Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 13
Practical Strategy for Treating Chronic Kidney Disease (CKD)-Associated with Hypertension
Authors Nagata D , Hishida E , Masuda T
Received 25 April 2020
Accepted for publication 11 June 2020
Published 7 July 2020 Volume 2020:13 Pages 171—178
DOI https://doi.org/10.2147/IJNRD.S259931
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Daisuke Nagata, Erika Hishida, Takahiro Masuda
Division of Nephrology, Department of Internal Medicine, Jichi Medical University, Tochigi, Japan
Correspondence: Daisuke Nagata Tel +81 285 58 7346
Fax +81 285 44 4869
Email [email protected]
Abstract: When renal function declines, blood pressure rises, which in turn causes the kidneys to deteriorate. In order to stop this vicious cycle, it is necessary to lower the blood pressure to a “moderate” level in patients who have chronic kidney disease (CKD)-associated hypertension. Such optimization is problematic, since tight control of blood pressure might worsen the prognosis in elderly patients with CKD, especially those with advanced arteriosclerosis. Although renin-angiotensin system (RAS) inhibitors, angiotensinogen converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs) are first-line drugs for hypertensive patients with diabetes, they should be used with caution depending on the patients’ conditions. Recently, there has been a focus on the preventive effects of sodium-glucose cotransporter 2 (SGLT2) inhibitors, anti-diabetic drugs that have been shown to have an impact, on heart and kidney complications. SGLT2 inhibitors increase the amount of sodium chloride delivered to the macular densa of the distal tubules and correct glomerular hyperfiltration by contraction of afferent arterioles via the tubule-glomerular feedback system. It might be one of the reasons why SGLT2 inhibitors show the renal- and cardio-protective effects; however, the mechanism behind their function remains to be elucidated.
Keywords: chronic kidney disease, CKD, hypertension, atherosclerosis, intensive blood pressure control, renin-angiotensin system inhibitors, RAS inhibitors, sodium-glucose cotransporter 2 inhibitors, SGLT2 inhibitors
Introduction
The kidney is the organ responsible for causing hypertension, but it is also the target organ of hypertension. If renal function declines, hypertension is caused, which in turn deteriorates renal function. When a condition like chronic kidney disease (CKD)-associated hypertension arises, it is difficult to discriminate the above two pathophysiological phenomena. Therefore, we focus on the extent to which blood pressure (BP) should be reduced in patients with CKD-associated hypertension.
The Kidney Disease Improving Global Outcomes (KDIGO) has defined CKD as abnormalities of kidney structure or functions, present for > 3 months, with implications for health in KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease (KDIGO CKD 2012).1 Criteria for CKD is known to be comprised of decreased GFR (<60 mL/min/1.73 m2) and markers of kidney damage such as albuminuria. KDIGO CKD 2012 clearly showed the recommendation of BP target ranges for CKD patients. In 2017, The American College of Cardiology and American Heart Association (ACC/AHA) published guidelines for the prevention, detection, evaluation, and management of high BP in adults.2 In 2018, the European Society of Cardiology and European Society of Hypertension (ESC/ESH) also published guidelines for the management of arterial hypertension.3 Both these guidelines provide recommendations for patients at risk of developing cardiovascular or renal disease. They are intended to define practices that meet the needs of patient care. The Japanese Society of Nephrology (JSN), which include the authors of this review as writing committee members, also published evidence-based clinical practice guidelines for chronic kidney disease in 2018 (JSN CKD 2018) consistent with KDIGO CKD 2012.4 The recommended protocols for BP control in CKD patients differ slightly among these four clinical guidelines (Table 1).
 |
Table 1 Comparisons of BP Target Ranges and Recommendations for Drug Treatment in KDIGO CKD 2012, JSN CKD 2018, ESC/ESH 2018, and ACC/AHA 2017 |
Although the reno-protective effects of renin-angiotensin system (RAS) inhibitors are widely known, it is well recognized that they might actually worsen renal function. In this review, we would like to explain the theoretical background behind these findings and suggest future strategies for renal protection in patients with CKD-associated hypertension.
