Back to Journals » Clinical Ophthalmology » Volume 16
Predictors for Dry Eye Diseases in Patients with Systemic Sclerosis
Authors Laovirojjanakul W , Yospaiboon Y , Anutarapongpan O, Mahakkanukrauh A, Suwannaroj S, Nanagara R, Foocharoen C
Received 6 September 2022
Accepted for publication 6 October 2022
Published 17 October 2022 Volume 2022:16 Pages 3447—3455
DOI https://doi.org/10.2147/OPTH.S387760
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Wipada Laovirojjanakul,1 Yosanan Yospaiboon,1 Orapin Anutarapongpan,1 Ajanee Mahakkanukrauh,2 Siraphop Suwannaroj,2 Ratanavadee Nanagara,2 Chingching Foocharoen2
1KKU Eye Center, Department of Ophthalmology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand; 2Division of Allergy-Immunology-Rheumatology, Department of Medicine, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
Correspondence: Orapin Anutarapongpan; Yosanan Yospaiboon, KKU Eye Center, Department of Ophthalmology, Faculty of Medicine, Khon Kaen University, 123 Mitraparb Highway, Khon Kaen, 40002, Thailand, Tel +66 4336-3010, Fax +66 4334-8383, Email [email protected]; [email protected]
Purpose: To evaluate the prevalence of dry eye disease (DED) in patients with both limited and diffuse subtypes of systemic sclerosis (SSc) and to determine the predictive factors associated with the occurrence of DED.
Patients and Methods: This was a prospective consecutive cross-sectional study of patients with SSc. Each patient underwent a comprehensive ophthalmological evaluation. The Ocular Surface Disease Index (OSDI) was used to assess the symptoms of DED. Tear break up time (TBUT), Schirmer I and ocular surface staining (OSS) were used for objective tests. Patients were diagnosed DED using the DEW II diagnostic method which included both symptom and objective tests. The collected data of patients with and without DED were compared to find out possible associated factors. Logistic regression analysis was used to determine the effects of these factors.
Results: Eighty-four SSc patients (25 limited and 59 diffuse subtypes) were studied. The prevalence of DED in SSc patients was 52.38%. The OSDI, tear break up time (TBUT), Schirmer I and ocular surface staining (OSS) were not significantly different between SSc subtypes. Among the 46 symptomatic patients, DED was noted in 44 (52.38%). Most patients (61.4%) were mixed aqueous deficiency-evaporative dry eye type (both TBUT and Schirmer I test positive). The mean age and the median of disease duration in DED patients were higher than those without DED with statistically significant difference (P = 0.004 and 0.019).
Conclusion: DED was common in patients with SSc. The predictors for the occurrence of DED were older age and longer disease duration. Therefore, OSDI and objective tests for DED should be evaluated in all SSc patients, particularly those with older age and longer disease duration.
Keywords: dry eye disease, systemic sclerosis, predictive factors
Introduction
Systemic sclerosis (SSc), or scleroderma, is a chronic multisystem disorder involving connective tissue with heterogeneous manifestations. The pathophysiology of SSc consists of autoantibody production, immune activation that leads to vascular abnormalities, and widespread fibrosis.1–4 SSc is distributed worldwide. The peak incidence appears in individuals between 30 and 60 years of age, with a female predilection.5–7 A review of the literature reported a wide variability of ophthalmic features in SSc, such as eyelid skin changes, conjunctival vascularization, shallow fornices, corneal abnormalities, and dry eyes.5,8 The most frequent ocular manifestation of SSc is dry eye disease (DED), which occurs in 37–79% of patients.5–12 However, most of these studies were small case series and varied in the method of diagnosing DED.9–12 To our knowledge, no study has determined the predictive factors associated with DED in SSc patients. Therefore, our study aimed to evaluate the prevalence of DED in both limited and diffuse subtypes of SSc using the DEW II diagnostic method developed by the Tear Flow and Ocular Surface Society (TFOS)13 and to determine the predictive factors associated with the occurrence of DED.
