Back to Journals » Clinical Ophthalmology » Volume 14
Preclinical Efficacy Comparison of Cyclosporine Ophthalmic Solution 0.09% vs Cyclosporine Ophthalmic Emulsion 0.05% vs Ciclosporin Ophthalmic Emulsion 0.1% in a NOD Mouse Model of Dry Eye Disease
Authors Burade V, Zalawadia R, Patel A, Ogundele A
Received 23 April 2020
Accepted for publication 20 August 2020
Published 21 September 2020 Volume 2020:14 Pages 2747—2755
DOI https://doi.org/10.2147/OPTH.S259331
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Vinod Burade,1 Rishit Zalawadia,1 Alpesh Patel,1 Abayomi Ogundele2
1Sun Pharmaceutical Industries Limited, Vadodara, Gujarat, India; 2Sun Pharmaceutical Industries, Inc, Princeton, NJ, USA
Correspondence: Abayomi Ogundele
Sun Pharmaceutical Industries, Inc, Princeton, NJ, USA
Tel +1 (609) 720-5389
Email [email protected]
Introduction: Cyclosporine ophthalmic solution 0.09% (CsA 0.09% sol) is approved to increase tear production in patients with keratoconjunctivitis sicca. This study evaluated the efficacy of CsA 0.09% sol vs cyclosporine ophthalmic emulsion 0.05% (CsA 0.05% eml) vs ciclosporin ophthalmic emulsion 0.1% (CsA 0.1% eml) in a NOD mice model.
Methods: Mice were randomized and administered placebo, CsA 0.09% sol twice daily, CsA 0.05% eml twice daily, CsA 0.09% sol once daily, or CsA 0.1% eml once daily in the conjunctival sac of both eyes for 60 days. Tear volume was measured with phenol red threads at baseline and 4 hours after treatment every 15 days. On day 58, the corneal surface was observed under a slit-lamp after staining with 3% lissamine green administered into the inferior lateral conjunctival sac. On day 61, mice were euthanized, globes excised, sliced into 4 μm sections in 3 areas per section, and stained. Total number of stained goblet cell/μm was counted, and the sum per eye was averaged. Lacrimal gland tissues were removed and interleukin (IL) 1-β cytokine levels estimated.
Results: Groups comprised 11 mice each, including an untreated normal and untreated diseased control group (7 groups total). CsA 0.09% sol twice daily significantly increased tear volume on day 30, 45, and 60 vs CsA 0.05% eml (P< 0.05, < 0.001, < 0.001, respectively) and vs CsA 0.1% eml on day 60 (P< 0.05); CsA 0.09% sol once daily significantly increased tear volume on day 45 vs CsA 0.05% eml (P< 0.05). Goblet cell density significantly increased with CsA 0.09% sol twice daily vs placebo and NOD control (P< 0.01 both). There was no significant difference in corneal staining and IL-1β levels with CsA 0.09% sol.
Conclusion: Sixty-day treatment with CsA 0.09% sol showed comparatively improved preclinical results vs CsA 0.05% eml and CsA 0.1% eml.
