Back to Journals » International Journal of Nanomedicine » Volume 13
Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies
Authors Qing Y, Cheng L, Li R , Liu G , Zhang Y, Tang X, Wang J , Liu H, Qin Y
Received 9 February 2018
Accepted for publication 9 April 2018
Published 5 June 2018 Volume 2018:13 Pages 3311—3327
DOI https://doi.org/10.2147/IJN.S165125
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
Yun’an Qing,1 Lin Cheng,2 Ruiyan Li,1 Guancong Liu,1 Yanbo Zhang,1 Xiongfeng Tang,1 Jincheng Wang,1 He Liu,1 Yanguo Qin1
1Orthopaedic Medical Center, The Second Hospital of Jilin University, Changchun 130041, People’s Republic of China; 2Department of Obstetrics and Gynecology, The Second Hospital of Jilin University, Changchun 130041, People’s Republic of China
Abstract: Infection, as a common postoperative complication of orthopedic surgery, is the main reason leading to implant failure. Silver nanoparticles (AgNPs) are considered as a promising antibacterial agent and always used to modify orthopedic implants to prevent infection. To optimize the implants in a reasonable manner, it is critical for us to know the specific antibacterial mechanism, which is still unclear. In this review, we analyzed the potential antibacterial mechanisms of AgNPs, and the influences of AgNPs on osteogenic-related cells, including cellular adhesion, proliferation, and differentiation, were also discussed. In addition, methods to enhance biocompatibility of AgNPs as well as advanced implants modifications technologies were also summarized.
Keywords: antibacterial mechanism, biocompatibility, osteogenic-related cells, orthopedic implants, silver nanoparticles, surface modification
Introduction
Implant-associated infection as a common postoperative complication of orthopedic surgery often results in patient suffering, financial burden, and even fatalities.1,2 Application of antibiotics is the most common approach to treat infection. However, the rise of resistant organisms has made routine antibiotic prophylaxis ineffective. What is worse, bacterium can rapidly form biofilm on the implant surface, making antibiotics unable to penetrate and develop antibacterial function.3 Thus, it is urgent to find an antibacterial agent that can kill drug-resistant bacteria and modify the prosthesis to prevent biofilm formation.
Silver (Ag) has been demonstrated to possess effective antibacterial effect and has been vastly used in medicine.4 Moreover, Ag can be manufactured into silver nanoparticles (AgNPs) through nanotechnology to have improved physical, chemical, and biological properties.5–7 Currently, application of AgNPs on orthopedic implant modification to prevent implant-associated infection has drawn more attention.8,9 In the field of orthopedic investigation, AgNP-coated external fixation pins, proximal femur or tibia mega-prostheses, and AgNPs containing bone cement have been innovated, and they show an infection inhibition trend.10–12 Since AgNP-coated orthopedic implants represent a promising approach to prevent infections, the specific antibacterial mechanism of AgNPs and its effect on osteogenic-related cells need to be understood.13
Many research studies have investigated the antimicrobial activity of AgNPs, but the possible antibiotic mechanisms and potential hazard are still unclear.14,15 Castiglioni et al concluded that AgNPs cause cytotoxicity in various cell lines in a dose-dependent manner.16 However, the effect of AgNPs on osteoblast and osteoclast, which are responsible for bone formation and resorption respectively, is not included. In addition, optimal dosage of AgNPs on osteogenic-related cells is still controversial. Hence, the effect of AgNPs on osteogenic-related cells needs to be considered in order to screen the optimal concentration. Although Prabhu et al summed up the potential toxicity of AgNPs, they did not propose how to reduce the toxicity effect.17 Biocompatibility is a precondition for the application in medicine. Aiming to enhance the biocompatibility of AgNPs, biosynthesis process can be applied to change the morphology and surface characteristics of AgNPs.18 Furthermore, recent studies have proven that surface modifications with biomolecules, polymers, or metal ions are effective strategies that can also fulfill this mission.19–21
In this review, we discuss the antibacterial mechanisms and antibiofilm activity of AgNPs, which are the fundamental of implants application. The disputed cellular effects of AgNPs on osteogenesis-related cells are discussed, and some advice for implants designed are provided. Methods such as biosynthesis, adjustments of physical properties, and combining with biomolecules to enhance the compatibility of AgNPs are highlighted. Finally, different modification methods of AgNP introduction into orthopedic implant are also summarized.
Antibacterial mechanisms and antibiofilm activity of AgNPs
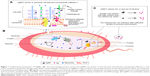
As described above, AgNPs can destroy multiple drug-resistant strains and prevent biofilm formation, indicating significant potential in antibacterial application.22 Although the antibacterial mechanisms of AgNPs have been discussed extensively, the exact effect of AgNPs on bacteria is still undefined. To our knowledge, two antibacterial mechanisms are widely accepted, namely contact killing and ion-mediated killing. In this section, specific antibacterial mechanisms and antibiofilm activity were described in detail.