Patients and Methods
This prospective consecutive cross-sectional study included 84 patients with SSc in the Scleroderma Clinic at Srinagarind Hospital, Faculty of Medicine, Khon Kaen University, Thailand. The study followed the tenets of the Declaration of Helsinki and was approved by the Khon Kaen University Ethics Committee for Human Research. Inclusion criteria were patients who fulfilled the American College of Rheumatology and European League Against Rheumatism (ACR/EULAR) criteria for the classification of SSc 201314 and signed informed consent forms. Exclusion criteria were unstable vital signs and inability to undergo slit-lamp biomicroscopic examination. Patients with evidence of Sjogren's syndrome or other connective tissue diseases were also excluded. All recruited patients had their skin score evaluated by the modified Rodnan skin score15 and were classified into limited and diffuse subtypes. Demographic data included age, gender, SSc subtype, skin tightness, and the total skin score. Since it was difficult to define the time of disease onset, we defined disease duration as the period from age at diagnosis to the time of the cross-sectional study.
After obtaining informed consent, each patient was interviewed by a trained interviewer using the Ocular Surface Disease Index (OSDI) questionnaire (Allergan, Irvine, CA, USA) and underwent a comprehensive ophthalmological evaluation, including slit-lamp examination, tear function tests, ocular surface staining, and intraocular pressure measurement. The sequence of ocular examination is of critical importance because the application of a vital dye or anesthetic eye drop to the ocular surface could alter the tear film and adversely affect subsequent results. For this reason, best-corrected visual acuity was assessed using the EDTRS chart. The patient underwent a complete ophthalmic evaluation by an ophthalmologist who was unaware of the SSc subtype. All ophthalmologists and technicians used the same evaluation technique. Slit lamp biomicroscopic examination was performed to detect abnormalities in the eyelids, lashes, conjunctivae, and corneal surface, which might influence DED. Subsequently, a simplified quantitative method for assessing dry eye was performed in the following sequence to avoid the effect of vital dyes on the ocular surface and tear film.16 The Schirmer I test without anesthesia was performed first, followed by instillation of fluorescein dye, tear break-up time (TBUT), and corneal staining was graded. Then, lissamine green dye was applied, and the conjunctival staining was graded immediately before the intensity of the staining diminished.16 Finally, an anesthetic eye drop (Tetracaine 0.5%, Alcon Laboratories, Texas, USA) was applied and intraocular pressure (IOP) was measured using a Goldmann applanation tonometer.
The Ocular Surface Disease Index (OSDI) was used to assess the symptoms of ocular irritation in dry eye disease and how they affect functioning related to vision. The OSDI consists of 12 questions to assess the symptoms experienced by patients in the last week. The questionnaire had three subscales: ocular symptoms, vision-related functions, and environmental triggers. The OSDI score was calculated using a total score that ranged from 0 to 100.17 The score determines the ocular surface as normal or asymptomatic (0–12 points), mild (13–22 points), moderate (23–32 points), or severe (33–100 points) dry eye symptoms.
A five-minute conventional Schirmer I test without anesthesia was performed to assess tear production using a commercially available 5×35 mm paper strip (Schirmer strips TM, 32 K SUPPLY Co, LTD, Bangkok, Thailand). The Schirmer stripsTM were inserted at the temporal fornices of the lower eyelids, and patients were instructed to gently close their eyes for 5 minutes. A Schirmer I test of ≤ 5 mm in 5 minutes in either eye was considered abnormal.
The tear break-up time (TBUT) was used to assess tear film stability. A fluorescein strip (Fluorescein Paper, 32 K SUPPLY Co, LTD, Bangkok, Thailand) moistened with a drop of non-preserved sterile saline solution (0.9%) and shaken to remove excess dye, was applied to the lower palpebral conjunctiva. They were then evaluated within two minutes after application of fluorescein dye under the setting of a 10x illumination high cobalt blue filter over the light source. TBUT, defined as the time in seconds between the patient’s last blink and the first appearance of a random dry spot on the corneal surface, was measured three times and the mean value was used for the analysis. A TBUT of less than 10 seconds in either eye was considered abnormal.