Pathophysiology of CKD-Associated Hypertension
Why does BP rise when kidney function declines? When the glomerular filtration rate (GFR) decreases, the ability of the kidney to excrete sodium and water decreases, and the amount of circulating plasma increases, thus causing hypertension (Figure 1). Although this is the main cause of hypertension, it also involves activation of the RAS, reduction of nitric oxide (NO) levels, enhancement of the sympathetic nervous system, and so on. Conversely, why does renal function deteriorate if hypertension lasts for a long time? Normally, the glomerular pressure is maintained at 50~60 mmHg by appropriately tightening the afferent arterioles.5 When the systemic BP is high or afferent arterioles cannot be adequately controlled due to disorders of the autonomic nervous system associated with diabetes, the glomerular BP increases. Since glomeruli are originally optimized for lower pressure, they will eventually be damaged if exposed to high pressure for a long time.
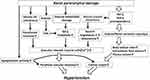 |
Figure 1 Mechanisms of hypertension induced by renal parenchymal damage. |
Under such conditions, a vicious cycle runs between renal insufficiency and hypertension. Moderate control of BP could interrupt this cycle; a suitable reduction of BP in hypertensive patients with CKD can suppress the decrease in renal function over long time periods.
Recommended BP Targets
JSN CKD 2018 recommends a BP target for patients with CKD-associated hypertension of < 140/90 mmHg for non-diabetic CKD patients without proteinuria under 75 years of age, and < 130/80 mmHg for other cases (Table 2). In patients aged 75 years or older, it is maintained at < 150/90 mmHg regardless of the CKD stage and presence/absence of diabetes mellitus, and at < 140/90 mmHg if there are no adverse events such as orthostatic hypotension (Table 2). Since there is no known benefit of lowering the systolic BP to < 110 mmHg in patients with CKD, this limit has not been suggested. It should be noted that the BP targets in this guideline are not necessarily universal. The medical practice guidelines for hypertension as per the European (ESC/ESH), American (ACC/AHA), KDIGO, and JSN CKD 2018 publications have been summarized in Table 1.
 |
Table 2 BP Treatment Target Ranges of JSN CKD 2018 |
When making the JSN CKD 2018, we looked back at major clinical trials to provide a basis for setting the BP targets. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study6 on patients with type 2 diabetes but without CKD stage 3–5, the incidence rates of cerebral vascular disorders and albuminuria, both of which were the primary outcomes of this study, were significantly lower in the strict BP target group (< 120 mmHg) than in the relaxed group (target systolic BP: < 140 mmHg). However, the incidence rate of renal dysfunction increased in the strict BP target group. A meta-analysis of 13 randomized controlled trials (RCTs)7 showed that maintaining systolic BP at < 130 mmHg reduced cerebrovascular disease but increased adverse events. A systematic review of three studies, the African American Study of Kidney Disease and Hypertension (AASK),8 Modification of Diet in Renal Disease (MDRD)9 and Ramipril Efficacy in Nephropathy-2 (REIN-2),10 which included only a few diabetes patients, showed that strict BP control suppressed renal events only in the group with proteinuria.11 Therefore, the rationale for the necessity of strict BP reduction patients with CKD and without proteinuria was considered to be weak. In a large-scale cohort study12 using data from a special health check-up in Japan, the incidence rates of CKD stages G3, 4, and 5 were significantly higher in proteinuria-positive cases with systolic BP ≥134 mmHg: however, in proteinuria-negative cases, a higher incidence rate of CKD was observed only when systolic BP ≥ 141 mmHg. In the SPRINT study13 which included only non-diabetic patients, usage of automated office BP (AOBP) measurement showed significant reductions in cardiovascular disease (CVD). Additionally, higher rates of all-cause mortality were observed in the strict hypotensive group (mean BP: 121.4 mmHg) when compared with the control group (mean BP: 136.2 mmHg). However, there was no evidence of suppression of end-stage renal failure in the strict BP target group. Furthermore, an increase in acute renal failure was observed in strict BP control patients. Post hoc analysis of Olmesartan Reducing Incidence of End-stage Renal Disease in Diabetic Nephropathy Trial (ORIENT),14 which included only diabetic patients, showed that renal events increased when systolic BP ≥ 131 mmHg, as compared to systolic BP of ≤ 130 mmHg.