Antibacterial mechanisms through direct contact with microorganisms
AgNPs have more outstanding physiochemical and biological properties beyond bulk silver. It has been reported that AgNPs can anchor to the bacterial cell wall and consequently infiltrate it. This action will cause physical changes in the bacterial membrane, like the membrane damage, which can lead to cellular contents leakage and bacterial death (Figure 1A).23,24 It was also demonstrated that the antibacterial effect of AgNPs on Gram-negative bacteria was stronger than Gram-positive bacteria. This phenomenon can be explained by the existing difference in the cell wall thickness between Gram-positive bacteria (30 nm) and Gram-negative bacteria (3–4 nm), which are mainly composed of peptidoglycan.25 In addition, it has been proved that the cellular membrane of bacteria has a negative charge due to the presence of carboxyl, phosphate, and amino groups.26 The positive charge confers electrostatic attraction between AgNPs and negatively charge cell membrane of the microorganisms, thereby facilitates AgNP attachment onto cell membranes.27 Hence, enhanced antibacterial effects can be obtained by altering the surface charge of AgNPs to achieve stronger attractive force.28
After adhesion to the bacterial wall, AgNPs can also penetrate the membrane and enter the bacteria. There is a size-dependent antibacterial effect, namely smaller nanoparticles has a large surface area in contact with the bacterial cells and can reach the cytoplasm more often than larger nanoparticles.24 When AgNPs penetrate inside the microbial cell, it may interact with cellular structures and biomolecules such as proteins, lipids, and DNA. Interaction between AgNPs and cellular structures or biomolecules will lead to bacterial dysfunction and finally death. In particular, AgNP interaction with ribosomes lead to their denaturation causing inhibition of translation and protein synthesis (Figure 1B). It is also speculated that AgNPs interact effectively with the carboxyl and thiol groups of β-galactosidase, inhibit intracellular biological functions, and lead cell death.29
Furthermore, the antibacterial mechanism of AgNPs is also due to their ability of producing high levels of reactive oxygen species (ROS) and free radical species such as hydrogen peroxide, superoxide anion, hydroxyl radical, hypochlorous acid, and singlet oxygen.30–32 Under normal circumstances, ROS generated in cells is limited and can be eliminated by antioxidant systems.33 AgNPs exert antibacterial effect through inactivation of respiratory chain dehydrogenases and eventual excess ROS generation, which inhibited respiration and growth of cells.34,35 AgNPs can downregulate the expression of antioxidant enzyme such as glutathione (GSH), superoxide dismutase, and catalase, which can accelerate the accumulation of ROS.22 Increased ROS lead to an apoptosis-like response, lipid peroxidation, depletion of GSH, and DNA damage.36,37 In addition, the antibacterial activity of AgNPs was also influenced by adenosine triphosphate (ATP)-associated metabolism and ROS.38
Antibacterial mechanisms mediated by the release of silver ions
There are several evidences suggesting that the released silver ions (Ag+) from AgNPs are important in the antibacterial activity.7,39–41 One of the important parameters of AgNPs against microbes is the surface area of the nanomaterials. AgNPs can sustainably release Ag+ in and out of bacteria. The highest concentration of released Ag+ was observed in the case of AgNPs with the highest surface area. The lowest concentration of Ag+ released was noted for AgNPs with the lowest surface area, resulting in weak antimicrobial property.42 The mechanism of the antimicrobial action of Ag+ is closely associated with its interface with sulfhydryl groups in enzymes and proteins (Figure 1C). For instance, Ag+ can bind to proteins that are present in the cell membrane to form stable bonds resulting in protein deactivation. The proteins are involved in transmembrane ATP generation and mediate ion transport across cell membranes.43 Besides, micromolar ranks of Ag+ have been described to uncouple respiratory electron transport from oxidative phosphorylation and limit respiratory chain enzymes or obstruct with the membrane penetrability to protons and phosphate.44,45 In addition, it has been found that Ag+ can form complex with nucleic acids, where it preferentially interact with the nucleosides. Ag+ intercalates between the purine and pyrimidine base pairs, disrupts the H-bonds between base pairs of the anti-parallel DNA strands, which prevent cell division and reproduction eventually.46,47
Ag+ as a heavy metal ion can cause the increase of cellular oxidative stress in microbes, which is another antibacterial mechanism. Long et al48 prepared four different AgNPs by coating with different ligand and investigated the mechanism of AgNP-dependent antibacterial activity. The released Ag+ from AgNPs was suggested to interact with respiratory chain proteins on the membrane, interrupt intracellular O2 reduction, and induce ROS production.48 The thioredoxin (Trx) system, which is composed of nicotinamide adenine dinucleotide phosphate, thioredoxin reductase (TrxR), and Trx, is one of the major disulfide reductase systems used by bacteria against oxidative stress.49 It was demonstrated that Ag+ binds to the active sites of Staphylococcus aureus TrxR and Trx and leads to oligomerization and functional disruption of TrxR as well as Trx. Ag+ also depleted intracellular thiol levels in S. aureus, disrupting bacterial thiol-redox homeostasis, and the increased ROS induced bacteria death finally.50 Although AgNPs can kill bacteria through the two different action modes mentioned above, the antibacterial mechanism is usually considered as the synergistic effect generated from AgNPs and Ag+.32
With the wide application of AgNPs, researchers start to worry about the potential development of bacterial resistance to AgNPs. In a recent report, it was found that AgNPs enhanced bacterial resistance to antibiotics by promoting stress tolerance through induction of intracellular ROS.51 In addition, Gram-negative bacteria Escherichia coli 013, Pseudomonas aeruginosa CCM 3955, and E. coli CCM 3954 can develop resistance to AgNPs after repeated exposure. This resistance was due to the production of flagellin, an adhesive protein of the bacterial flagellum, which caused the aggregation of AgNPs and thereby eliminated their antibacterial effect.52 Indeed, bacterial resistance exists, and the mechanism is the aggregation of AgNPs. However, AgNPs are always incorporated into the implant surface in a dispersed state. Thus, further studies are needed to verify whether bacterial resistance develop in AgNP-coated implant surface.