Ocular surface staining was performed to assess damage to the ocular surface. Corneal staining was evaluated after 2–3 minutes of staining with fluorescein and then counted and scored following the DEW II diagnostic method.13 The Conjunctival staining was evaluated by one drop of 1% lissamine solution.18 The solution prepared using four strips of lissamine green (OpGreen, Ophtechnics Unlimited, Haryana, India) incubated in four drops (200 μL) of non-preserved sterile saline solution (0.9%) for 1 minute.18 The lissamine solution was instilled into the inferior conjunctivae fornices of both eyes, and patients were asked to blink several times. After instillation, the conjunctivae were examined with a slit lamp at 10× magnification using a neutral density filter, and the staining spot was counted.16 The horizontal length of the lid wiper, extending from the superior punctum to the lateral canthus, and the sagittal width of the lid wiper, extending from just proximal to the line of Marx to the subtarsal fold, were examined. Staining > 5 corneal spots, > 9 conjunctival spots, or upper lid margin staining (LWE) ≥ 2 mm in horizontal length and/or ≥ 25% sagittal width (excluding Marx’s line) in either eye was considered abnormal.13
Patients were classified into the DED group, no DED group, pre-clinical ocular surface disease group, and patients with prodromal signs. Symptomatic patients without one of the above-mentioned tests did not fall into the DED group but were defined as having preclinical ocular surface disease. Conversely, asymptomatic patients with positive tests were defined as those with prodromal signs who may be at risk of developing manifest DED with time or provocation. Finally, asymptomatic patients without any positive tests were classified as definitely no DED.
Statistical Analyses
The collected data were analyzed using Stata version 16.1 (StataCorp, College Station, TX77845 USA). Continuous variables were expressed as mean and standard deviation for data with normal distribution or median and interquartile range for data with non-normal distribution. Categorical variables were expressed as frequencies and percentages. Parametric (Student’s t-test) and non-parametric (Mann–Whitney U-test) analyses were used to compare continuous variables according to data distribution. Two-sided Fisher’s exact test was used to compare categorical variables. Logistic regression analysis was used to determine the effects of associated factors. Statistical significance was set at p <0.05.
Results
Eighty-four patients were enrolled in the study. The study included 57 women and 27 men. Age ranged from 20 to 79 years old, with a mean age of 55.58 ± 10.63 years (Table 1). There were 59 (70%) patients with diffuse SSc and 25 (30%) with a limited subtype. The mean age in limited and diffuse subtypes were 54.16 ± 11.21 and 56.19 ± 10.39 years old respectively (p=0.43). Regarding the underlying diseases, systemic hypertension, diabetes mellitus, and dyslipidemia were noted in 12 (14.3%), 3 (3.6%), and 15 (17.9%) patients, respectively. One of the patients had a history of congenital ptosis. One patient had mild non-proliferative diabetic retinopathy.
 |
Table 1 Demographic Data of Patients in the Study |
The majority (72.6%) of patients had BCVAs of 45 letters in the ETDRS chart or better. The mean BCVA in right eye was 46.48 ± 12.26, 45.12 ± 11.38, and that in left eye was 46.64 ± 9.17, 44.19 ± 13.79 in the limited and diffuse subtypes, respectively. There was no significant difference in mean BCVA between both subtypes in the right eye (p=0.63) and left eyes (p=0.42). Two patients had zero-letter BCVA in both eyes because of bilateral dense cataracts. One patient had low visual acuity due to pathological myopia with posterior staphyloma in both eyes.
Mean IOP value in right eye was 13.16 ± 3.85, 12.39 ± 3.13 mmHg and that of left eye was 13.24 ± 3.8, 12.53 ± 3.14 mmHg in limited and diffuse subtypes, respectively. These differences were not statistically significant. A value exceeding 21 mmHg was observed in 5 patients and two of them had a large cup disc ratio of >0.5. These patients were then scheduled for further detailed reevaluation and appropriate treatment. It was noted that these five patients were all in the diffuse subtype. Table 1 presents the demographic data of the study population. There were no significant differences in the demographic data between the two subtypes, except for the total skin score. Patients with the diffuse subtype had more skin tightness (p=0.05) and a significantly higher skin score than those with the limited subtype (p=0.002).
