetic foot characteristics chart was used for documentation of relevant information, which checks for age, sex, body mass index, smoking, duration of diabetes, diabetic control therapy, associated hypertension, cardiac diseases, stroke, chronic renal impairment, renal replacement therapy (hem-dialysis), and history of diabetes-related complication in both feet prior to the study period. The predictors for major lower limb amputations were compared between groups using chi-square test, and binary logistic regression was used to determine the factors associated with major amputation.
Results: Twenty-seven limbs underwent major amputations with an overall rate of major amputation of 11.6%. The following predictors were found to be associated with the higher incidence of major lower limb amputations: duration of diabetes ≥15 years, HbA1c ≥8%, patients on insulin, with hypertension, cardiac diseases, chronic renal impairment, stroke, having gangrene, higher number of components, higher Wagner classification, and ischemia. However, the rate did not differ significantly between men and women.
Conclusion: Presentation with gangrenous tissue and poor glycemic control are the important risks and significant predictive factors for type 2 diabetes-related major lower limb amputations.
Keywords: diabetes mellitus, amputations, diabetic foot complication, gangrene
Introduction
The feet of diabetic patients are at risk of developing a wide spectrum of clinical conditions that result from the interaction of several diabetes-related complications; these clinical conditions are collectively known as diabetic foot syndrome (DFS).1–3 DFS is the major cause of hospitalization for diabetes-related complications.4 Feet deformity, secondary to motor and autonomic neuropathy,1,5 loss of protective sensation, and impaired vision increase the susceptibility for minor feet trauma, which results in diabetic foot ulceration with or without subsequent infection.5,6 Peripheral arterial disease (PAD) is a major cause of impaired ulcer/wound healing and gangrenous diabetic foot.2 Impaired immunity related to chronic hyperglycemia and subsequent superadded infections will result in septic diabetic foot.7,8
The primary objective in caring for patients with DFS is to prevent progression to more complex stages by early detection of foot at risk and evidence-based management to assure functionally intact feet with healed wounds and to minimize the need for subsequent major lower extremity amputations. There are global variations in the reported rate of lower extremity amputation related to DFS9; these reported variations might be explained by differences in design and population of the studies, severities of DFS, standard of medical care, and accesses to care.1,10,11
A wide range of risk factors for major lower extremity amputations related to DFS had been reported, which included microvascular diseases, PAD, infections, long duration of diabetes, poor glycemic control, old age, and associated cardiovascular comorbidities.1,3,12–15
Even though diabetes mellitus is common in Jordan with a prevalence of 17% among adult population;16 however, only few studies have been conducted on the prevalence and predictors of amputations in a population of type 2 diabetic patients.17
This study was conducted to determine the risk factors of major lower extremity amputations in type 2 diabetic patients referred for hospital care with DFS.
Patients and methods
The approval for this retrospective study was obtained from the King Abdullah University Hospital Review Board. This study was based on the review of medical records of consecutive type 2 diabetic patients referred for management with DFS at King Abdullah University Hospital (KAUH) in the period between January 2014 and December 2015. Type 2 diabetic patients with intermittent claudication/rest pain without feet infections/tissue loss were excluded from this study.
Diabetic patients with DFS were admitted under the care of vascular surgeon according to KAUH policy. Cardiac and endocrine consultations were made at the time of hospital admission to optimize patients’ medical status and to prepare them for surgical intervention (tissue debridement, incision, and drainage). Orthopedic, nephrology, infectious, plastic and special diabetic nursing consultations were obtained as needed according to patients’ progress through hospital stay.
Admitted diabetic patients with DFS were managed according to an updated management protocol consistent with guidelines and international recommendations.7,15 A written informed consent was obtained from all patients on admission for the use of data for research purposes, and a written informed consent for each individual intervention.
Our objective was the salvage of functional healed foot wounds, with adoption of early assessment for associated comorbidities and amputation risk factors: ulcer characteristics (infection and depth, site) and diabetic peripheral neuropathy (DPN; 10 g Semmes–Weinstein monofilaments test). Associated PAD was assessed by a clinical and noninvasive method (history, physical examination, and Ankle Brachial Pressure Index [ABI]). Early interventions to deal with infective complication were taken as a priority: starting broad-spectrum antibiotics, incision and drainage, wide necrotic tissue debridement including toes amputation if needed, tissue culture, and sensitivity that guided the antimicrobial agents all through the course of the disease.
Computed tomography angiography was used as the diagnostic modality for patients with ABI (<0.9 and >1.2) with normal creatinine (<112 mmol/L) to plan treatment. The decision for subsequent early revascularization was discussed between vascular surgeons and an interventional radiologist for an individual patient.