Antibiofilm activity of AgNPs
Biofilms are communities of microorganisms attached to a solid surface. Once the biofilm is formed on the implant surface, it protects microorganisms from antibiotic treatment and causes serious consequences.53,54 The antibiofilm activity of AgNPs has been demonstrated in a number of studies. One pioneering study was performed to analyze the interactions of AgNPs with Pseudomonas putida biofilms. The results suggested that biofilms are impacted by the treatment with AgNPs.54 Du et al55 synthesized AgNPs by using benzoin gum extract and tested their antibiofilm effect by using E. coli. The AgNPs exhibited the antibiofilm activity at concentrations up to 10 μg/mL.55 AgNPs were fabricated in situ and immobilized on the titanium surface. This modified surface can reduce bacterial biofilm formation in vitro by inhibiting bacterial adhesion and icaAD transcription. In addition, the antibiofilm activity of the immobilized AgNPs is independent of silver release, and AgNPs can defend several cycles of bacterial exposure in vitro and reduce implant-associated periprosthetic infection in vivo.56 In another study, AgNPs were incorporated into porous titanium implants in the grown oxide layer and to create a micro-/nanoporous structure on the surface of the implants. Antimicrobial assays showed strong antimicrobial activity against methicillin-resistant S. aureus including released activity, surface antimicrobial activity, and prevention of biofilm formation.57 These evidences showed that implant can be endowed with antibiofilm activity with AgNP incorporation.
Cellular effects of AgNPs on osteogenesis-related cells
Biocompatibility of AgNPs on osteogenesis-related cells, especially osteoblast, osteoclast, and mesenchymal stem cells (MSCs) should be concerned due to their key roles in bone regeneration.58,59 In this section, we discuss the influence of AgNPs on the abovementioned cell activity, adhesion, proliferation, and differentiation.
Effects of AgNPs on osteoblast and osteoclast
Bone metabolism is a critical factor during implants relative to bone integration, in which the osteoblast and osteoclast are responsible for bone formation and absorption during the integration, respectively.59 AgNPs could be uptake into osteoblasts and could cause the first manifestation of cell injury through generation excessive nitric oxide, that is, swelling of the endoplasmic reticulum.60 AgNPs were reported showing a cytotoxicity effect on osteoblasts in a dose-dependent manner and impaired cell viability at a concentration of 10 μg/g of AgNPs.61 In addition, higher cytotoxicity concentrations of AgNPs were observed from other studies, which was 25 μg/mL and 50 μM, respectively.16,62 However, from the results mentioned above, the proper concentration of AgNPs was concluded to be 10 μg/mL aiming for medical application, possessing effective antibacterial and good biocompatibility simultaneously. Furthermore, size-dependent cytotoxicity effect was also observed. When several cell lines were treated with three different characteristic sized AgNPs, the smaller particles exhibited stronger cytotoxic effects on osteoblast, which is due to the size and surface area discrepancy release of Ag+ from AgNPs.63,64
Despite the side effect, AgNPs were demonstrated to possess the capacity of enhancing mineralization and alkaline phosphatase (ALP) expression in MC3T3-E1 cells at a concentration of 20 μg/mL. The underlying mechanisms were the miRNA regulation of expression of mothers against decapentaplegic (Smad) transcription factor 1 and 5, and Runt-related transcription factor 2 (Runx2), which were related to osteogenesis.65 Furthermore, some results indicated that the incorporation of AgNPs into biomaterials might lead to decreased cytotoxicity by reducing the cellular uptake of AgNPs.66 In addition, cell spreading is suggested to be beneficial to osteoblast differentiation and also results in better cell–cell communication, which is reported being critical to coordinate cell behavior.67 When AgNPs were incorporated into TiO2 nanotube and cultured with MC3T3-E1 cells, some favorable effects on promoting cell spreading were observed from cell morphology assay after culturing for 3 days.68 Another study indicated the same trend, and no significant cytotoxicity was observed when the osteoblast-like MG63 and MC3T3 cells were exposed to AgNPs, and the MG63 cell even promoted cellular proliferation.69 Furthermore, nanostructure properties of implant surface were enhanced by the incorporation of AgNPs, which is a benefit for promoting osteogenesis with increased cell attachment, viability, and osteogenic gene expression (ALP, Runx2, and OCN).70
Cellular effects of AgNPs on viability and differentiation of MSCs
MSCs act as multipotent precursors of various cells, which can differentiate into osteoblasts, adipocytes, and chondrocytes by a proper induction condition. Due to the key role of MSCs in bone regeneration, some researchers reported different effects of AgNPs on MSCs.
Similar to the effect on osteoblast and osteoclast, AgNPs exert cytotoxic effects on MSCs in a dose- and time-dependent manner. Greulich et al71 investigated the biocompatibility of 100 nm AgNPs in human mesenchymal stem cells (hMSCs). The cytokine level of interleukin-8 (IL-8) was significantly higher than that of IL-6 and VEGF at concentrations of 5 μg/mL and above.71 In addition, AgNPs can be absorbed into cells and then induce DNA damage, cell death, and functional impairment of MSCs. Distribution analysis showed that AgNPs were mainly located in the cytoplasm and the nucleus. Cytotoxic and genotoxic effects were positively correlated with dose. Interestingly, the migration ability of hMSCs was not impaired at subtoxic concentrations.72 In terms of MSC proliferation, some studies have demonstrated that AgNPs can promote cell proliferation at nontoxic concentration. Jung et al73 proved that AgNPs increased cell proliferation at subtoxic concentrations and decreased cell proliferation at concentrations above 10 μg/mL. In addition, hypoxia-inducible factor-1a acting as a transcription factor in regulating metabolism, development, proliferation, and pathology under hypoxic conditions plays a key factor in AgNPs-mediated cell proliferation in hMSCs.73
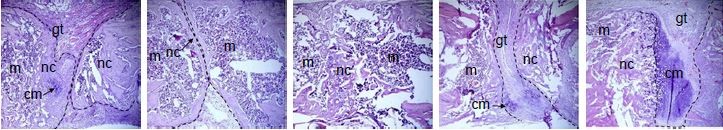