The ocular surface disease index (OSDI) was completed in all patients. Thirty-eight patients (45.2%) with OSDI score of 0–12 were classified as asymptomatic and 46 patients (54.8%) with an OSDI score > 12 were defined as symptomatic, whereas mild, moderate, and severe dry eye symptoms were noted in 28 (33.3%), 10 (11.9%), and 8 (9.5%) patients, respectively (Table 2).
 |
Table 2 Dry Eye Symptoms and Signs in Patients with Limited and Diffuse SSc |
Abnormal TBUT (< 10 mm in at least one eye) was observed in 59 patients (70.2%). This finding was detected in 40 cases of diffuse SSc (67.8%) and 19 cases of limited SSc (76.0%). There were no significant differences in TBUT between subtypes. An abnormal Schirmer I test (≤ 5 mm after 5 min in at least one eye) was noted in 38 (45.2%) patients. This finding was observed in 11 of 25 patients with limited SSc (44.0%) and 27 of 59 patients with diffuse SSc (45.8%), and the difference was not statistically significant. Abnormal ocular surface staining was noted in 18 of the 25 patients with limited SSc (72%) and 36 of the 59 patients with diffuse SSc (61%). This difference was also not statistically significant (Table 2).
DED was confirmed based on the DEW II diagnostic methodology, which requires a positive symptom score and one or more positive homeostatic marker results (TBUT, Schirmer I, or OSS).13 Among the 46 symptomatic patients, DED was noted in 44 (52.38%). Two symptomatic (2.38%) patients without any positive tests possibly had preclinical ocular surface disease. In contrast, 31 (36.91%) asymptomatic patients with positive tests were not diagnosed with DED but were defined as patients with prodromal signs, and seven (8.33%) asymptomatic patients with all negative tests were defined as having no DED (Table 3). Among 44 confirmed DED patients, 13 out of 44 patients (29.5%) had only TBUT positive or evaporative dry eye (EDE), and 4 out of 44 patients (9.1%) had only Schirmer I test positive or aqueous - deficient dry eye (ADDE). Most patients (27/44, 61.4%) had both TBUT and Schirmer I test positive or mixed ADDE-EDE.
 |
Table 3 Clinical Signs and Symptoms of Patients in the Study |
We compared SSc patients with and without confirmed DED to determine factors associated with the occurrence of DED (Table 4). The result demonstrated that the mean age of patients with DED was older than those without DED with statistically significant difference (56.9 ± 7.5 vs 45 ± 18.7 years, P = 0.004). The median of disease duration of patients with DED was also longer than those without DED with statistically significant difference (72 vs 24 months, P = 0.019). However, multivariate logistic regression analysis revealed that only age factors were statistically significant (Odds Ratio 1.1, 95% CI 1.01 to 1.20, P 0.023) (Table 5). There were no significant differences between the two groups in terms of age at diagnosis, gender, disease subtype, eyelid stiffness, skin tightness, total skin score, and disease duration. Mean OSDI score were 22.8 ± 6.8 (15–39) in DED group and 5.1 ± 4.5 (0–12) in no DED group (P < 0.0001). Mean Schirmer 1 test were 9.16 ± 6.96 (0–35) in DED group and 16.3 ± 10.13 (6–35) in no DED group (P < 0.0003). Mean TBUT were 6.77 ± 3.17 (0–16) in DED group and 10.3 ± 0.47 (10–11) in no DED group (P < 0.0001).