A structured customized diabetic foot data collection form with diabetic foot characteristics chart was used for documentation of relevant information, which checks for age, sex, body mass index (BMI), smoking, duration of diabetes, diabetic control therapy, associated hypertension, cardiac diseases, stroke, chronic renal impairment (abnormal creatinine >112 mmol/L or glomerular filtration rate <90 mL/min/1.73 m2), renal replacement therapy (hemo-dialysis), and history of diabetes-related complication in both feet prior to the study period.
The special diabetic foot chart was designed for documentation of diabetic foot characteristic and risk variables (perfusion, neuropathy, ulcer depth, infections, and ulcer site), which facilitate staging of ulcers/wounds according to Wagner’s classification of diabetic foot,18 and classification of chronic ischemia in consistence with modified Rutherford chronic ischemic criteria and ABI reading,19 and infections according to the modified International Working Group for Diabetic Foot criteria (Table S1).7
The glycated hemoglobin level (HbA1c) and erythrocyte sedimentation rate (ESR) were retrieved from the hospital database.
Initial surgical interventions – tissue debridement, incision and drainage, and amputation and revascularizations – were retrieved from the operative note. The modality and date of primary revascularization, secondary revascularization, discharge diagnosis, time to wound healing, type and date of amputation, cause and date of death, and last follow-up were all extracted from the patients’ medical records.
Lower extremity amputation is defined as resection of any segment of the lower extremity with removal of bone. Minor amputation was defined as any amputation that preserves the ankle joint with an intact healed wound. Major amputation was defined as any amputation that interferes with the ankle joint.20,21 For the purpose of this study, major amputation refers to amputation performed for the presenting diabetic foot problem. Amputation performed later on due to another reason were excluded.
Statistical analysis was performed using IBM SPSS version 22. Data were described using frequencies, percentages, and means. The rate of major amputation was compared between groups using chi-square test. Multivariate was used to determine the independent factors associated with major amputation. A P-value ≤0.05 was considered statistically significant.
Results
This study included a total of 225 type 2 diabetic patients (153 males and 72 females) with DFS. A total of 10 patients had bilateral DFS, giving a total of 235 DFSs managed in the study period. One-third (34.7%) of patients were <55 years of age. About 43.6% had BMI ≥25 kg/m2. Nearly half of them (48.9%) had diabetes for ≥15 years and 52.4% had HbA1c level ≥8%. The mean duration of diabetes was 14.3 (6.8) years and the mean HbA1c was 8.7% (2.2%).
Clinical evidence of infection was found in 173 limbs (76.9%), of which 67 limbs (28.5%) had evidence of infections without ulcer or gangrene. Evidence of ischemia was found in 119 (52.9%) of limbs, and neuropathy was found in 122 (54.2%) of limbs. Ulcer was found in 102 (45.3%) of the feet, of which 23% had ischemic ulcer. Ischemic neuropathic foot was found in 103 (43.8%). Single component (ischemia, neuropathy, or infection) was found in 49 (21.8%) of DFS, two components were found in 160 (71.1%) of DFS, and 17 (7.2%) of DFS had three components (neuropathy, ischemia, and infections). According to Wagner’s classification, none of the feet had grade 0 or 1, and 37 (16.4%) of the limbs were not classified on admission (cellulites without tissue loss at presentation).
Tissue debridement was the initial surgical intervention for 152 (64.7%) of the limbs. Incision and drainage was needed for 12 (5.1%) of the feet (deep-seated infections). No surgical intervention was needed for 21 (8.9%) of the feet (cellulites). Toes amputation was needed in 62 (26.4%) of DFS. A minor amputation was performed in 31 (16.6%), and a major amputation to attain healing was needed for 26 limbs (11.6%). Primary revascularization was needed for 108 (46.0%) of the DFS.
The overall rate of major amputation was 11.1%. Table 1 shows the rate of major amputation according to patients’ characteristics, and Table 2 shows the rate of major amputation in relation to foot characteristics. The rate did not differ significantly in relation to gender, age group, ESR, BMI, neuropathy, ulcer, and infections.
  | Table 2 The rate of major amputation according to foot characteristics Abbreviation: DFS, diabetic foot syndrome. |
Those who had diabetes for ≥15 years had a significantly higher rate compared with those with shorter duration (20.0% vs 3.5%). Patients with HbA1c ≥8% had higher rate of major amputation compared with patients with HbA1c <8% (17.8% vs 4.7%). The rate was much higher among patients on insulin, with hypertension, cardiac diseases, chronic renal impairment, stroke (Table 1), gangrene, higher number of components, higher Wagner classification, and ischemia (Table 2).