Use of Antihypertensive Reagents
A number of meta-analyses and RCTs have shown that ACE inhibitors and ARBs reduce the risk of progression to end-stage renal failure and death, regardless of diabetic status or CKD stage.15,16 On the other hand, it has not been concluded that RAS inhibitors are superior to other antihypertensive drugs in terms of suppressing cardiovascular events.15,17 Several meta-analyses have reported that proteinuria is inhibited by RAS inhibitors.17,18 Although RAS inhibitors have not been shown to significantly reduce cardiovascular events, they are believed to ameliorate the risk of end-stage kidney disease (ESKD). As such, ACE inhibitors and ARBs have been recommended as first-line drugs for CKD patients with diabetes and proteinuria. For CKD patients without proteinuria and non-diabetic CKD, ACE inhibitors, ARBs, calcium antagonists, and thiazide diuretics are recommended (Table 2). There have been no RCTs conducted specifically in elderly patients aged 75 years or older with CKD. Considering their vulnerability to dehydration and ischemia, calcium antagonists are recommended for CDK stages G4 or 5 elderly patients as per the JSN CKD 2018.
Is It Better to Control BP Strictly in Patients with CKD-Associated Hypertension?
There has been much debate over which antihypertensive drugs should be used and the extent to which BP should be lowered in CKD-associated hypertension. We must pay equal attention to these two issues: 1) the clinical outcomes that should be adopted to reach a consensus and 2) whether evidence obtained from large-scale RCTs should always be top priority.
If we exclude cardiovascular death, there are two main outcomes for determining the effects of antihypertensive therapy: cardiovascular events and renal prognosis. The choice of antihypertensive drugs and BP targets have largely been set using these indicators. Unfortunately, with respect to antihypertensive therapy for CKD patients, the suppression of cardiovascular events and renal protection do not often coincide. A typical example is the SPRINT study.13 The strict BP control group (mean systolic BP: 121.4 mmHg) was reported to have significantly fewer cardiovascular events than did the control group (mean BP: 136.2 mmHg), but had a higher incidence of acute renal injury. In a subsequent CKD sub-analysis,19 composite endpoints such as myocardial infarction, heart failure, and cardiovascular death were not suppressed by strict BP control in CKD patients. It has also been shown that drop in estimated glomerular filtration rate (eGFR) is significantly greater in patients with strict BP control. Furthermore, Li et al20 reported that a strict BP level, compared with a conventional BP control, could lead to a decreased risk of stroke in hypertensive patients with CKD; however, no significant suppressive trend of CKD progression was found in the strict BP control group. When aiming for optimal anti-hypertensive levels, the perspectives of cardio and renal protection are often inconsistent, making it very difficult to determine the highest-priority clinical outcome.
The next problem is the kind of antihypertensive drugs that must be used. JSN CKD 2018 recommends calcium antagonists in CKD stage G4 and 5 for elderly individuals aged 75 years and over. However, there are no meta-analyses or large-scale clinical trials showing that calcium antagonists improve clinical outcomes when compared to ACE inhibitors and ARBs in this class of elderly patients. A large cohort study of younger patients with CKD stage G5 (mean age: 64.7 years) reported that administration of ACE inhibitors or ARBs improved renal prognosis as compared to other antihypertensive drugs.21 Nevertheless, JSN CKD 2018 recommends calcium antagonists because elderly people with severe atherosclerosis are vulnerable to dehydration and ischemia, and ACE inhibitors and ARBs may cause rapid renal dysfunction. These inconsistencies show the difficulty of applying the results of large-scale trials in a scenario of the real clinical settings and the importance of balancing the risks and benefits of medications.
