Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 9
Prediction of efficacy for conversion from adjunctive therapy to monotherapy with eslicarbazepine acetate 800 mg once daily for partial-onset epilepsy
Authors Sunkaraneni S, Passarell JA, Ludwig EA, Fiedler-Kelly J, Pitner JK, Grinnell TA, Blum D
Received 3 February 2017
Accepted for publication 3 April 2017
Published 27 June 2017 Volume 2017:9 Pages 65—72
DOI https://doi.org/10.2147/CPAA.S133815
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Arthur E. Frankel
Soujanya Sunkaraneni,1 Julie A Passarell,2 Elizabeth A Ludwig,2 Jill Fiedler-Kelly,2 Janet K Pitner,1 Todd A Grinnell,1 David Blum1
1Sunovion Pharmaceuticals Inc., Marlborough, MA, USA; 2Cognigen Corporation (a SimulationsPlus company), Buffalo, NY, USA
Purpose: Eslicarbazepine acetate (ESL) is a once-daily (QD) oral antiepileptic drug (AED) indicated for partial-onset seizures (POS). Clinical studies of gradual conversion to ESL 1,200 and 1,600 mg QD monotherapies were previously conducted in patients with POS who were not well-controlled by 1 or 2 AEDs. This report describes modeling and simulation of plasma eslicarbazepine (primary active metabolite of ESL) concentrations and time to monotherapy study exit to predict efficacy for conversion to ESL monotherapy at a lower dose of 800 mg, as an option for patients requiring or not tolerating higher doses since this regimen is effective in adjunctive therapy for POS.
Patients and methods: A previously developed population pharmacokinetic model for ESL monotherapy was used to predict minimum plasma eslicarbazepine concentration (Cmin) in 1,500 virtual patients taking 1 (n=1,000) or 2 (n=500) AEDs at baseline, treated with ESL 400 mg QD for 1 week, followed by 800 mg QD for 17 weeks (similar to ESL monotherapy trials where the other AEDs were withdrawn during the first 6 weeks following titration to the randomized ESL dose). Model-predicted Cmin as a time-varying covariate and number of baseline AEDs were used to determine the weekly probability of each patient meeting exit criteria (65.3% threshold) indicative of worsening seizure control in 500 simulated ESL monotherapy trials. A previously developed extended Cox proportional hazards exposure–response model was used to relate time-varying eslicarbazepine exposure to the time to study exit.
Results: For virtual patients receiving ESL monotherapy (800 mg QD), the 95% upper prediction limit for exit rate at 112 days of 34.9% in patients taking 1 AED at baseline was well below the 65.3% threshold from historical control trials, while the estimate for patients taking 2 AEDs (70.6%) was slightly above the historical control threshold.
Conclusion: This model-based assessment supports conversion to ESL 800 mg QD monotherapy for POS in adults taking 1 AED. For patients taking 2 concomitant AEDs, however, prescribers should consider maintenance doses of 1,200 or 1,600 mg ESL QD to reduce the likelihood of seizure worsening if conversion to ESL monotherapy is contemplated.