 |
Table 4 Univariate Analysis of the Factors Associated with the Occurrence of DED |
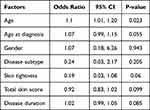 |
Table 5 Multivariate Analysis of the Factors Associated with the Occurrence of DED |
Discussion
Dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film and is accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.13 DED has been reported to be the most frequent ocular manifestation in patients with SSc.5–12 However, these studies varied in their methods of diagnosing DED. Horan et al reported reduced tear secretion in 11 of 23 patients with SSc (48%) diagnosed using the Schirmer test and rose Bengal staining.9 The criterion for positive Schirmer test results was < 5 mm wetting at 5 min. West and Barnett also reported deficient tear secretion in 14 of 38 SSc patients (36.7%) diagnosed using the Schirmer test and rose bengal staining, but the criteria for positive Schirmer test were < 10 mm wetting after 5 minutes.10 Gomes et al performed a cross-sectional study in 45 patients with SSc and demonstrated keratoconjunctivitis sicca in 22 patients (48.9%).11 OSDI, TBUT, Schirmer I, and rose bengal staining were tested in all patients, and the diagnosis was confirmed by positive results in two or more objective tests. Abnormal test values were TBUT < 10 seconds, Schirmer test score ≤ 5 mm at 5 minutes, and rose Bengal staining score > 3. Szucs et al revealed DED in 64.7% of 51 SSc patients diagnosed with OSDI, TBUT < 10 seconds, and Schirmer tests < 10 mm in 5 minutes.12 Adiguzel et al compared 32 patients with SSc and 31 healthy subjects using the OSDI, TBUT, Schirmer 1 test, and ocular surface staining with fluorescein (Oxford score).19 Schirmer 1 test ≤ 5 mm at 5 minutes and TBUT ≤ 10 seconds were considered abnormal in their study.
According to DEW II, developed by the Tear Flow and Ocular Surface Society (TFOS), diagnostic methods for DED include screening for symptoms and homeostasis marker tests.13 Dry eye symptoms plus one of the marker tests confirmed the diagnosis of DED. A dry eye questionnaire (DEQ-5) score ≥6 or an OSDI score ≥13 was considered symptomatic. Homeostasis markers in DEW II included TBUT < 10 seconds and tear osmolarity ≥ 308 mOsm/L in either eye and ocular surface staining (OSS) > 5 corneal spots, > 9 conjunctival spots, or lid margin (≥ 2 mm length and ≥ 25% width). Tear osmolarity was not measured in this study. We used an OSDI score ≥13 and a positive test in one of the tests (TBUT < 10 seconds, Schirmer I test ≤ 5 mm, or ocular surface staining positive) to confirm the diagnosis of DED. In our study, using the DEW II diagnostic method, the prevalence of DED was 52.38% as compared to the prevalence using only the marker test (TBUT, 70.2%). However, this correlates well with previous studies that reported a prevalence ranging from 37% to 79% in patients with SSc.5–12
DED is a consequence of SSc. Fibrotic changes in the lacrimal gland lead to a decreased production of aqueous tears. In addition, reducing the number of goblet cells in the conjunctival epithelium causes a decrease in tear film mucin layer secretion. Consequently, excessive evaporation of tears from the eye surface causes DED. Previous studies have shown that most DED in SSc patients was ADDE, as confirmed by the Schirmer test,9,10 Other studies revealed that EDE was the most common type in SSc patients, as demonstrated by abnormal TBUT.11,12,19 The results of our study showed that the most common type of DED was mixed ADDE-EDE. This correlates well with the DEW II report on the spectrum of DED, from ADDE to EDE. This may be attributed to alterations in the connective tissue and microvascular changes in patients with SSc, which influence both the eyelids and ocular surface. As a result, fibrotic changes in the lacrimal gland lead to ADDE, and the loss of goblet cells in the conjunctival epithelium results in lipid tear instability and EDE.
Previous studies have compared DED in SSc patients with healthy subjects9,19 and found that DED was more common in SSc patients than in the healthy population. However, there has been little evidence for the comparison of DED and no DED in SSc patients and no study on the factors associated with the occurrence of DED. We analyzed the data of 44 confirmed DED and 7 definite no DED in SSc patients (Table 4). Patients with DED were significantly older than those without. In addition, the age at diagnosis was higher in patients with DED than in those without DED; however, the difference was not statistically significant.
There is strong evidence of a female predilection in patients with SSc. Previous studies have reported that women were more common than men, with the overall reported female-to-male ratio ranging from 1:1 to 14:1.5–7 Our study also reported more female patients in the limited (72%) and diffuse (66%) subtypes. When compared between the DED and no DED groups, both groups also showed a female predilection (DED, 73.2% and no DED, 60%).