Keywords: cyclosporine A, goblet cell density, keratoconjunctivitis sicca, preclinical, tear production
Introduction
Dry eye disease or keratoconjunctivitis sicca (KCS) is a multifactorial disease affecting the ocular surface. It is characterized by a loss of homeostasis of the tear film, and is accompanied by ocular symptoms in which tear film instability and hyperosmolarity, and ocular surface inflammation and damage play etiological roles.1 KCS is often initiated with a change in tear hyperosmolarity, leading to a release of inflammatory cytokines and proteases, resulting in a vicious cycle of ocular surface damage marked by tear film instability, and goblet cell and epithelial cell loss and damage.2
Topical anti-inflammatory agents such as cyclosporine A (CsA) provide a targeted approach to treat and suspend the inflammatory cascade of KCS.3 CsA inhibits calcineurin, preventing T-cell activation and inflammatory cytokine production.4 Treatment with CsA improves objective measures of KCS including decreased corneal fluorescein staining (CFS) and improved Schirmer’s test.5–7 Current CsA formulations approved to treat KCS include cyclosporine ophthalmic solution 0.09% (CEQUA™; Sun Pharmaceutical Industries, Inc., Princeton, NJ, USA), cyclosporine ophthalmic emulsion 0.05% (RESTASIS®; Allergan, Inc., Irvine, CA, USA), and ciclosporin ophthalmic emulsion 0.1% (Ikervis®; Santen SAS, Evry, France).8–10 There are significant variations in the formulations of the approved treatments. Cyclosporine ophthalmic emulsion 0.05% is a preservative-free anionic oil-in-water emulsion with CsA dissolved in castor oil and emulsified with polysorbate 80,11 while ciclosporin ophthalmic emulsion 0.1% is a cationic nanoemulsion intended to achieve ocular bioavailability through the net positive charge of the oil nanodroplets.11 Cyclosporine ophthalmic solution 0.09% is a clear aqueous nanomicellar formulation that encapsulates hydrophobic CsA within its micelle core and is surrounded by a water-soluble outer shell, allowing enhanced ocular delivery of cyclosporine.5,8,12
Here we report the comparative efficacy of cyclosporine ophthalmic solution 0.09% vs cyclosporine ophthalmic emulsion 0.05% (herein referred to as reference) vs ciclosporin ophthalmic emulsion 0.1% (herein referred to as ciclosporin) in a NOD.B10.H2b (NOD) mice model. NOD mice spontaneously develop Sjögren’s syndrome—resulting in the typical features of dry eye and secretory dysfunction, among other manifestations—without the occurrence of diabetes.13,14
Materials and Methods
Experimental Design
Overall, 75 male NOD and 15 C57Bl6/J mice were obtained from Laboratory Animal Resources (LAR; Sun Pharmaceutical Advanced Research Company Ltd., Vadodara, India). Mice, aged 16–19 weeks, were randomized into 7 groups with 11 mice per group (C57Bl6/J [normal control], NOD diseased control, placebo twice daily, cyclosporine ophthalmic solution 0.09% twice daily, cyclosporine ophthalmic solution 0.09% once daily, reference twice daily, and ciclosporin once daily). With the exception of the cyclosporine ophthalmic solution 0.09% once-daily group, treatment frequency was based on the approved dosing frequency for the cyclosporine ophthalmic formulations included. All treatment groups had matched baseline (day −1) tear volume as measured by phenol red-impregnated cotton threads. The nanomicelle formulation vehicle was the placebo in this study. Mice received 10 µL of assigned treatment topically in the conjunctival sac of both eyes either once or twice daily (at approximately 12-hour intervals) for 60 days. Assessments included tear production at day 15, 30, 45, and 60, corneal staining at day 58, and conjunctival goblet cell density and interleukin (IL) 1-β estimation at day 61 (Figure 1).
 |
Figure 1 Study design. |
The study proposal was reviewed and approved by the Institutional Animal Ethics Committee in India, which is overseen by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). The CPCSEA is a statutory body formed by the Act of the Indian Parliament under the Prevention of Cruelty to Animals Act 1960. All recommendations by the Institutional Animal Ethics Committee concerning animal care and handling were followed. All mice were housed under standard conditions and allowed an acclimation period of one week following receipt from LAR. At day 61, animals were euthanized with carbon dioxide inhalation, and tissue collection was performed.
Assessments
Tear Volume
Tear volume was measured in all groups at day −1, and 4 hours after treatment at days 15, 30, 45, and 60 using Zone-Quick phenol red thread (FCI Ophthalmics, Pembroke, MA). Mice were lightly anesthetized with isoflurane. A phenol red-impregnated thread was inserted into the lateral canthus of each eye and held in place with sterile jeweler forceps (without touching the ocular surface) for 60 seconds. After removing the thread, the wetted length of the thread was measured in millimeters (mm) and recorded.
Corneal Staining
On day 58, the mice were lightly anesthetized with isoflurane 4 hours after treatment, and 1 drop of 3% lissamine green B dye was instilled into the inferior lateral conjunctival sac of each eye. The corneal surface was examined with a Zeiss slit-lamp microscope with a white filter and scored from 0 to 3 (0 = no punctate staining, 1 = less than one-third of the cornea stained, 2 = two-thirds or less of the cornea stained, 3 = more than two-thirds of the cornea stained).