Keywords: eslicarbazepine, monotherapy, simulations, antiepileptic
Introduction
Eslicarbazepine acetate (ESL, Aptiom®, Sunovion Pharmaceuticals Inc., Marlborough, MA, USA; Zebinix®, BIAL – Portela & Ca, S.A., S. Mamede do Coronado, Portugal) is a once-daily (QD) oral antiepileptic drug (AED) approved as adjunctive treatment for partial-onset seizures (POS) for adults in the USA, Europe, and Canada and as monotherapy for POS in the USA. ESL was also recently approved in Europe as monotherapy for POS in adults with newly diagnosed epilepsy. A study of ESL as monotherapy for newly diagnosed partial-onset seizures showed noninferiority to carbamazepine; however, this study was conducted in a different population than the trials for conversion to monotherapy in treatment-resistant patients.1 Following oral dosing, ESL is rapidly and extensively metabolized to the active metabolite, eslicarbazepine,2 which is thought to act primarily by preferentially stabilizing the inactivated state of voltage-gated sodium channels.3
Conversion to ESL monotherapy (1,200 and 1,600 mg QD) has been studied in 2 Phase 3 studies (093-045 and 093-046) in adults with POS not adequately controlled on 1 or 2 AEDs.4–6 To avoid the need for a placebo/pseudo-placebo arm, a historical control comparator was used in both studies as described by French et al.7 The historical control comparator exit rate was determined from the placebo/pseudo-placebo (suboptimal maintenance dose of a safe and effective antiepileptic) groups of 8 historical conversion-to-monotherapy trials.7 For both doses examined (1,200 and 1,600 mg), conversion to ESL monotherapy was found to be effective (superior to a historical control) and well tolerated.4–6
The US Food and Drug Administration-recommended maintenance dosage range for ESL is 800–1,600 mg QD, when administered as adjunctive therapy or as monotherapy.8 A maintenance dosage of 800 mg QD may be considered for patients on ESL monotherapy who are unable to tolerate a dosage of 1,200 mg QD.8 In the trials reported herein (Studies 093-045 and 093-046 conversion to monotherapy), the starting dose was 400 or 600 mg QD, with subsequent titration to 1,200 or 1,600 mg QD, respectively. The higher doses (compared to the lowest effective dose of 800 mg QD seen in adjunctive trials) were selected due to plans to convert subjects to monotherapy by withdrawal of other AEDs.
The most common treatment-emergent adverse events in conversion to monotherapy Studies 093-045 and 093-46 were headache, dizziness, nausea, somnolence, and fatigue; however, there was no clear relationship between incidence of these adverse events and ESL dose.4–6 A greater proportion of patients reported treatment-emergent adverse events during the titration period for ESL (72%4 and 42%5), compared to the AED conversion phase (tapering of ESL; 61%4 and 37%5) or monotherapy period (49%4 and 38%5). In a 1-year, retrospective, multicenter, observational study in 253 patients aged >18 years who had failed first AED monotherapy and then received ESL, the 1-year retention rate was 92.9% and the final median dose of ESL was 800 mg daily. During follow-up, 31.6% of the patients reported ESL-related adverse events, most commonly somnolence (8.7%) and dizziness (6.1%), and 3.6% discontinued due to adverse events.9
This report describes the use of a time-to-event model as a basis for simulations estimating the efficacy of conversion to ESL monotherapy 800 mg QD in patients previously receiving 1 or 2 AEDs as this lower dose was not examined as a maintenance dose regimen in the Phase 3 monotherapy studies. Efficacy outcomes were estimated for comparison between patients converting from either 1 or 2 AEDs to ESL (~70% of patients were taking 1 AED during the baseline period6). Since this report describes model-based simulations based on the Phase 3 conversion to monotherapy trials specifically designed to convert patients from their prior AED therapy to ESL, the resulting dosing recommendations should not be extrapolated for use in patients receiving ESL 800 mg QD monotherapy as initial treatment of POS.
Methods
Study design and patient population
Population pharmacokinetics (PK) and time-to-event models used for the simulations described in this report were previously developed using data from patients who participated in the 2 Phase 3 conversion-to-ESL-monotherapy studies (Studies 093-045 and 093-046 of identical design) as previously reported.4,5 Patients aged 16–70 years with POS were eligible for study participation if the following criteria were met: documented electroencephalogram recording consistent with POS; ≥4 POS during the 8 weeks before screening with no seizure-free period ≥4 weeks; treatment with stable doses of 1 or 2 AEDs in the 4 weeks before screening (if receiving 2 AEDs at screening, only 1 AED could be a sodium channel blocker (ie, phenytoin, carbamazepine, oxcarbazepine, or lamotrigine) and only 1 could be in the upper dose range (greater than approximately two-thirds of its defined daily dose);10 and no additional/potential health complications (elderly patients [65–70 years] only).