In this study, disease subtypes, total skin score, and eyelid tightness had no significant association with the occurrence of DED. However, SSc patients with DED had a longer disease duration than those without DED. Interestingly, both older age and longer disease duration were associated with time. This implies that during SSc progression, the alteration in skin stiffness and microvascular changes gradually increases with time and results in DED with a progressive increase in severity.
In previous studies, there were more limited SSc than diffuse SSc patients,9–11 and it was proposed that diffuse SSc was more severe and had more extensive skin fibrotic changes.11 In this study, there was a higher prevalence of diffuse SSc than the limited subtype, and the total skin score in diffuse SSc was significantly higher than that in the limited subtype. A higher total skin score was likely to indicate more severe skin involvement and a higher chance of developing DED. However, our study did not demonstrate a significant difference in the dry eye test results between the limited and diffuse subtypes (Table 2). In addition, Table 4 demonstrates that differences in subtype, skin tightness, and total skin score did not significantly affect the occurrence of DED in our study.
Previous studies have evaluated the correlation between the subjective symptoms and objective signs of DED in patients with SSc.20,21 In a cross-sectional study, Gomes et al evaluated the OSDI, TBUT, Schirmer test, and rose bengal staining in 45 patients with SSc.20 Twenty-two patients were diagnosed with DED based on abnormal results in two or more of the tests (TBUT, Schirmer, or rose bengal), and the other 23 patients were diagnosed with non-DED. There was no significant difference in the OSDI scores between patients with and without DED. In contrast, our study demonstrated significantly higher OSDI scores in patients with DED than in those without DED. This difference can be explained by the differences in the diagnostic methods of DED. Based on DEW II diagnostic methodology, we used both the OSDI score and one of the marker tests for the diagnosis of DED, whereas Gomes et al used two or more objective tests for diagnosis. Moreover, using OSDI questionnaires, Gomes et al found that dry eye symptoms did not correlate well with clinical signs. Rentka et al also revealed a weak association between the OSDI score as a subjective parameter and clinical objective tests.21 Our study also demonstrated that the OSDI did not correlate well with dry eye evaluation tests. There were 31 patients (36.91%) without dry eye symptoms, but the objective tests were positive and, by the DEW II definition, were classified as patients with prodromal signs, who may be at risk of developing manifest DED with time or provocation. Since DED is common in patients with SSc, it is recommended to look for symptoms and signs in these patients.
Our study has several strengths. To our knowledge, this is the first study of DED in patients with SSc using the DEW II diagnostic method to confirm definite DED and no DED. In addition, determining the factors possibly associated with the occurrence of DED, which have never been reported before, is also a strength of this study. However, the limitations of the study include the small sample size. Although 84 SSc patients are appropriate for the study, grouping by DEW II diagnostic method makes the no DED subgroup too small to reach statistical significance by logistic regression analysis in some factors such as disease duration and skin tightness. Other limitations include a lack of data in highly advanced SSc patients who have unstable vital signs and cannot undergo complete ocular examination. Moreover, there were no follow-up clinical data due to the cross-sectional study design. Further studies with a larger series of SSc patients and additional data are needed to confirm these predictors.
In conclusion, our study found that the prevalence of DED in patients with SSc using the DEW II diagnostic method was 52.38%. The most common type of DED was mixed ADDE-EDE. The predictive factors associated with the occurrence of DED were older age and longer disease duration by univariate analysis. The diffuse subtype and a higher total skin score had no effect on DED. Dry eye symptoms do not correlate well with dry eye evaluation tests; therefore, DED should be evaluated in all SSc patients, particularly those with older age and longer disease duration, so that early treatment can prevent the risk of serious complications.