Conjunctival Goblet Cell Density
At day 61, all mice were euthanized and whole globes, including the superior and inferior forniceal conjunctiva, were excised and fixed in 4% paraformaldehyde. The tissues were sectioned into 4 µm-thick segments through both the superior and inferior conjunctival fornices and stained with periodic acid-Schiff (PAS) reagent (Merck Millipore, catalog number: 1016460001). Stained sections were examined with a Zeiss Axio A1 microscope with 100× magnification, and the total number of stained conjunctival goblet cells per 100 µm were counted per 3 different areas of each section per eye. The sum for each eye was then averaged to determine the mean number of goblet cells per mouse. Slide numbers for tissue sections were assigned a new randomized code prior to microscopic examination.
Interleukin 1-β Assay
After euthanasia, the exorbital lacrimal glands of both eyes were dissected, snap frozen in liquid nitrogen, and stored at −70° C in a microcentrifuge tube. The frozen lacrimal tissues were weighed and cocktail protease inhibitor (1 mL 0.1 M phenylmethylsulfonyl fluoride, 50 µL aprotinin [1 mg/mL], 50 µL Triton X 100 and 50 mL phosphate buffer saline [PBS, pH 7.4] for volume) added to prepare a 10% w/v tissue homogenate. Each lacrimal gland tissue was homogenized for 30 to 90 seconds at 19,000 to 26,000 rotations per minute (rpm) in a 5 mL cryovial in a homogenizer in an ice bath. Following each run, the homogenizer probe was washed with 70% v/v isopropyl alcohol and PBS (pH 7.4) and wiped dry. All homogenized tissue samples were centrifuged at 10,000 rpm for 10 minutes at 4° C; the resulting supernatant was separated in microcentrifuge tubes, snap frozen in liquid nitrogen, and stored at −70° C. IL 1-β estimation in the supernatant was performed using mouse enzyme-linked immunosorbent assay kits (R&D Systems; catalog number: MLB00C) according to the manufacturer’s instructions.
Statistical Analysis
All statistical analyses were performed with Prism (GraphPad version 5.03, December 10, 2009) and P <0.05 was statistically significant. Data were analyzed by 2-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test for tear production; a 1-way ANOVA followed by Bonferroni’s multiple comparison test was used for corneal staining, goblet cell density, and IL 1-β assay assessments.
Results
Each treatment group consisted of 11 mice for a total of 77 mice in the study.
Tear Production
The baseline mean (standard deviation [SD]) wetting length (mm) for the normal control, NOD diseased control, placebo twice daily, ciclosporin once daily, reference twice daily, cyclosporine ophthalmic solution 0.09% twice daily, and cyclosporine ophthalmic solution 0.09% once daily was 4.8 (2.2), 4.9 (1.6), 4.9 (1.3), 4.7 (1.0), 4.7 (1.2), 5.0 (1.3), and 4.7 (1.0), respectively. Overall, tear production significantly increased with cyclosporine ophthalmic solution 0.09% twice daily vs cyclosporine ophthalmic emulsion 0.05% twice daily on day 30, 45, and 60 (P <0.05, P <0.001, and P <0.001, respectively) and on day 60 vs ciclosporin ophthalmic emulsion 0.1% once daily (P <0.05, Table 1). Cyclosporine ophthalmic solution 0.09% once daily significantly increased tear volume vs cyclosporine ophthalmic emulsion 0.5% twice daily on day 45 (P <0.05).
 |
Table 1 Phenol Red-Impregnated Thread Wetting Length (mm) in Both Eyes from Baseline to Day 60 |
Cyclosporine ophthalmic solution 0.09% twice daily significantly improved tear volume mean change from baseline (SD) vs cyclosporine ophthalmic emulsion 0.05% twice daily at day 45 and 60; 6.5 (3.6) vs 2.3 (3.7), and 7.9 (4.3) vs 4.1 (2.7), respectively (P<0.01 for both; Figure 2). Cyclosporine ophthalmic solution 0.09% twice daily significantly increased tear volume vs NOD diseased control on day 30, 45, and 60 (P<0.001) and vs placebo twice daily onday 30, 45, and 60 (P<0.01, P<0.01, P<0.001, respectively). Similarly, cyclosporine ophthalmic solution 0.09% once daily had a significant increase in tear volume vs NOD diseased control on day 30, 45, and 60 (P<0.001 for all) and vs placebo twice daily on day 60 (P<0.01). There was no significant difference in tear production between cyclosporine ophthalmic solution 0.09% once daily and ciclosporin ophthalmic emulsion 0.1% once daily at any time point.