After an 8-week baseline period, eligible patients were randomized 2:1 to receive oral ESL (1,600 or 1,200 mg tablets QD), and began the 18-week double-blind treatment period (2-week ESL titration, 6-week withdrawal of concomitant AEDs, 10-week ESL monotherapy). The primary efficacy endpoint was study exit based on meeting ≥1 of 5 prospectively defined exit criteria (signifying worsening seizure control) between the start of the AED withdrawal period and the end of the monotherapy period.
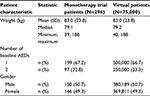
Seizure diaries were completed daily by each patient or their caregiver throughout the study. Blood samples were collected prior to in-clinic study dose administration at Weeks 8, 11, 14, and 18 after randomization for measurement of eslicarbazepine concentrations. In these studies, PK and seizure frequency measurements were available from 296 patients, of whom 199 patients converted to monotherapy from 1 AED and 97 patients converted from 2 AEDs.
Studies 093-045 and 093-046 were conducted according to the US Office of Federal Regulations, the principles of Good Clinical Practice, the World Medical Association Declaration of Helsinki (1989), and the International Conference on Harmonization guidelines and in accordance with national, state, and local laws of the pertinent regulatory authorities. All subjects were required to provide written informed consent prior to study enrollment.
Simulation of eslicarbazepine exposure
The virtual patient population of 1,500 was generated by random resampling of the patient characteristics previously shown to be significant predictors of PK (weight and gender)11 from the 296 patients in the 2 Phase 3 studies. Resampling was used to ensure that the distribution of demographic characteristics in the virtual population was similar to the observed distribution from the actual patient population. The number of baseline AEDs was randomly assigned in a 2:1 ratio (1 AED at baseline: 2 AEDs at baseline) based on the observed distribution of use. Five hundred clinical trials were simulated.
Biweekly estimates of eslicarbazepine exposure (minimum plasma eslicarbazepine concentration [Cmin]) during the 18 weeks of treatment with ESL (1 week of ESL 400 mg QD and 17 weeks of ESL 800 mg QD; same study design as in monotherapy Studies 093-045 and 093-046) were calculated for each virtual patient based on simulation using the population PK model.11 Plasma eslicarbazepine concentrations were described by a 1-compartment model with first-order absorption and first-order linear elimination, with covariate effects of body weight and gender on apparent clearance (CL/F) and apparent distribution volume (V/F). The magnitude of these PK parameters increased with increasing body weight, and females were predicted to have lower CL/F and V/F as compared to males of the same body weight.11 Exploratory graphical analysis showed largely similar trends in the eslicarbazepine concentrations over time during the monotherapy phase regardless of prior AED medication.11 Integration of the simulated plasma concentration–time profile was performed using NONMEM® (ICON plc., Dublin, Ireland), version 7.1.2 (ICON Development Solutions 2010) to obtain the individual estimates of eslicarbazepine exposure.
Simulation of study exit
In the conversion-to-monotherapy trial design, patients met the primary endpoint of exiting the study if 1 or more predefined exit criteria indicative of worsening seizure control4–6 applied: 1 episode of status epilepticus; 1 secondary generalized partial seizure (for patients without generalized seizures during 6 months prior to screening); twofold increase from baseline in consecutive 28-day seizure rate; twofold increase from baseline in consecutive 2-day seizure rate; or worsening of seizures or increase in seizure frequency (as judged by the investigator).
The time to study exit model used to evaluate the exposure–response relationship for ESL efficacy in patients administered ESL 1,200 or 1,600 mg QD was previously developed with data from the conversion-to-monotherapy trials.12 The exposure–response model was characterized using an extended Cox proportional hazards model to relate time-varying eslicarbazepine exposure (Cmin) and number of AEDs at baseline to the hazard of study exit. The hazard function describing the risk of study exit is expressed as 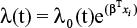 .
.