In the multivariate analysis, patients who were on insulin had higher odds of major amputation compared with those who were taking oral hypoglycemic agents (odd ratio [OR]=6.9; 95% CI: 1.9–25.4). HbA1c was significantly associated with increased odds of major amputation (OR=4.0; 95% CI: 1.3–12.4). Longer duration of diabetes (≥15 years; OR=6.0), renal impairment (OR=3.5), and gangrene (OR=4.2) were significantly associated with increased risk of major amputation (Table 3).
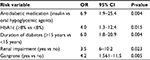  | Table 3 Multivariate analysis of factors associated with major amputation Abbreviations: HbA1c, glycated hemoglobin level; OR, odds ratio. |
Discussion
DFS refers to a wide spectrum of complex and dynamic clinical conditions affecting the feet of diabetic patients, which results from the interaction of several diabetes-related complications, DPN and PAD, precipitated by minor trauma that leads to ulceration or gangrene with subsequent variable degree of super-added infections.1,3,6
The complex nature of DFS is demonstrated in this study where clinical evidence of infection was found in 76.9% of the DFS referred for hospital care. The majority (78.2%) of limbs had two or more components of the triad incriminated in pathogenesis of DFS (ischemia, neuropathy, and infection). Ischemic neuropathic feet were found in 28.1% of limbs. However, ulcer was found at presentation in less than half of DFS in this study. This complex nature of DFS has been reported in previous studies, where admitted patients with DFS had infection in 82% of cases, diabetic neuropathy in 86%, and PAD in 49% of DFS. About 31% had infection and ischemia.23
Nearly half of DFS (49.4%–52.3%) were ischemic neuropathic in nature,24,25 even though the majority of diabetes-related lower extremity amputations are preceded by unhealed ulcers/wounds.26,20,22 Ulceration, as part of DFS, does not occur spontaneously; rather, it is a manifestation of underlying micro- and macrovascular complications of diabetes that are uncovered by minor trauma.5
The interaction of diabetes-related complications is responsible for the increased risk of major lower extremity amputation in diabetics by 10–20-fold compared with nondiabetics.27,28
A major lower limb amputation was needed in 11.1% of patients with DFS. Hospital-based data reported an amputation rate as low as 4.2% and as high as 27%.24,8 However, a rate of 2%–16% has been reported from different studies.1 The complex nature of DFS and the wide spectrum of clinical presentations might explain the differences in amputation rates reported in this study from other studies, as there are global variations in the reported amputation rate.9 The global variation in amputation rate was explained on the bases of differences in population and design of the studies, as well as due to differences in severity, the standard of managements, and access to medical care.24,10
Previous studies reported several risk factors for lower extremity amputation related to DFS including DPN, PAD,5,29,30 long diabetic duration, high HbA1c,13,12,22 treatment with insulin,31 old age, male gender,12,30 infections,5,2,8,7,23 associated cardiovascular comorbidities,31 and diabetic nephropathy.29,14
Our study showed several independent predictor factors for lower extremity amputation including gangrene as the presenting characteristic, chronic renal impairment/end-stage renal failure on hem-dialysis, duration of diabetes ≥15 years, and increased HbA1c ≥8%.
Gangrene as the presenting characteristics was found in 31.1% of DFS in our study. Similar results were reported by others.8 Gangrenous tissue at presentation was found to be an independent risk factor for amputation in comparison with nongangrenous DFS (P-value=0.001). Similar to the finding in other studies,13,17 impairment of arterial perfusion may act as an isolated cause for amputation in diabetics, and it is one of the major causes of gangrenous DFS.14,26 Ischemia was diagnosed in 52.9% of this group. However, revascularization was performed in 84% of DFS with ischemia. Gangrene can be a result of PAD, and it can be a result of deep-seated infections and impaired cutaneous microcirculation,32 even though early diagnosis and management of ischemia as a risk factor may aid in avoiding limb loss due to ischemia, as percutaneous transluminal angioplasty (PTA) became a feasible modality with promising outcome.33
Diabetic nephropathy, in general, and patients on renal replacement therapy (hemodialysis) were found to be predictors of major amputation. Diabetic nephropathy is one of the major microvascular complications of diabetes with subsequent end-stage renal disease.34 Diabetic nephropathy is associated with an increased risk of neuropathy and PAD,35 which can explain the impact of chronic renal impairment on lower extremity amputation in patients hospitalized for DFS.26,32,36
The duration of diabetes for ≥15 years, insulin therapy, and increased level of HbA1c ≥8% were found as independent predictor factors for major lower limb amputation in this study.