 |
Figure 2 Change from baseline in tear production through 60 days of treatment. |
Corneal Staining
At day 58, mean corneal staining score (SD) for NOD diseased control, placebo twice daily, ciclosporin ophthalmic emulsion 0.1% once daily, cyclosporine ophthalmic emulsion 0.05% twice daily, cyclosporine ophthalmic solution 0.09% twice daily, and cyclosporine ophthalmic solution 0.09% once daily was 2.2 (0.6), 2.1 (0.5), 1.8 (0.3), 2.1 (0.5), 1.6 (0.4), and 1.8 (0.35), respectively. Corneal staining scores were not estimable in the normal control group due to the black background in the eye. The cyclosporine ophthalmic solution 0.09% twice-daily and cyclosporine ophthalmic solution 0.09% once-daily groups had numerically better corneal scores compared with mice receiving ciclosporin ophthalmic emulsion 0.1% once daily and cyclosporine ophthalmic emulsion 0.05% twice daily, but the difference was not statistically significant (Supplemental Figure 1).
Conjunctival Goblet Cell Density
At day 60, the mean (SD) number of goblet cells for normal control, NOD diseased control, placebo twice daily, ciclosporin ophthalmic emulsion 0.1% once daily, cyclosporine ophthalmic emulsion 0.05% twice daily, cyclosporine ophthalmic solution 0.09% twice daily, and cyclosporine ophthalmic solution 0.09% once daily was 277.2 (100.6), 73.2 (42.6), 72.1 (30.1), 138.5 (55.9), 110.2 (41.7), 174.6 (86.1), and 138.9 (50.1), respectively. Overall, mice in the cyclosporine ophthalmic solution 0.09% twice-daily group had significantly higher goblet cell density compared with mice in the placebo and NOD diseased control groups after 60 days of treatment (P <0.01 for both, Figures 3 and 4). Similarly, the cyclosporine ophthalmic solution 0.09% twice-daily group had numerically greater number of goblet cells vs ciclosporin ophthalmic emulsion 0.1% once daily and cyclosporine ophthalmic emulsion 0.05% twice daily at day 60. In addition, mice receiving cyclosporine ophthalmic solution 0.09% once daily had numerically higher mean number of goblet cells vs cyclosporine ophthalmic emulsion 0.05% twice daily.
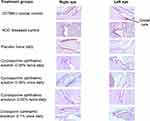 |
Figure 3 Representative periodic acid Schiff stained conjunctival goblet cells (100x magnification) at day 60.Abbreviation: NOD, NOD.B10.H2b. |
 |
Figure 4 Total number of goblet cells in the conjunctiva at day 60. |
Interleukin 1-β Assay
At day 60, mean (SD) IL 1-β lacrimal gland homogenate for normal control, NOD diseased control, placebo twice daily, ciclosporin ophthalmic emulsion 0.1% once daily, cyclosporine ophthalmic emulsion 0.05% twice daily, cyclosporine ophthalmic solution 0.09% twice daily, and cyclosporine ophthalmic solution 0.09% once daily was 95.6 (32.2), 111.9 (68.8), 110.2 (36.3), 82.5 (27.6), 79.1 (29.7), 94.7 (33.5), and 82.5 (27.6), respectively. Although there were no statistically significant differences between the study groups, the cyclosporine ophthalmic solution 0.09% once-daily group had numerically decreased levels of IL 1-β compared to ciclosporin ophthalmic emulsion 0.1% once daily and cyclosporine ophthalmic emulsion 0.05% twice daily (Supplementary Figure 2).
Discussion
This preclinical study provides relevant comparative efficacy data for eye care practitioners using immunosuppressants (particularly cyclosporine A ophthalmic formulations) for the treatment of KCS. Efficacy measurements used in this study parallel similar assessments included in previous clinical studies.