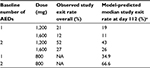
where λ0(t) is the baseline hazard estimated using the 1,200 and 1,600 mg data; βT is the vector of parameter estimates for eslicarbazepine Cmin and the baseline number of AEDs; and xi is the vector of covariates for an individual patient. The parameter vector βT was estimated using maximum partial likelihood in SAS® (version 9.2, Cary, NC, USA, 2009). The parameter estimates and corresponding precision of the estimates for the exposure–response model of time to study exit are provided in Table 1. To confirm the predictive ability of the time to study model as a basis for the current analysis, the study exit rates at Day 112 for the 1,200 and 1,600 mg regimens by baseline number of AEDs were predicted.
Although both eslicarbazepine Cmin and baseline number of AEDs were statistically significant predictors of the time to study exit (p=0.0018 and p=0.0001, respectively), the effect size for the baseline number of AEDs was of considerably larger magnitude. The Cox hazard ratio for the effect of Cmin on time to study exit was 0.905 (95% confidence interval [CI]: 0.850, 0.963), indicating that with increasing eslicarbazepine exposure, or more specifically, for each increase of 1,000 ng/mL of Cmin, the hazard for study exit was decreased by 9.5%. The Cox hazard ratio for the effect of 1 baseline AED on time to study exit was 0.380 (95% CI: 0.232, 0.624), indicating that the risk of study exit for 1 baseline AED is 38% the risk for 2 baseline AEDs. Using this model of the time to study exit, the predicted Cmin on selected weeks and the number of baseline AEDs (1 or 2) were used to determine the probability of study exit (leaving the trial on each week) for each virtual patient in each virtual trial.
The 95% prediction interval for the probability of survival (and exit rate = 1– survival) was determined every other week for virtual patients converting to ESL 800 mg QD monotherapy who were on 1 or 2 AEDs at baseline. A schematic diagram of the overall study design and simulation methods is shown in Figure 1.
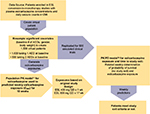  | Figure 1 Application of the population pharmacokinetic model and the time-to-event model to predict probability of survival (not exiting the study) during conversion to ESL monotherapy (800 mg QD). Notes: The development of the population PK model11 and the PK/PD time to study exit model12 has been previously described. Abbreviations: AED, antiepileptic drug; Cmin, minimum plasma eslicarbazepine concentration; ESL, eslicarbazepine acetate; n, number of patients; PK, pharmacokinetic; PK/PD, pharmacokinetic/pharmacodynamic; QD, once daily; wk, week. |
Comparison with historical control
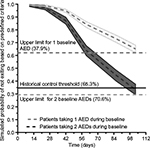
A historical control, representative of the placebo/pseudo-placebo groups in 8 historical conversion-to-monotherapy trials, is currently the standard for comparison in conversion-to-monotherapy AED trials.7 To compare the exit rate for the historical control with that for a new AED, the key statistic is the lower bound of the 95% prediction interval of the overall historical control exit rate; at a type I error rate of ≤5%, this equates to an exit rate of 65.3% at 112 days for the historical control (based on 8 studies).13 The same statistic was used to compare the simulated outcome for ESL 800 mg QD with that for the historical control; if the 95% upper prediction limit of the simulated exit rate is ≤65.3%, then the null hypothesis (that the exit rate for ESL 800 mg QD is equal to that for the historical control) would be rejected.
Results
Table 2 provides the summary statistics of the demographic characteristics of the patients included in the monotherapy trials and the virtual patients from the 500 simulated clinical trials. Based on the resampling technique implemented, the distributions of the virtual patient demographic characteristics are similar to the actual population of patients in the clinical trials used as a basis for these simulations.
Since the time-to-event model was developed with data from ESL 1,200 and 1,600 mg QD regimens using the semi-parametric extended Cox proportional hazards model, the underlying assumption of risk over time (baseline survival) inherent within the estimated model was dependent upon the observed data from these dosing regimens. To illustrate the predictive ability of the model, Table 3 provides the observed overall study exit rates from the Phase 3 trials and the corresponding model-predicted study exit rates at Day 112 for the 1,200 and 1,600 mg regimens (assuming the median Cmin for each dose group) by baseline number of AEDs. There is good concordance between the observed and model-predicted study exit rates for the 1,200 and 1,600 mg doses, thus demonstrating that the time to study exit model was unbiased and predicted the data well, except for a slight underprediction bias of the 1,200 mg regimen with 2 baseline AEDs.