A long diabetic duration was found to be a predictor for diabetes-related major lower extremity amputations.31,14 The need for insulin in the control of type 2 diabetes tend to increase with longer duration of diabetes, and it was reported as an independent predictor factor for major amputation in other studies.31,11,12,15 Increased level of HbA1c was found to be associated with increased risk of amputation.37,14,12,8
The strong association of HbA1c with lower extremity amputations could reflect the pathogenic role of chronic hyperglycemia, probably via neuropathy, impaired wound healing, PAD, and increased susceptibility to infection.38,39,40,32 The limitation of this study is being a retrospective study, and the long-term outcome was not included.
Conclusion
DFS is a complex wide spectrum of feet problem related to diabetic complications with different range of severity. Ulceration is part of the spectrum of DFS, and presentation with gangrenous tissue and poor glycemic controls are the important independent risk factors for diabetes-related major lower extremity amputations.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Frykberg RG, Zqonis T, Armstrong DG, et al; American College of Foot and Ankle Surgeons. Diabetic foot disorders: a clinical practice guideline (2006 revision). J Foot Ankle Surg. 2006;45(5 Suppl):S1–S66. | ||
Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care. 1990;13(5):513–521. | ||
Jeffloate WJ, Macfarlane RM, Fletcher EM. The description and classification of diabetic foot lesions. Lancet. 2005;366:1719–1724. | ||
Singh N, Armestrong DG, Lipsky BA. Foot ulcer in patients with diabetes. JAMA. 2005;293(2):217–228. | ||
Reiber GE, Vileikyte L, Boyko EJ, et al. Causal pathways for incident lower extremity ulcers in patients with diabetes from two settings. Diabet Care. 1999;22(1):157–162. | ||
Jude EB, Boulton AJM. End stage complication of diabetic neuropathy. Diabet Rev. 1999;7:395–410. | ||
Lipsky BA, Berendt AR, Cornia PB et al; Infectious Diseases Society of America. 2012 Infectious disease society of Americas clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132–e173. | ||
Nather A, Bee CS, Huak CY, et al. Epidemiology of diabetic foot problems and predictive factors for limb loss. J Diabetes Complications. 2008;22(2):77–82. | ||
Moxey PW, Gogalniceanu P, Hinchliffe RJ, et al. Lower extremity amputations – a review of global variability in incidence. Diabet Med. 2011;28(10):1144–1153. | ||
Jeffcoate WJ, Van Hountum WH. Amputation as a marker of the quality of foot care in diabetes. Diabetologia. 2004;47(12):2051–2058. | ||
van Hauttum P, Schaper N, Prompers L, et al. Differences in minor amputation rate in diabetic foot disease throughout Europe are in part explained by differences in disease severity at presentation. Diabet Med. 2011;28(2):199–205. | ||
Pscherer S, Dippel FW, Lauterbach S, Kostev K. Amputation rate and risk factors in type 2 patients with diabetic foot syndrome under real-life conditions in Germany. Prim Care Diabetes. 2012;6(3):241–246. | ||
Pemayun TG, Naibaho RM, Novitasari D, Amin N, Minuljo TT. Risk factors for lower extremity amputation in patients with diabetic foot ulcers: a hospital-based case control study. Diabet Foot Ankle. 2015;6:29629. | ||
Davis WA, Norman PE, Bruce DG, Davis TM. Predictors, consequences and costs of diabetes-related lower extremity amputation complicating type 2 diabetes: the Fremantle Diabetes Study. Diabetologia. 2006;49(11):2634–2641. | ||
Wukich DK, Armstrong DG, Attinger CE, et al. Inpatient management of diabetic foot disorders: a clinical guide. Diabetes Care. 2013;36(9): 2862–2871. | ||
Ajlouni K, Kader YS, Batieh A, Ajlouni H, El-Khateeb M. An increase in the prevalence of diabetes in Jordan over 10 years. J Diabetes Complications. 2008;22(5):317–324. | ||
Jbour AS, Jarrah NS, Radideh AM, et al. Prevalence and predictors of diabetic foot syndrome in type 2 diabetes mellitus in Jordan. Saudi Med J. 2003;24(7):761–764. | ||
Wagner FW Jr. The dysvascular foot: a system for diagnosis and treatment. Foot Ankle. 1981;2(2):64–122. | ||
Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26(3):517–538. | ||