In the aspect of differentiation, Sengstock et al observed that AgNPs attenuate the adipogenic and osteogenic differentiation of hMSCs even at nontoxic concentrations, whereas chondrogenic was unaffected.74 Conversely, Samberg et al exposed human adipose-derived stem cells in both undifferentiated and differentiated states to various concentrations from 0.1 to 100 μg/mL with 10 or 20 nm AgNPs, and no significant effect was observed on cell differentiation.75 Qin et al76 revealed that 4 μg/mL of AgNPs was safe to urine-derived stem cells (USCs). At this concentration, AgNPs can promote osteogenic differentiation of USCs, induce actin polymerization, increase cytoskeletal tension, and activate RhoA.76 In another study, AgNPs promoted MSC differentiation even at a much higher concentration (4–20 μM). Furthermore, AgNPs were also considered to be able to promote the formation of fracture callus and induce early closure of the fracture gap in vivo (Figure 2). The mechanisms may be via multiple routes: 1) chemoattraction of MSCs and fibroblasts to migrate to the fracture site; 2) induction of the proliferation of MSCs; 3) induction of osteogenic differentiation of MSCs via induction/activation of TGF-β/BMP signaling in MSCs.77
  | Figure 2 (A) AgNPs increase MSC proliferation and do not reduce cell viability at low concentration. (A1) Cells were cultured with different concentrations of AgNPs for 2 days to test cell proliferation. (A2) Cell viability assay was performed to test the effect of AgNps on MSC. (B) ALP activity (B1) and Alizarin red staining (B2) indicated that AgNPs promote osteogenic differentiation of MSC in vitro. (C) AgNPs promote fracture healing in vivo. Plain X-ray radiograph of the fracture sites; broken line demarcates the unfilled fracture gap (C1). Hematoxylin and eosin staining of the middle section of the fracture site of each treatment group was shown. Broken lines indicate the two ends of the fracture femoral bone (C2). The areas of the facture gap of each treatment groups at different postoperative days were quantified and shown in (C3). |
As discussed above, these findings suggest that AgNPs could be used as a biocompatible agent within a particular dosage window. Maybe AgNPs ≤10 μg/mL is a safe dose window for osteogenesis-related cells. The cellular effects of AgNPs on osteogenesis-related cells with respect to size of particles, exposure doses, type of cell line, major outcome of each study, and their mechanisms were summarized in Table 1.
Methods to improve biocompatibility of AgNPs
Due to great application of AgNPs, their biocompatibility should be paid attention for medical use. In this part, we focus three methods to enhance the biocompatibility of AgNPs, namely biosynthesis process, adjustment of physical properties, and biomolecule combination.
Biosynthesis to improve biocompatibility
The traditional chemical and physical methods are widely used for AgNP synthesis. However, these methods always have associated risks, such as chemical precursor contamination, solvent toxicity, and hazardous byproduct formation, which make alternative synthetic methods imperative.78 At present, bio-enthused synthesis of nanoparticles provides advantages over chemical and physical methods as it is environment-friendly, no need to use high-pressure, high temperature, and no toxic chemicals are needed in biological method.79
There are many resources such as bacteria, yeast, fungi, and various parts of plants can be used in the nanoparticle synthesis. Plant extracts frequently offer good manipulation and control over crystal growth and stabilization.80 The major advantage of using plant extracts for biosynthesis is that they are easily available, safe, and nontoxic. In most cases, there are various metabolites that can be used to reduce Ag+.17 Through adjustment reaction parameters, AgNPs can be achieved with better yield, controlled size, shape, greater particle stability, more biocompatibility, scalability, and applicability.78,81 For example, AgNPs can be green synthesized by using Artemisia tournefortiana Rchb ethanol extract; these AgNPs with spherical shape showed powerful antibacterial ability.82 In addition, Mangifera indica inflorescence aqueous extract was used to reduce AgNO3 to produce green AgNPs. These particles with an average diameter of 40 nm showed powerful killing effect for Gram-negative (Klebsiella pneumoniae, P. aeruginosa, and E. coli) and Gram-positive (Staphylococcus mutans and S. aureus) strains. Importantly, AgNPs exhibited no significant toxic effect on HeLa cell line below 25 μg/mL (Figure 3).83 Kasithevar et al synthesized AgNPs by using aqueous leaf extract of Alysicarpus monilifer; these green synthesized AgNPs were biocompatible with normal Vero cell line and have high antibacterial activity.84 In conclusion, biosynthesis approach is a promising method to produce AgNPs with both significant antibacterial effect and biocompatibility.
  | Figure 3 (A) Graphical presentation of green silver nanoparticles biosynthesis. (B) Characterization of green AgNPs: (a) ultraviolet-visible spectra indicated the synthesis of AgNPs were extremely stable. (b) Nanophox particles size analyzer graph showed the size of the particle was ranging between 30 and 70 nm with an average particle size of 40 nm. (c) Field-emission scanning electron microscope image. (d and e) Transmission electron microscope images. *P<0.05. (C) Visualization of the biofilm inhibition: (a and b) images display control and treated biofilm stained by crystal violet. (c and d) images show the difference in biofilm after treatment as visualized by the SEM, (e and f) are confocal laser scanning microscope images of the control and treated biofilms. Red arrows indicate the damage in the bacterial cells due to action of AgNPs. (D) Cell line (HeLa) toxicity assessment of the AgNPs. |
Adjustment of physical properties
The fundamental characteristics of metallic nanoparticles strongly depend on their shapes, sizes, configurations, crystallinity, and structure whether they are in solid form or in hollow geometries. Thus, controlling such parameters can achieve the desired properties of the nanoparticles.18 In this section, we discuss the role of different sizes and shapes of AgNPs, which are the two main properties in biomedical application.