When the baseline survival based on the 1,200 and 1,600 mg dose groups in the Phase 3 trials was used to simulate outcomes with 800 mg QD, the predicted study exit rate was similar to that of the 1,200 mg QD dose regimen (21%). In order to simulate responses for the lower dose regimen of 800 mg that were more predictive of the expected higher risk associated with a lower dose, the baseline survival (risk over time) was adjusted by an empirical factor of 1.75 ([1200 + 1600]/2 = 1400 mg/800 mg) for extrapolation of the lower dose regimen.
Simulated median exit rates (95% prediction intervals) at 112 days for virtual patients receiving ESL monotherapy at a dose of 800 mg QD were 34.9% (32.0%–37.9%) for patients taking 1 AED at baseline and 66.6% (62.4%–70.6%) for those taking 2 AEDs at baseline. The 95% upper prediction limit for the exit rate at 112 days for patients taking 1 AED at baseline of 37.9% was below the 65.3% threshold based on historical control. However, the 95% upper limit for the exit rate at 112 days for those patients taking 2 AEDs at baseline (ie, 70.6%) was above the historical control threshold as shown in Figure 2. Thus, the model-based simulations suggest that ESL 800 mg QD reduces seizure-related study exits compared with the historical control in patients who convert from 1 previous AED.
Figure 3 shows the median probability of survival (remaining in the study) over time for patient subgroups defined by quartiles of eslicarbazepine Cmin for patients converting from 1 baseline AED (left panel) or 2 baseline AEDs (right panel). The results indicate that during conversion to ESL 800 mg QD, the probability of survival is greater for patients who convert from 1 baseline AED, than for patients who convert from 2 previous AEDs, and in addition, patients with higher eslicarbazepine exposure (Cmin) are predicted to have a higher probability of survival as compared to those with lower eslicarbazepine exposure (Cmin).
Discussion
The efficacy of conversion to ESL monotherapy as treatment for POS was demonstrated in 2 clinical trials where study exit by meeting predefined exit criteria (signifying worsening seizure control) was the primary endpoint.4,5 Subjects whose POS were medically uncontrolled on 1 or 2 AEDs were converted to ESL 1,200 or 1,600 mg QD monotherapy. Pooled exit rates from the 18-week, double-blind treatment periods in the 2 studies were 20.6% (95% CI: 15.6%–26.7%) and 30.8% (95% CI: 23.0%–40.5%) for the 1,600 and 1,200 mg dose regimens at 112 days, respectively.6 These rates are well below the study exit rate for the historical control, representative of placebo/pseudo-placebo exit rates in 8 withdrawal-to-monotherapy trials, of 85.1%, with a lower bound of the 95% prediction interval of 65.3%.7 Use of these historical control data represented, at the time of these trials, the only FDA-accepted path to monotherapy approval that is also considered acceptable to the epilepsy community.7,14
This report describes use of an extended Cox proportional hazards model to perform simulations of the exposure–response relationship with respect to time to study exit for ESL 800 mg QD monotherapy. This type of PKPD model was required due to the time-varying nature of eslicarbazepine exposure (Cmin) resulting from titration to the maximal dose and accumulation to steady-state conditions. In this analysis, predicted study exit rates for subjects converting from 1 or 2 AEDs for treatment of POS to ESL monotherapy using a dosing regimen of ESL 800 mg QD were evaluated. Even when considering the worst-case scenario, the 95% upper prediction limit for the exit rate at 112 days was below the 65.3% threshold for patients taking 1 AED at baseline (37.9%) but above the threshold for those taking 2 AEDs at baseline (70.6%). This difference in predicted exit rates between patients converting from 1 versus 2 AEDs is not unexpected given this patient population with refractory partial epilepsy. It is likely that patients who had received 2 AEDs had required more drug in an effort to provide adequate seizure control, consistent with the presence of more treatment refractory disease, relative to those subjects who received 1 baseline AED.15 It should be noted that patients receiving 2 AEDs at baseline were only included in the Phase 3 monotherapy trials if they were not receiving maximum doses of both drugs, otherwise one might expect the likelihood of success to be even lower, thereby requiring higher doses of ESL. This consideration is somewhat hypothetical, as it would not be anticipated that a patient receiving maximum doses of 2 antiepileptics in a clinical practice setting would be considered to convert to monotherapy.