RAS Inhibitors are Not Always General-Purpose Medicines for Patients with CKD
While ACE inhibitors and ARBs have long-term renal protection effects, there is a risk of rapid deterioration of renal function and hyperkalemia, as mentioned above, because ACE inhibitors and ARBs reduce intraglomerular BP by dilating the efferent arterioles. In the absence of arteriosclerosis, for example, even if the mean systemic arterial pressure drops from 160 mmHg to 80 mmHg with RAS inhibitors, the decrease in glomerular pressure is small (Figure 2, white arrow). However, if the sclerotic change in afferent arterioles is severe, there would be an insufficient increase in glomerular pressure. This would cause both the mean arterial pressure and glomerular pressure to drop sharply (Figure 2, black arrow), in turn inducing a sudden decline of GFR and possible increase in the risk of hyperkalemia. In a study examining renal biopsy tissue from patients with CKD-associated diabetes, diabetic pathological findings such as nodular lesions or mesangiolysis often consisted of nephrosclerosis associated with hypertension and aging.22 With such heterogeneous conditions, administration of ACE inhibitors or ARBs, which uniformly dilate efferent arterioles and lower intraglomerular pressure, has a positive effect on hyperfiltrated glomeruli but not on sclerotic or ischemic glomeruli.
Promise of SGLT2 Inhibitors
Sodium-glucose cotransporter 2 (SGLT2) inhibitors, originally a therapeutic agent for diabetes, have recently attracted the attention of clinicians treating renal disorders. First results of the empagliflozin cardiovascular outcome event trial in type-2 diabetes mellitus patients – removing excess glucose (EMPA-REG OUTCOME)23,24 suggested that SGLT2 inhibitors could improve renal prognosis in patients with atherosclerotic disease. Second, in the subsequent canagliflozin cardiovascular assessment study (CANVAS) program,25 dapagliflozin effect on cardiovascular events – thrombolysis in myocardial infarction 58 (DECLARE-TIMI 58),26 and canagliflozin and renal events in diabetes with established nephropathy clinical evaluation (CREDENCE) trials,27 the morbidity of atherosclerotic diseases was 65.6%, 40.6% and 50.4%, respectively. However, CREDENCE is slightly different from the other three trials, and the primary outcomes are renal events (doubling of serum creatinine, ESKD, and kidney-related death). Table 3 provides a summary of the characteristics of these four major clinical trials of SGLT2 inhibitors. Meta-analysis of the three composite endpoints of decreased renal function (such as doubling of serum creatinine and reduction of eGFR by ≥ 40%), end-stage renal failure, and renal-related death from the above four trials is shown in Figure 3. The results show that renal composite endpoints are significantly suppressed, regardless of baseline renal function or if eGFR is limited ≤ 60 mL/min/1.73 m2. The reason why almost the same analysis results can be obtained regardless of renal function is an issue to be solved in the future.
 |
Table 3 Randomized Clinical Trials of Sodium-Glucose Cotransporter-2 Inhibitors |
SGLT2 inhibitors are thought to selectively inhibit the sodium-glucose cotransporter 2, which is expressed in the S1 segment of proximal tubules28 and to promote hypotensive activity in addition to glucose suppression.29,30 SGLT2 inhibitors also increase the amount of sodium chloride (NaCl) delivered to the macular densa of the distal tubules and correct glomerular hyperfiltration by contraction of afferent arterioles via the tubule-glomerular feedback (TGF) mechanism.29,31 The TGF mechanism by SGLT2 inhibitor administration is not expected to decrease GFR in ischemic glomeruli that are not in the hyperfiltration stage since the increase in NaCl reaching the macula densa is what triggers TGF. Other actions of SGLT2 inhibitors on individual glomeruli via the TGF mechanism may explain some of the reno- protective effects that have been revealed in large-scale clinical trials.32–35
Although SGLT2 inhibitors frequently cause polyuria and natriuresis, which potentially activate the RAS in the early stages of treatment, the effects of SGLT2 inhibitors on RAS activity are not straightforward. Ansary et al36 published an instructive systematic review, which includes three animal experiments and six clinical studies. They suggested that chronic administration of SGLT2 inhibitors might not necessarily activate intrarenal RAS in type 2 diabetic patients.