In this study, the NOD diseased control group consistently had decreased tear production and reduced goblet cell density compared with the normal control group. Overall, increased tear production was the most significant change in NOD mice receiving cyclosporine ophthalmic solution 0.09% vs mice receiving ciclosporin ophthalmic emulsion 0.1%, cyclosporine ophthalmic emulsion 0.05%, or placebo. Tears constantly bathe the corneal surface creating an unbroken film to protect the eye from desiccating stress and injury. Disruptions in tear production can lead to tear film instability, ocular inflammation, and damage to the ocular surface.15 Tear volume is a reliable measure of both dry eye disease and response to treatment given that many therapeutic strategies for KCS are indicated to increase tear production.8,9 Interestingly, the normal control group demonstrated an increase in tear production from day 45 to day 60. Possible explanations for this increase may be due to small sample size and inherent genetic variability. Several studies observed similar increases in tear volume in C57BL/6 mice with increased age and attributed it to genetic background.16,17
Previous clinical studies demonstrate the efficacy of cyclosporine ophthalmic solution 0.09% in improving corneal staining scores and increasing tear volume in patients with KCS.5,18–20 In the phase 2b/3 study and the phase 3 study, a significantly greater number of patients receiving cyclosporine ophthalmic solution 0.09% twice daily had an increase of ≥10 mm from baseline to day 84 in Schirmer’s test scores compared to patients receiving vehicle (P <0.007 and P <0.001, respectively).5,20 Cyclosporine ophthalmic solution 0.09% twice daily significantly improved corneal fluorescein staining scores vs vehicle at day 84 of treatment in the phase 2b study (P <0.0003) and at days 28, 56, and 84 in the phase 3 study (P <0.01 for all).5,20
Clinical studies of comparator cyclosporine A formulations show improvements in CFS and Schirmer’s test following treatment. In the phase 4 study for cyclosporine ophthalmic solution 0.05%, after 6 months of treatment, total corneal and ocular surface staining scores were significantly improved from baseline (P <0.001%), and 18.9% of patients experienced a ≥10 mm improvement in Schirmer's score.21 Similarly, in the phase 4 study of ciclosporin ophthalmic emulsion 0.1%, mean adjusted CFS scores significantly improved from baseline to 6 months (P = 0.037) in patients treated with ciclosporin ophthalmic emulsion 0.1% vs vehicle; there was no statistical significance in Schirmer’s test for patients receiving ciclosporin ophthalmic emulsion 0.1% vs vehicle.22
NOD mice receiving cyclosporine ophthalmic solution 0.09% twice daily had significantly greater number of goblet cells compared to placebo and NOD diseased control. This suggests cyclosporine ophthalmic solution 0.09% improved ocular surface hydration compared to the groups that received placebo or no treatment. Goblet cells secrete gel-forming mucins, which transform aqueous tears into a mucoaqueous gel that contributes to the majority of the preocular tear film and maintains hydration of the ocular surface.15 Mucins secreted from conjunctival goblet cells do not adhere to the epithelial surface of the cornea—creating a soluble mucous layer that spreads over the ocular surface through blinking and other involuntary ocular movements.23,24 Resultantly, mucins provide a lubricated surface allowing smooth movement of the globe and eyelid and assisting with formation of the glycocalyx—a carbohydrate-enriched coating essential for protecting the corneal and conjunctival surface.24 In addition, mucins protect ocular surface health through inhibiting bacterial attachment and removing contaminants and pollutants from the ocular surface.24 Moreover, conjunctival goblet cells are essential to maintaining homeostasis and immune tolerance of the ocular surface,25 and several studies point to decreased goblet cells as an early sign of KCS.26,27
Although there were no significant differences in corneal staining and IL 1-β levels between cyclosporine ophthalmic solution 0.09% once and twice daily vs ciclosporin ophthalmic emulsion 0.1% once daily and cyclosporine ophthalmic solution 0.05% twice daily, cyclosporine ophthalmic solution 0.09% twice daily demonstrated numerically better corneal scores and lower levels of IL 1-β.