In the simulations, study exit rate was predicted every other week to be consistent with the protocol-specified study visits scheduled according to defined study periods in the design of the ESL monotherapy clinical trials. To assess whether the frequency of study exit rate prediction influenced the simulation results, alternative frequencies of prediction (eg, every week) were also evaluated. However, the findings from testing of these other prediction frequencies indicated that the use of frequencies of assessment other than every other week had little influence on the simulation results.
Prior to incorporation of the empirical dose-related adjustment to the baseline survival distribution, initial simulations resulted in an apparent overprediction of efficacy from the eslicarbazepine 800 mg QD regimen such that the predicted exit rate at 112 days was similar to the observed rate for the 1,200 mg dose regimen. While model-based extrapolation is fraught with some inherent risk of inappropriate conclusion, after consideration of the marginal contributions of the baseline survival distribution, the number of AEDs at baseline, and the effect of eslicarbazepine exposure on the predicted survival probability from the time-to-event model, it was determined that the baseline survival distribution likely exerted a greater influence on the model than eslicarbazepine exposure. This may be due to the use of data from only 2 dose levels (ie, 1,200 and 1,600 mg QD) with overlapping exposure distributions in the development of the time-to-event model. Therefore, the baseline survival distribution required some adjustment to achieve logical and expected results for the 800 mg QD regimen. Although there would be no a priori expectation that the baseline survival distribution would differ based on randomized dose, the observed baseline survival distribution differed for the 1,200 and 1,600 mg QD dose groups. As such, sensitivity analyses were performed to select an empirical adjustment factor for the baseline survival distribution, appropriate for the 800 mg QD regimen. Ultimately, a factor of 1.75 was selected for the baseline survival distribution for 800 mg QD relative to the 1,200 and 1,600 mg regimens (consistent with the ratio of the average of the observed doses to the 800 mg dose) to extrapolate the model to appropriately simulate responses for the lower-dose regimen.
Overall, this simulation analysis was associated with several limitations that required assumptions to be made as described above. The risk of study exit over time (baseline survival) was dependent on the observed data, but it was shown that the observed and model-predicted study exit rate exhibited reasonable concordance. Since no actual observed data were available after administration of ESL 800 mg QD, an empirical adjustment of the baseline survival was required to allow for extrapolation to the lower 800 mg dosage regimen. In addition, predicted study exit rates for the ESL dose regimens were compared to the rate historical controls rather than actual placebo-treated subjects in the ESL conversion to monotherapy trials. This approach was considered the standard of practice at the time these studies were conducted.7
Conclusion
Overall, the results of the simulations provide evidence that conversion to ESL 800 mg QD monotherapy may be possible for adults with POS who are taking 1 AED. Patients who are taking 2 AEDs are predicted to be more likely to meet the monotherapy clinical trial exit criteria (due to seizure worsening) when converted to a simulated 800 mg maintenance dose, so maintenance doses of ESL 1,200 or 1,600 mg QD should be considered if conversion from 2 AEDs to ESL monotherapy is contemplated. Since these results are based on clinical trials specifically designed to convert patients from their prior AED therapy to ESL monotherapy, the dosing recommendations provided herein should not be extrapolated for use in patients receiving ESL 800 mg QD monotherapy as initial treatment of POS.