Dekkers et al37 reported that dapagliflozin decreased urinary excretion of proximal tubular marker KIM-1 and inflammatory marker IL-6. They found that the observed reduction in albuminuria correlated positively with the decrease in eGFR, and also with the decrease in KIM-1 excretion, and concluded that the albuminuria-lowering effect of dapagliflozin could be the result of a reduction in glomerular pressure and improved proximal tubular cell integrity. Although SGLT2 inhibitors have a positive effect on the progression of CKD, there is a concern that they might cause acute kidney injury (AKI) due to their pharmacological effect of lowering GFR. Menne et al38 performed a meta-analysis and reported that SGLT2 inhibitors might reduce the occurrence of AKI in diabetic patients.
SGLT2 inhibitors also have a cardioprotective effect,39 mainly in suppressing heart failure. Further elucidation on the mechanism of action as a cardiorenal protectant, which is likely different from that of RAS inhibitors, remains wanting.
Conclusions
An overview of the pathophysiology of CKD-associated hypertension and its treatment strategy was provided by explaining important points when revising the JSN CKD 2018. To date, RAS inhibitors have been the main tools of antihypertensive therapy for CKD patients with hypertension and will continue to remain so for some time. However, one should be fully aware that the risk of rapid renal function deterioration and hyperkalemia is high when accessing long-term renal protection. We should pay minute attention to clinical manifestations in patients using RAS inhibitors, especially in the elderly. New anti-diabetic reagents, SGLT2 inhibitors, which selectively inhibit the sodium-glucose cotransporter 2, have been shown to improve cardiac and renal prognosis. The mechanisms by which SGLT2 inhibitors play a protective role in the heart and kidney may be unveiled in the near future.
Acknowledgments
We thank Ms. Keiko Fukuda for her technical support. This review was supported in part by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP18ek0310010 (to DN) and JSPS KEKENHI Grant Number JP19K08685 (to DN). We would like to thank Editage for English language editing.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kidney Disease Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150.
2. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American college of cardiology/American Heart association task force on clinical Practice Hypertension. J Am Coll Cardiol. 2018;71:e13–e115.
3. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104.
4. Japanese Society of Nephrology. Essential points from evidence-based clinical practice guidelines for chronic kidney disease 2018. Clin Exp Nephrol. 2019;23(1):1–15. doi:10.1007/s10157-018-1648-1
5. Yu A, Chertow G, Luyckx V, Marsden P, Skorecki K, Taal M. Brenner and Rector’s the Kidney 11th Edition. Elsevier; 2019:90–91
6. Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585.
7. Sim JJ, Shi J, Kovesdy CP, et al. Impact of achieved blood pressures on mortality risk and end-stage renal disease among a large, diverse hypertension population. J Am Coll Cardiol. 2018;283(6):588–597. doi:10.1016/j.jacc.2014.04.065
8. Wright JJT. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease -results from the AASK trial-. JAMA. 2002;288:2421. doi:10.1001/jama.288.19.2421
9. Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med. 1994;330:877–884. doi:10.1056/NEJM199403313301301
10. Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939–946.