Limitations of this study include the lack of baseline scores for goblet cell density, IL 1-β estimation, and corneal scoring, as well as the limited number of corneal staining assessments performed. In addition, corneal scores were not estimable in the normal control group due to poor visibility against a dark iris background. Dark iris color affects contrast appreciated with lissamine green B dye and may influence detection of mild corneal surface changes in the normal control group.28 Another limitation includes extrapolation of these preclinical results to findings in a clinical setting; further comparator studies in patients with KCS are required to directly assess results among current cyclosporine A formulations.
Conclusions
Overall, cyclosporine ophthalmic solution 0.09% twice daily showed comparatively greater results for increases in tear volume and goblet cell density in a dry eye phenotype mice model compared to cyclosporine ophthalmic emulsion 0.05% and ciclosporin ophthalmic emulsion 0.1%. The improvements demonstrated in this preclinical study support the efficacy of cyclosporine ophthalmic solution 0.09% in treating patients with KCS.
Compliance with Ethics Guidelines
The study proposal was reviewed and approved by the Institutional Animal Ethics Committee in India, which is overseen by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA). The CPCSEA is a statutory body formed by the Act of the Indian Parliament under the Prevention of Cruelty to Animals Act 1960. All recommendations by the Institutional Animal Ethics Committee concerning animal care and handling were followed.
Acknowledgments
This study was sponsored and funded by Sun Pharmaceutical Industries, Inc. Writing and editorial support for manuscript preparation were provided by Zehra Gundogan, VMD, of AlphaBioCom, LLC, and funded by Sun Pharmaceutical Industries, Inc. All authors met the International Council of Medical Journal Editors criteria and received neither honoraria nor payment for authorship. Portions of this manuscript were presented at the 2020 Association for Research in Vision in Ophthalmology annual meeting as a poster presentation with interim findings. The poster’s abstract was published in Investigative Ophthalmology and Visual Science, June 2020 (https://iovs.arvojournals.org/article.aspx?articleid=2766337).
Disclosure
Vinod Burade, Rishit Zalawadia, and Alpesh Patel are employees of Sun Pharmaceutical Industries, Ltd. Abayomi Ogundele is an employee of Sun Pharmaceutical Industries, Inc. The authors report no other conflicts of interest in this work.
References
1. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. doi:10.1016/j.jtos.2017.05.008
2. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II report executive summary. Ocul Surf. 2017;15(4):802–812. doi:10.1016/j.jtos.2017.08.003
3. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575–628. doi:10.1016/j.jtos.2017.05.006
4. Pflugfelder SC. Antiinflammatory therapy for dry eye. Am J Ophthalmol. 2004;137(2):337–342. doi:10.1016/j.ajo.2003.10.036
5. Tauber J, Schechter BA, Bacharach J, et al. A Phase II/III, randomized, double-masked, vehicle-controlled, dose-ranging study of the safety and efficacy of OTX-101 in the treatment of dry eye disease. Clin Ophthalmol. 2018;12:1921–1929. doi:10.2147/OPTH.S175065
6. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107(4):631–639. doi:10.1016/S0161-6420(99)00176-1
7. Baudouin C, de la Maza MS, Amrane M, et al. One-Year Efficacy and Safety of 0.1% Cyclosporine a Cationic Emulsion in the Treatment of Severe Dry Eye Disease. Eur J Ophthalmol. 2017;27(6):678–685. doi:10.5301/ejo.5001002
8. Sun Pharmaceutical Industries, Inc. CEQUA™ (Cyclosporine Ophthalmic Solution 0.09%). Full Prescribing Information. Cranbury, NJ: Sun Pharmaceutical Industries, Inc; 2018.
9. Allergan. RESTASIS® (Cyclosporine Ophthalmic Emulsion) 0.05% for Topical Ophthalmic Use. Full prescribing information. Irvine, CA: Allergan; 2017.
10. Santen SAS. IKERVIS® (Ciclosporin Ophthalmic Emulsion) 1 Mg/Ml, for Topical Ophthalmic Use. Full prescribing information. Evry, France: Santen SAS; 2015.