Acknowledgments
The authors appreciated the insightful review of this manuscript provided by Raju Poola, MS, PhD, Associate Director, Sunovion Pharmaceuticals, Inc. Sunovion Pharmaceuticals Inc. provided financial support to Cognigen Corporation (a SimulationsPlus company) to perform the reported analyses and for manuscript preparation.
Disclosure
Funding for these analyses and for manuscript preparation was provided by Sunovion Pharmaceuticals, Inc. to Cognigen Corporation (a SimulationsPlus company). S Sunkaraneni, JK Pitner, TA Grinnell, and D Blum are employees of Sunovion Pharmaceuticals, Inc. JA Passarell, EA Ludwig, and J Fiedler-Kelly are employees of Cognigen Corporation.
References
Trinka E, Kowacs B, Ben-Menachem E, et al. Efficacy of eslicarbazepine acetate versus controlled-release carbamazepine as monotherapy of newly diagnosed partial-onset seizures. Poster presented at: Second Congress of the European Academy of Neurology; May 28, 2016; Copenhagen, Denmark. | ||
Almeida L, Potgieter JH, Maia J, Potgieter MA, Mota F, Soares-da-Silva P. Pharmacokinetics of eslicarbazepine acetate in patients with moderate hepatic impairment. Eur J Clin Pharmacol. 2008;64(3):267–273. | ||
Hebeisen S, Pires N, Loureiro AI, et al. Eslicarbazepine and the enhancement of slow inactivation of voltage-gated sodium channels: a comparison with carbamazepine, oxcarbazepine and lacosamide. Neuropharmacology. 2015;89:122–135. | ||
Sperling MR, Harvey J, Grinnell T, et al. Efficacy and safety of conversion to monotherapy with eslicarbazepine acetate in adults with uncontrolled partial-onset seizures: a randomized historical-control phase III study based in North America. Epilepsia. 2015;56(4):546–555. | ||
Jacobson MP, Pazdera L, Bhatia P, et al. Efficacy and safety of conversion to monotherapy with eslicarbazepine acetate in adults with uncontrolled partial-onset seizures: a historical-control phase III study. BMC Neurol. 2015;15:46. | ||
Sperling MR, French J, Jacobson MP, et al. Conversion to eslicarbazepine acetate monotherapy: a pooled analysis of 2 phase III studies. Neurology. 2016;86(12):1095–1102. | ||
French JA, Wang S, Warnock B, et al. Historical control monotherapy design in the treatment of epilepsy. Epilepsia. 2010;51(10):1936–1943. | ||
Sunovion APTIOM® (eslicarbazepine acetate) Full Prescribing Information, 2015. Sunovion Pharmaceuticals Inc., Marlborough, MA, USA. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/022416s001lbl.pdf. Accessed February 19, 2016. | ||
Villanueva V, Bermejo P, Montoya J, et al. EARLY-ESLI study: long-term experience with eslicarbazepine acetate after first monotherapy failure. Acta Neurol Scand. Epub 2016 Dec 9. | ||
WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD index. Available from: http://www.whocc.no/atc_ddd_index/. Accessed June 21, 2016. | ||
Abou-Khalil B, Ali I, Shah A, et al. Eslicarbazepine acetate monotherapy: a population pharmacokinetic analysis. Epilepsy Curr. 2015; 15(Suppl 1; Abstract 1.321):150–151. | ||
Rogin J, Cole AJ, Strom L, et al. Relationship between exposure and efficacy of eslicarbazepine acetate monotherapy. Epilepsy Curr. 2015; 15(Suppl 1; Abstract 1.314):145–146. | ||
U.S. Food and Drug Administration. Ling X. Lamictal® XR™(lamotrigine) historical-controlled trial: Peripheral and Central Nervous System Drugs Advisory Committee Meeting 2011. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PeripheralandCentralNervousSystemDrugsAdvisoryCommittee/UCM246068.pdf. Accessed March 14, 2016. | ||
Brodie MJ. Road to refractory epilepsy: the Glasgow story. Epilepsia. 2013;54(Suppl 2):5–8. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.