Many studies have demonstrated that smaller AgNPs showed better antimicrobial activity. For instance, monodispersed AgNPs with sizes of 25, 35, 45, 60, and 70 nm were obtained, and cell viability test was performed by using human lung fibroblast. The smaller AgNPs can cause severe cell apoptosis and necrosis and generation of high levels of ROS.85 Similar results were observed in other in vitro studies. Compared with larger AgNPs, increased apoptosis, activation of cytokines/chemokines IL-8, IL-1β, tumor necrosis factor, macrophage inhibitory protein, and ROS production were observed in macrophages treated with smaller AgNPs.86
The shape of AgNPs can impact the degree of particle toxicity. In order to comprehensively investigate these facts, spherical, rod-shaped, truncated triangular AgNPs, and their skin penetration capabilities were studied. From the results, it was found that triangular AgNPs could be an ideal candidate for topical applications which can reduce systemic toxicity, compared to the rod-shaped and spherical AgNPs.87 In another study, spherical, rectangular, penta, and hexagonal AgNPs of different dimensions were biosynthesized, and the results showed that the spherical AgNPs possessed more antimicrobial effect than other shaped AgNPs.88 The electron charge transfer properties are also affected by different types of AgNPs. Regardless of the size required, narrow size distributions and more “spherical” shapes can improve sample quality.89 In a word, the above studies have highlighted the role of size and shape in potentiating AgNP-based cellular effect.
Combination with biomolecules
Surface modification of nanomaterials is essential where the surface layer facilitates the reduction of surface energy. It can also provide a protective coating that prevents nanoparticles from agglomeration, thus increasing their long-term stability.90 Many organic molecules can be used for surface functionalization. Examples include small molecules like lipids, vitamins, peptides, and sugar and larger ones such as natural polymers including proteins, enzymes, DNA, and RNA.91 In this section, we discuss several biomolecules especially chitosan (CS), which is commonly used in surface modification.
CS is a linear polysaccharide, which has randomly distributed β-(1-4)-linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit).92 It is a highly biocompatible biodegradable material and shows antibacterial properties against pathogenic bacteria, with potential application as an antimicrobial agent.93 These properties make CS suitable for surface modification of AgNPs. The hydrogels based on CS and modified with AgNPs exhibit toxicity in relation to S. aureus and did not exert any negative impact on the cells of the dermis.94 AgNPs embedded in CS are capable to interact with amine and hydroxyl groups of the CS molecule, subsequently form complexes and produce stable nanostructured film. From the results, the CS/nAg nanocomposites showed enhanced antibacterial and biocompatibility properties.93 Low-molecular-weight CS (LMWC) has better water solubility and biological activity than higher degree of polymerization of CS. Compared with AgNPs coated with high-molecular-weight CS, AgNPs coated with LMWC were more effective against methicillin-resistant S. aureus and showed better biocompatibility and lower body absorption characteristics.95 In another study, an agarose composite embedded with CS-coated AgNPs was synthesized. This scaffold showed good swelling ratio, excellent hemocompatibility, and appreciable antibacterial activity. Moreover, it also showed good biocompatibility to several cell lines and benefits for cell sustained growth.20 These studies suggest that CS is a good material for surface modification of AgNPs.
Except CS, there are many other biomolecules that can be used in combination with AgNPs for surface functionalization. Polyethylene glycol (PEG) can reduce osponization process in which nanoparticles are directed to liver through macrophages.21 In addition, Kwon et al demonstrated that polyvinylpyrrolidone was a more suitable surfactant than PEG for AgNP capping, which could decrease aggregation and reduce cytotoxicity.96 Furthermore, silk fibroin (SF) as a natural polymer shows the ability of reduction and osteogenic regeneration. It can reduce Ag+ to Ag0 by the Tyr residues with strong electron-donating property and also exposes to disperse and stabilize the produced AgNPs to maintain the stability of the SF-AgNPs composite solution. The SF-AgNPs composite showed good biocompatibility and improved osteogenic differentiation by decreasing the Ag+ release from AgNPs (Figure 4).97 Advantages of combination with biomolecules is that the surface chemistry properties of AgNPs can be easily controlled to realize multifunctionalization. Many biomolecules can meet our requirements to enhance the biocompatibility of AgNPs for medical use, which is worthy to investigate in vivo reaction in future study.
  | Figure 4 (A) Schematic of in situ preparation of SF/AgNPs/Gen composite solution and the possible reduction mechanism of Ag+ by SF as well as the interaction between AgNPs and gentamicin. (B) Illustrative diagram of fabrication process for PD-S-Ag/g coatings on titanium substrate, scheme is not in real scale. (C) SF-coated AgNPs shows good biocompatibility and improved osteogenic differentiation. (a) Fluorescent images for 3 and 5 days with actin stained with FITC (green) and nuclei stained with DAPI (blue). (b) Collagen secretion on different specimens for 28 days. (c) Calcium deposition (red arrow) on different specimens for 28 days. (D) Mechanism on the AgNPs/Gen-contained SF-based coatings. |
Modification of orthopedic implants by using AgNPs
Over the past decades, metallic implants have been widely used in orthopedic fields. However, it is biologically inert and cannot induce new bone regeneration or inhibit bacterial activity. AgNPs as an antibacterial agent are usually used to modify orthopedic implant surface to obtain antibacterial and potential osteointegration ability. In this section, we discuss various surface modification techniques for AgNP coating onto implants surface (Figure 5), as well as their effects on antibacterial and bone formation.