Acknowledgments
The authors thank Dr. Kaewjai Thepsuthammarat, Clinical Epidemiology Unit, Faculty of Medicine, Khon Kaen University for assistance with statistical analyses. This study was supported by invitation research grant (IN60329) from the Faculty of Medicine, Khon Kaen University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sakkas LI. New development in pathogenesis of systemic sclerosis. Autoimmunity. 2005;38:113–116. doi:10.1080/16066350500095415
2. Guiducci S, Distler O, Distler JH, Matucci-Cerinic M. Mechanism of vascular damage is SSc-implications for vascular treatment strategies. Rheumatology. 2008;47(Suppl 5):v18–v20. doi:10.1093/rheumatology/ken267
3. Jinnin M. Mechanisms of skin fibrosis in systemic sclerosis. J Dermatol. 2010;37:11–25. doi:10.1111/j.1346-8138.2009.00738.x
4. Fujimoto M, Sato S. B lymphocytes and systemic sclerosis. Curr Opin Rheumatol. 2005;17:746–751. doi:10.1097/01.bor.0000179945.73518.28
5. Tailor R, Gupta A, Herrick A, Kwartz J. Ocular manifestations of scleroderma. Surv Ophthalmol. 2009;54:292–304. doi:10.1016/j.survophthal.2008.12.007
6. Chifflot H, Fautrel B, Sordet C, et al. Incidence and prevalence of systemic sclerosis: a systematic literature review. Semin Arthritis Rheum. 2008;37(4):223–235. doi:10.1016/j.semarthrit.2007.05.003
7. Gagliano C, Visalli E, Toro MD, et al. Dry eye in systemic sclerosis patients: novel methods to monitor disease activity. Diagnostics. 2020;10(6):404. doi:10.3390/diagnostics10060404
8. Kozikowska M, Lubon W, Kucharz E, Kominek EM. Ocular manifestations in patients with systemic sclerosis. Reumatologia. 2020;58(6):401–406. doi:10.5114/reum.2020.102004
9. Horan EC. Ophthalmic manifestations of progressive systemic sclerosis. Br J Ophthalmol. 1969;53:388–392. doi:10.1136/bjo.53.6.388
10. West RH, Barnett AJ. Ocular involvement in scleroderma. Br J Ophthalmol. 1979;63:845–847. doi:10.1136/bjo.63.12.845
11. Gomes BF, Santhiago MR, Magalhães P, Kara-Junior N, Azevedo MN, Moraes HV. Ocular findings in patients with systemic sclerosis. Clinic. 2011;66(3):379–385.
12. Szucs G, Szekanecz Z, Aszalos Z, et al. A wide spectrum of ocular manifestations signify patients with systemic sclerosis. Ocul Immunol Inflamm. 2019;29(1):81–89. doi:10.1080/09273948.2019.1657467
13. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II Report Executive Summary. Ocul Surf. 2017;15(4):802–812. doi:10.1016/j.jtos.2017.08.003
14. Pope JE, Johnson SR. New classification criteria for systemic sclerosis (scleroderma). Rheum Dis Clin North Am. 2015;41(3):383–398. doi:10.1016/j.rdc.2015.04.003
15. Khanna D, Furst DE, Clements PJ, et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J Scleroderma Relat Disord. 2017;2(1):11–18. doi:10.5301/jsrd.5000231
16. Whitcher JP, Shiboski CH, Shiboski SC, et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjögren’s Syndrome International Registry. Am J Ophthalmol. 2010;149(3):405–415. doi:10.1016/j.ajo.2009.09.013
17. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118:615–621. doi:10.1001/archopht.118.5.615
18. Stock M, Salvay D, Shoss B, et al. Obtaining lissamine green 1% solution for clinical use. Cornea. 2015;34(11):1523–1525. doi:10.1097/ICO.0000000000000588
19. Adiguzel S, Palamar M, Yargucu F, Oksel F, Yagci A. Evaluation of ocular surface and meibomian glands in patients with scleroderma. Cornea. 2021;40(8):977–981. doi:10.1097/ICO.0000000000002551
20. Gomes BAF, Santhiago MR, de Azevedo MN, Moraes HV. Evaluation of dry eye signs and symptoms in patients with systemic sclerosis. Graefes Arch Clin Exp Ophthalmol. 2012;250(7):1051–1056. doi:10.1007/s00417-012-1938-3
21. Rentka A, Nagy A, Harsfalvi J, et al. Association between objective signs and subjective symptoms of dry eye disease in patients with systemic sclerosis. Rheumatol Int. 2017;37(11):1835–1845. doi:10.1007/s00296-017-3794-2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