11. Lallemand F, Schmitt M, Bourges JL, Gurny R, Benita S, Garrigue JS. Cyclosporine A delivery to the eye: A comprehensive review of academic and industrial efforts. Eur J Pharm Biopharm. 2017;117:14–28. doi:10.1016/j.ejpb.2017.03.006
12. Vaishya RD, Khurana V, Patel S, Mitra AK. Controlled ocular drug delivery with nanomicelles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(5):422–437. doi:10.1002/wnan.1272
13. Xiao F, Han M, Wang X, et al. Animal models of Sjogren’s syndrome: an update. Clin Exp Rheumatol. 2019;37(3):209–216.
14. Vitali C, Del Papa N. Classification criteria for Sjögren’s syndrome. In: Sjögren’s Syndrome Elsevier. 2016;47–60.
15. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510. doi:10.1016/j.jtos.2017.05.011
16. Marko CK, Menon BB, Chen G, Whitsett JA, Clevers H, Gipson IK. Spdef null mice lack conjunctival goblet cells and provide a model of dry eye. Am J Pathol. 2013;183(1):35–48. doi:10.1016/j.ajpath.2013.03.017
17. McClellan AJ, Volpe EA, Zhang X, et al. Ocular surface disease and dacryoadenitis in aging C57BL/6 mice. Am J Pathol. 2014;184(3):631–643. doi:10.1016/j.ajpath.2013.11.019
18. Malhotra R, Devries DK, Luchs J, et al. Effect of OTX-101, a Novel Nanomicellar Formulation of Cyclosporine A, on Corneal Staining in Patients With Keratoconjunctivitis Sicca: A Pooled Analysis of Phase 2b/3 and Phase 3 Studies. Cornea. 2019;38(10):1259. doi:10.1097/ICO.0000000000001989
19. Sheppard J, Kannarr S, Luchs J, et al. Efficacy and Safety of OTX-101, a Novel Nanomicellar Formulation of Cyclosporine A, for the Treatment of Keratoconjunctivitis Sicca: Pooled Analysis of a Phase 2b/3 and Phase 3 Study. Eye Contact Lens. 2020;46(1):S14–S19. doi:10.1097/ICL.0000000000000636
20. Goldberg DF, Malhotra RP, Schechter BA, et al. 3, Randomized, Double-Masked Study of OTX-101 Ophthalmic Solution 0.09% in the Treatment of Dry Eye Disease. Ophthalmology. 2019;126(9):1230–1237. doi:10.1016/j.ophtha.2019.03.050
21. Stonecipher KG, Torkildsen GL, Ousler GW
22. Leonardi A, Messmer EM, Labetoulle M, et al. Efficacy and safety of 0.1% ciclosporin A cationic emulsion in dry eye disease: a pooled analysis of two double-masked, randomised, vehicle-controlled Phase III clinical studies. Br J Ophthalmol. 2019;103(1):125–131. doi:10.1136/bjophthalmol-2017-311801
23. Gipson IK. Goblet cells of the conjunctiva: A review of recent findings. Prog Retin Eye Res. 2016;54:49–63. doi:10.1016/j.preteyeres.2016.04.005
24. Dartt DA, Hodges RR, Serhan CN. Immunoresolvent Resolvin D1 Maintains the Health of the Ocular Surface. Adv Exp Med Biol. 2019;1161:13–25.
25. Ko BY, Xiao Y, Barbosa FL, de Paiva CS, Pflugfelder SC. Goblet cell loss abrogates ocular surface immune tolerance. JCI Insight. 2018;3(3). doi:10.1172/jci.insight.98222
26. Kunert KS, Tisdale AS, Gipson IK. Goblet cell numbers and epithelial proliferation in the conjunctiva of patients with dry eye syndrome treated with cyclosporine. Arch Ophthalmol. 2002;120(3):330–337. doi:10.1001/archopht.120.3.330
27. Ralph R. Conjunctival goblet cell density in normal subjects and in dry eye syndromes. Invest Ophthalmol Vis Sci. 1975;14(4):299–302.
28. Hamrah P, Alipour F, Jiang S, Sohn JH, Foulks GN. Optimizing evaluation of Lissamine Green parameters for ocular surface staining. Eye. 2011;25(11):1429–1434. doi:10.1038/eye.2011.184
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