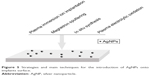  | Figure 5 Strategies and main techniques for the introduction of AgNPs onto implants surface. |
Plasma immersion ion implantation (PIII)
PIIIs is proven to be economical and effective and have already been widely applied in the modification of semiconductors and biomedical industry. The process involved accelerating positive ion incorporation vertically into the negative potential surfaces in the electric field.98 It can deposit different ions on many biomaterials, including metal, ceramics, and polymers.99 Moreover, PIII can efficiently control the distribution and concentration of incorporated element in material by regulating the modification parameters.100
AgNPs can be incorporated into the near-surface of implant by PIII process. This technique significantly improved the stability of surface coating with a negligible amount of Ag+ release. The immobilized AgNP implant surface not only inhibited bacterial adhesion and biofilm formation in vitro but also reduced implant-associated infection in vivo. While, the biocompatibility of titanium implants was also enhanced due to the change of implant surface physical topography.101,102 In addition, Ag-PIII-treated titanium accelerated the osteointegration of titanium implant by initiating the ERK1/2 signal.102 In another study, similar results have been observed by activating the integrin-α5-orchestrated MAPK/ERK signal cascade.103 Moreover, by increasing the PIII process time (1, 2, and 3 h), the diameter of AgNPs becomes larger, thus increasing implant surface roughness. The concentration of AgNPs can be effectively controlled within the safe range by changing the fabrication time.104 Furthermore, two or more different metallic ions can be introduced on the implant surface through PIII process to obtain more biological functions.105,106 For instance, Zn and Ag were simultaneously and sequentially implanted into titanium by PIII. The advantages of Zn and Ag are preserved, the Zn/Ag microgalvanic couple formed on Zn/Ag dual-ion coimplanted titanium shows the best osseointegration as well as good antibacterial properties.106
Magnetron sputtering
Magnetron sputtering constitutes one of the physical vapor deposition techniques that have been widely used in applications involving the metal-mechanic industry with great success. The sputtering technique has advantages of coating thinly, thickness uniformity, and high bonding strength to metal. Many stable bioactive coating can be deposited on various implant surface uniformly through magnetron sputtering.107,108 Magnetron sputtering requires simple equipment and thus is easy to control. In addition, it has low substrate temperature, high film forming rate, and strong film adhesion.109
AgNPs coating on M2 tool steel and Si substrate by the sputtering technique, the antibacterial efficiency, and tribological properties of the substrate were significantly enhanced.110,111 By controlling magnetron sputtering time, coating morphological shapes could be controlled, as could the quantity of deposited structures.112 In addition, the stable coating on the implant surface can achieve long-term antibacterial effect through magnetron sputtering technique. Furthermore, AgNPs incorporated on the implant surface by sputtering can increase water contact angle and improve hydrophobicity of the substrate surface.113 It was found that with a decreased sputtering time, surface roughness and wettability were higher. An increasing roughness value is well known to be associated with increased osteoblast adhesion. However, it is also beneficial to bacterial adhesion.114 In Liu et al study,115 the homogeneous AgNP coating was uniformly distributed on the surface of the polyetheretherketone (PEEK) samples via magnetron sputtering. The AgNP-coated samples had a significant increase in surface roughness (P<0.05) as the thickness of their AgNP coating increased. Their antibacterial rates were above 99%, indicating that the AgNP-coated PEEK implant materials have strong antibacterial and bactericidal effects against S. mutans and S. aureus in the oral cavity.115
In situ synthesis
In situ synthesis is a method through chemical reaction on the implant surface to incorporate different nanoparticles. This technology is simple, economical, and available for surface modification. However, adhesion between nanoparticles and substrate surface is not tight.
AgNPs have been obtained on different TiO2 substrates, through reduction in AgNO3 solutions. The amount of deposited nanoparticles depends on the concentration of the silver nitrate solution and reactive time.116–118 There is no doubt that the released Ag+ showed powerful antibacterial effect, but the key point is how to prolong the release time. To enhance the adhesion on the substrate and prolong antibacterial time, an initial layer on Ti surfaces using phase-transited lysozyme was established. Then, AgNPs were synthesized on CS and hyaluronic acid layer, and multilayer coatings were prepared on Ti surfaces via a layer-by-layer self-assembly technique. This system showed obvious antibacterial effect even in 14 days.7 In another study, a mussel-inspired self-polymerized polydopamine anchor was employed, and the released time was up to 28 days.119 A titanate nanowire film was produced on Ti substrate by an alkali hydrothermal reaction and subsequently doped by AgNPs through an ultraviolet light chemical reduction. The release of Ag+ was detrimental for the growth, proliferation, and differentiation of MC3T3. However, the additional CS will alleviate this cytotoxicity effect.120 During bacterial infection, pH level around the peri-implant surface decreases as low as pH 5.5.121 Dong et al122 construct a pH-dependent AgNP releasing titanium implant to control peri-implant infection. Once the pH was doped to 5.5, the robust released AgNPs from implant system which efficiently controlled bacterial growth. Moreover, this system was biocompatible and showed osteoinductive properties.122 In situ synthesis is a simple method, but the key point is how to design a safe and suitable system to achieve long-term antibacterial effect.
Plasma electrolytic oxidation (PEO)
PEO also known as micro-arc oxidation is a method that develops ceramic-like surfaces on the implant surface. The oxidation layer can also offer a wide variety of mechanical, biomedical, tribological, and antibacterial properties through the incorporation of several ions and particles.123,124 Nowadays, this method is widely used in the modification of implants to prepare bioactive coatings.
PEO can dope the surface of the implants with fully dispersed and firmly attached AgNPs all within the span of a few minutes. In addition, factors such as voltage and time were not affected significantly due to the addition of AgNPs in electrolyte.125 AgNPs were immobilized in the oxide layer on titanium implant to create a micro-/nanoporous structure through PEO technique. The coating showed strong antimicrobial activity without any signs of cytotoxicity, because AgNPs are entrapped in an in-depth growing oxide layer which fully immobilizes them and prevents them from freely circulating through the blood stem.57 Furthermore, the AgNPs were found to be very stable in biological fluids with material loss, as a result of dissolution, to be <0.07% for the silver nanocoatings after 24 h in a modified Krebs-Ringer bicarbonate buffer, which shows a stable coating with a significant effect for preventing bacterial growth.126
Three-dimensional printing (3DP) silver-containing scaffolds
Additive manufacturing technology, also called 3DP, has emerged recently. It can precisely fabricate scaffolds with defined shape, size, porosity, and pore size distribution, which is beneficial for bone growth.127,128 In addition, more and more complex orthopedic diseases can be solved based on this technique.129 In this section, we discuss the silver-containing 3D scaffolds in orthopedic field.