11. Upadhyay A, Earley A, Haynes SM, et al. Systematic review: blood pressure target in chronic kidney disease and proteinuria as an effect modifier. Ann Intern Med. 2011;154(8):541–548. doi:10.7326/0003-4819-154-8-201104190-00335
12. Hirayama A, Konta T, Kamei K, et al. Blood pressure, proteinuria, and renal function decline: associations in a large community-based population. Am J Hypertens. 2015;28(9):1150–1156. doi:10.1093/ajh/hpv003
13. Wright JT
14. Imai E, Ito S, Haneda M, et al. Effects of blood pressure on renal and cardiovascular outcomes in Asian patients with type 2 diabetes and overt nephropathy: a post hoc analysis (ORIENT-blood pressure). Nephrol Dial Transplant. 2016;31(3):447–454. doi:10.1093/ndt/gfv272
15. Xie X, Liu Y, Perkovic V, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67(5):728–741. doi:10.1053/j.ajkd.2015.10.011
16. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–869. doi:10.1056/NEJMoa011161
17. Nistor I, Bolignano D, Haller MC, et al. Why creating standardized core outcome sets for chronic kidney disease will improve clinical practice. Nephrol Dial Transplant. 2017;32(8):1268–1273. doi:10.1093/ndt/gfv365
18. Casas JP, Chua W, Loukogeorgakis S, et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet. 2005;366(9502):2026–2033. doi:10.1016/S0140-6736(05)67814-2
19. Obi Y, Kalantar-Zadeh K, Shintani A, et al. Estimated glomerular filtration rate and the risk-benefit profile of intensive blood pressure control amongst nondiabetic patients: a post hoc analysis of a randomized clinical trial. J Intern Med. 2018;283(3):314–327. doi:10.1111/joim.12701
20. Li Y, Liang M, Jiang C, et al. Impact of achieved blood pressure on renal function decline and first stroke in hypertensive patients with chronic kidney disease. Nephrol Dial Transplant. 2018;33(3):409–417. doi:10.1093/ndt/gfx267
21. Hsu T-W, Liu J-S, Hung S-C, et al. Renoprotective effect of renin-angiotensin-aldosterone system blockade in patients with predialysis advanced chronic kidney disease, hypertension, and anemia. JAMA Intern Med. 2014;174(3):347–354. doi:10.1001/jamainternmed.2013.12700
22. Furuichi K, Shimizu M, Okada H, et al. Clinico-pathological features of kidney disease in diabetic cases. Clin Exp Nephrol. 2018;22(5):1046–1051. doi:10.1007/s10157-018-1556-4
23. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi:10.1056/NEJMoa1504720
24. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323–334. doi:10.1056/NEJMoa1515920
25. Mahaffey KW, Neal B, Perkovic V, et al. Canagliflozin for primary and secondary prevention of cardiovascular events. Circulation. 2018;137(4):323–334. doi:10.1161/CIRCULATIONAHA.117.032038
26. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi:10.1056/NEJMoa1812389
27. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi:10.1056/NEJMoa1811744
28. Wright EM, Loo DDF, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91(2):733–794. doi:10.1152/physrev.00055.2009
29. Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60(2):215–225. doi:10.1007/s00125-016-4157-3
30. Masuda T, Watanabe Y, Fukuda K, et al. Unmasking a sustained negative effect of SGLT2 inhibition on body fluid volume in the rat. Am J Physiol Renal Physiol. 2018;315(3):F653–f664. doi:10.1152/ajprenal.00143.2018
31. Cherney DZI, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129(5):587–597. doi:10.1161/CIRCULATIONAHA.113.005081
32. Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39(7):1115–1122. doi:10.2337/dc16-0542
33. Baartscheer A, Schumacher CA, Wüst RCI, et al. Empagliflozin decreases myocardial cytoplasmic Na+ through inhibition of the cardiac Na+/H+ exchanger in rats and rabbits. Diabetologia. 2017;60(3):568–573. doi:10.1007/s00125-016-4134-x
34. Kamezaki M, Kusaba T, Komaki K, et al. Comprehensive renoprotective effects of ipragliflozin on early diabetic nephropathy in mice. Sci Rep. 2018;8(1):4029. doi:10.1038/s41598-018-22229-5
35. Nespoux J, Vallon V. SGLT2 inhibition and kidney protection. Clin Sci. 2018;132:1329–1339. doi:10.1042/CS20171298
36. Ansary TM, Nakano D, Nishiyama A. Diuretic effects of sodium glucose cotransporter 2 inhibitors and their influence on the renin-angiotensin system. Int J Mol Sci. 2019;20(3):629. doi:10.3390/ijms20030629
37. Dekkers CCJ, Petrykiv S, Laverman GD, et al. Effects of the SGLT-2 inhibitor dapagliflozin on glomerular and tubular injury markers. Diabetes Obes Metab. 2018;20(8):1988–1993. doi:10.1111/dom.13301
38. Menne J, Dumann E, Haller H, et al. Acute kidney injury and adverse renal events in patients receiving SGLT2-inhibitors: a systematic review and meta-analysis. PLoS Med. 2011;154(12):e1002983. doi:10.1371/journal.pmed.1002983
39. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31–39. doi:10.1016/S0140-6736(18)32590-X
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