Alvarsson A, Sandgren B, Wendel C, Alvarsson M, Brismar K. A retrospective analysis of amputation rates in diabetic patients: can lower extremity amputation be farther prevented? Cardiovasc Diabetol. 2012;11:18. | ||
Bakker K, Apelqvist J, Lipsky BA, Van Netten JJ; International Working Group on Diabetic Foot. The 2015 IWGDF guidance documents on prevention and management of foot problems in diabetes: development of an evidence-based global consensus. Diabetes Metab Res Rev. 2016;32(Suppl 1):S2–S6. | ||
McNeely MJ, Boyko EJ, Ahroni JH, et al. The independent contributions of diabetic neuropathy and vasculopathy in foot ulceration. How great are the risks? Diabetes Care. 1995;18(2):216–219. | ||
Pompers L, Schapper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The (EUROFIALE) study. Diabetologia. 2008;51(5):747–755. | ||
Thewjitcharoen Y, Krittiyawong S, Porramatikul S, et al. Outcomes of hospitalized diabetic foot patients in a multi-disciplinary team setting: Thailand’s experience. J Clin Transl Endocrinol. 2014;1(4):187–191. | ||
Oyibo SO, Jude EB, Voyatzoglou D, Boulton AJM, Clinical characteristics of patients with diabetic foot problems: changing patterns of foot ulcer presentation. Pract Diabet Int. 2002;19(1):10–12. | ||
Adler AI, Boyko EJ, Ahroni JH, Smith DG. Lower-extremity amputation in diabetes. The independent effect of peripheral vascular disease, sensory neuropathy and foot ulcer. Diabet Care. 1999;22(7):1029–1035. | ||
Van Houtum WH, Lavery LA. Outcomes associated with diabetes related amputations in The Netherlands and in the state of California, USA. J Intern Med. 1996;240(4):227–231. | ||
Armstrong DG, Larvery LA, Harkless LB. Validation of a diabetic wound classification system. The contribution of depth, infection and ischemia to risk of amputation. Diabetes Care. 1998;21(5):855–859. | ||
Shajaiefard A, Khorgami Z, Larijani B. Independent risk factors for amputation in diabetic foot. Int J diabetes Dev Ctries. 2008;28(2):32–37. | ||
Burn C, Siersma V, Guassora AD, Holstein P, de Fine Olivarius N. History of amputations and foot ulcers in patients newly diagnosed with type 2 diabetes mellitus and observed for 19 years: the role of age gender and comorbidities. Diabet Med. 2013;30(8):964–972. | ||
Lee JS, Lu M, Lee VS, Russel D, Bahr C, Lee ET. Lower extremity amputation: incidence, risk factors and mortality in the Oklahoma Indian Diabetes Study. Diabetes. 1993;42(6):876–882. | ||
Abbott CA, Carrington AL, Ashe H, et al; North-West Diabetes Foot Care Study. The North-West Diabetes Foot Care Study: incidence of, and risk factors for new diabetic foot ulceration in a community-based patient cohort. Diabet Med. 2002;19(5):377–384. | ||
Forsythe RO, Brownrigg J, Hinchliffe RJ. Peripheral arterial disease and revascularization of diabetic foot. Diabetes Obes Metab. 2015;17(5):435–444. | ||
Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28(1):164–176. | ||
AggersPW,Gohdes D, Pugh J. Nontraumatic lower extremity amputations in the Medicare end-stage renal disease population. Kidney Int. 1999;56(4):1524–1533. | ||
Margolis DJ, Hofstad O, Feldman HI. Association between renal failure and foot ulcer or lower-extremity amputation in patients with diabetes. Diabetes Care. 2008;31(7):1331–1336. | ||
Zhou ZY, Liu YK, Chen HL, Yang HL, Liu F. HbA1c and lower extremity amputation risk in patients with diabetes: a meta-analysis. Int J Low Extrem Wounds. 2015;14(2):168–177. | ||
Adler AI, Erqou S, Lima TA, Robinson AH. Association between glycated hemoglobin and the risk of lower extremity amputation in patients with diabetes mellitus review and meta-analysis. Diabetologia. 2010;53(5):840–849. | ||
Rai NK, Suryabhan, Ansari M, Kumar M, Shukla VK, Tripathi K. Effect of glycemic control on apoptosis in diabetic wounds. Int J Low Extrem Wounds. 2015;14(2):168–177. | ||
Klein R. Hyperglycemia and microvascular and macrovascular disease in diabetes. Diabetes Care. 1995;18(2):258–268. |
Supplementary material
  | Table S1 Diabetic foot characteristics chart Abbreviations: ABI, Ankle Brachial Pressure Index; CT, computed tomography; MRI, magnetic resonance imaging; OR, odds ratio; US, ultrasound. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