Many materials can be used to produce scaffold via this technology, such as metal, polymer, and inorganic materials. 3D scaffolds with a porosity structure is suitable for loading ions, nanoparticles, and biomolecules to achieve multifunctional application.130 In Correia et al study,131 the tricalcium phosphate (TCP)/sodium alginate (SA) scaffolds were produced by 3DP. Subsequently, AgNPs were incorporated into scaffolds through two different methods, direct incorporation and physical adsorption. Both of them present appropriate mechanical properties, biocompatibility, and bactericidal activity, which is suitable for being used in bone tissue regeneration (Figure 6).131 Similarly, graphene oxide and AgNPs nanocomposites were successfully modified on the β-TCP scaffolds by a simple soaking method to achieve biofunctions with antibacterial and osteogenic activity.132 However, through soaking method, AgNP burst was released from the 3D scaffolds in short time, and this manner easily causes cytotoxicity. To control the release, a mixture of nanocomposites such as bioceramic and polyvinyl alcohol in different compartment can achieve different release kinetics.133 Notably, a growing number of researchers focus on the bio-printing technology, which is a bio-printer that is used to dispense “bioinks,” consisting of cells, scaffolds, and biomolecules, in a spatially controlled manner.134 Biological structures fabricated via bio-printing can encapsulate cells and bioactive agents directly, which include various biomimetic tissues such as the blood vessels, liver, heart, and tumors.135 Furthermore, 3DP technology is a continuous development, and it will play a bigger role in regenerative medicine.
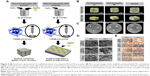  | Figure 6 (A) Schematic representation of scaffold production by using the DI and PA processes. (B) Macroscopic images of the scaffolds produced by DI, PA, and without AgNPs (TCP/SA) (front and side view). (C) Characterization of the bactericidal activity of the produced scaffolds, DI process (C1), PA process (C2), TCP/SA scaffold (C3). (D) SEM images of osteoblast morphology in the presence of the scaffolds. (E) Qualitative evaluation of mineralization on the 3D scaffolds by human osteoblast through alizarin red staining. |
Conclusion
Although orthopedic implants have made huge improvement for functional reconstruction of patients with bone fractures or defects, however, implant failure and revision surgeries are needed once the infection occurs. AgNPs with strong antibacterial efficacy are being used extensively in implant surface modification to prevent implant-related infection. The potential antibacterial mechanisms of the AgNPs were summarized, and their roles in optimization of orthopedic implants to provide some advice for implant designing were highlighted in this review. Future directions should focus on stability and long-term AgNP release, exploration of suitable size, shape, as well as the novel method of surface modification, such as 3DP technology.
Acknowledgments
Support from National Natural Science Foundation of China (Grant No 81772456, 51627805), Norman Bethune Program of Jilin University (Grant No 2012216), Science and Technology Development program of Jilin province (Grant No 20150414006GH, 20170520120JH), Special fund project of Jilin provincial industrial innovation (No 2016C037), Training Program of Outstanding Doctoral Student by Norman Bethune Health Science Center of Jilin University (No YB201501), Graduate Innovation Fund of Jilin University (No 2017089) is highly appreciated.
Disclosure
The authors report no conflicts of interest in this work.
References
Cochis A, Azzimonti B, Della Valle C, et al. The effect of silver or gallium doped titanium against the multidrug resistant Acinetobacter baumannii. Biomaterials. 2016;80:80–95. | ||
Gao A, Hang R, Huang X, et al. The effects of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts. Biomaterials. 2014;35(13):4223–4235. | ||
Kuehl R, Brunetto PS, Woischnig AK, et al. Preventing implant-associated infections by silver coating. Antimicrob Agents Chemother. 2016;60(4):2467–2475. | ||
Brennan SA, Fhoghlú CN, Devitt B, O’Mahony FJ, Brabazon D, Walsh A. Silver nanoparticles and their orthopaedic applications. Bone Joint J. 2015;97(5):582–589. | ||
Kelkawi AHA, Abbasi Kajani A, Bordbar AK. Green synthesis of silver nanoparticles using Mentha pulegium and investigation of their antibacterial, antifungal and anticancer activity. IET Nanobiotechnol. 2017;11(4):370–376. | ||
Li Y, Lin Z, Zhao M, et al. Silver nanoparticle based codelivery of oseltamivir to inhibit the activity of the H1N1 influenza virus through ROS-mediated signaling pathways. ACS Appl Mater Interfaces. 2016;8(37):24385–24393. | ||
Zhong X, Song Y, Yang P, et al. Titanium surface priming with phase-transited lysozyme to establish a silver nanoparticle-loaded chitosan/hyaluronic acid antibacterial multilayer via layer-by-layer self-assembly. PLoS One. 2016;11(1):e0146957. | ||
Xiang Y, Li J, Liu X, et al. Construction of poly(lactic-co-glycolic acid)/ZnO nanorods/Ag nanoparticles hybrid coating on Ti implants for enhanced antibacterial activity and biocompatibility. Mater Sci Eng C Mater Biol Appl. 2017;79:629–637. | ||
Nandi SK, Shivaram A, Bose S, Bandyopadhyay A. Silver nanoparticle deposited implants to treat osteomyelitis. J Biomed Mater Res B Appl Biomater. 2018;106(3):1073–1083. | ||
Hardes J, Henrichs MP, Hauschild G, Nottrott M, Guder W, Streitbuerger A. Silver-coated megaprosthesis of the proximal tibia in patients with sarcoma. J Arthroplasty. 2017;32(7):2208–2213. | ||
Wickens DJ, West G, Kelly PJ, et al. Antimicrobial activity of nanocomposite zirconium nitride/silver coatings to combat external bone fixation pin infections. Int J Artif Organs. 2012;35(10):817–825. | ||
Prokopovich P, Leech R, Carmalt CJ, Parlin IP, Perni S. A novel bone cement impregnated with silver–tiopronin nanoparticles: its antimicrobial, cytotoxic, and mechanical properties. Int J Nanomedicine. 2013;8:2227–2237. | ||
Wang J, Li J, Qian S, et al. Antibacterial surface design of titanium-based biomaterials for enhanced bacteria-killing and cell-assisting functions against periprosthetic joint infection. ACS Appl Mater Interfaces. 2016;8(17):11162–11178. | ||
Durán N, Durán M, de Jesus MB, Seabra AB, Fávaro WJ, Nakazato G. Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomedicine. 2016;12(3):789–799. | ||
Velusamy P, Kumar GV, Jeyanthi V, Das J, Pachaiappan R. Bio-inspired green nanoparticles: synthesis, mechanism, and antibacterial application. Toxicol Res. 2016;32(2):95–102. | ||
Castiglioni S, Cazzaniga A, Locatelli L, Maier JAM. Silver nanoparticles in orthopedic applications: new insights on their effects on osteogenic cells. Nanomaterials (Basel). 2017;7(6):124. | ||
Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett. 2012;2(32):1–12. | ||
Ahmed KBR, Nagy AM, Brown RP, Zhang Q, Malghan SG, Goering PL. Silver nanoparticles: significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol In Vitro. 2017;38:179–192. | ||
Cheng H, Xiong W, Fang Z, et al. Strontium (Sr) and silver (Ag) loaded nanotubular structures with combined osteoinductive and antimicrobial activities. Acta Biomater. 2016;31:388–400. | ||
Kumar N, Desagani D, Chandran G, et al. Biocompatible agarose-chitosan coated silver nanoparticle composite for soft tissue engineering applications. Artif Cells Nanomed Biotechnol. 2018;46(3):1–13. | ||
Muhammad Z, Raza A, Ghafoor S, et al. PEG capped methotrexate silver nanoparticles for efficient anticancer activity and biocompatibility. Eur J Pharm Sci. 2016;91:251–255. | ||
Yuan YG, Peng QL, Gurunathan S. Effects of silver nanoparticles on multiple drug-resistant strains of Staphylococcus aureus and Pseudomonas aeruginosa from mastitis-infected goats: an alternative approach for antimicrobial therapy. Int J Mol Sci. 2017;18(3):569. | ||
Seong M, Lee DG. Silver nanoparticles against Salmonella enterica serotype typhimurium: role of inner membrane dysfunction. Curr Microbiol. 2017;74(6):661–670. | ||
Khalandi B, Asadi N, Milani M, et al. A review on potential role of silver nanoparticles and possible mechanisms of their actions on bacteria. Drug Res (Stuttg). 2017;67(2):70–76. | ||
Chatterjee T, Chatterjee BK, Majumdar D, Chakrabarti P. Antibacterial effect of silver nanoparticles and the modeling of bacterial growth kinetics using a modified Gompertz model. Biochim Biophys Acta. 2015;1850(2):299–306. | ||
Van Der Wal A, Norde W, Zehnder AJB, Lyklema J. Determination of the total charge in the cell walls of Gram-positive bacteria. Colloids Surf B Biointerfaces. 1997;9(1–2):81–100. | ||
Abbaszadegan A, Ghahramani Y, Gholami A, et al. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: a preliminary study. J Nanomater. 2015;16(1):53. | ||
Mandal D, Kumar Dash S, Das B, et al. Bio-fabricated silver nanoparticles preferentially targets Gram positive depending on cell surface charge. Biomed Pharmacother. 2016;83:548–558. | ||
You C, Han C, Wang X, et al. The progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicity. Mol Biol Rep. 2012;39(9):9193–9201. | ||
Zhao R, Lv M, Li Y, et al. Stable nanocomposite based on PEGylated and silver nanoparticles loaded graphene oxide for long-term antibacterial activity. ACS Appl Mater Interfaces. 2017;9(18):15328–15341. | ||
Gomaa EZ. Silver nanoparticles as an antimicrobial agent: a case study on Staphylococcus aureus and Escherichia coli as models for Gram-positive and Gram-negative bacteria. J Gen Appl Microbiol. 2017;63(1):36–43. | ||
Siritongsuk P, Hongsing N, Thammawithan S, et al. Two-phase bactericidal mechanism of silver nanoparticles against Burkholderia pseudomallei. PLoS One. 2016;11(12):e0168098. | ||
Ramalingam B, Parandhaman T, Das SK. Antibacterial effects of biosynthesized silver nanoparticles on surface ultrastructure and nanomechanical properties of gram-negative bacteria viz. Escherichia coli and Pseudomonas aeruginosa. ACS Appl Mater Interfaces. 2016;8(7):4963–4976. | ||
Quinteros MA, Cano Aristizábal V, Dalmasso PR, Paraje MG, Páez PL. Oxidative stress generation of silver nanoparticles in three bacterial genera and its relationship with the antimicrobial activity. Toxicol In Vitro. 2016;36:216–223. | ||
Su HL, Chou CC, Hung DJ, et al. The disruption of bacterial membrane integrity through ROS generation induced by nanohybrids of silver and clay. Biomaterials. 2009;30(30):5979–5987. | ||
Korshed P, Li L, Liu Z, Wang T. The molecular mechanisms of the antibacterial effect of picosecond laser generated silver nanoparticles and their toxicity to human cells. PLoS One. 2016;11(8):e0160078. | ||
Lee W, Kim KJ, Lee DG. A novel mechanism for the antibacterial effect of silver nanoparticles on Escherichia coli. Biometals. 2014;27(6):1191–1201. | ||
Hwang IS, Hwang JH, Choi H, Kim KJ, Lee DG. Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. J Med Microbiol. 2012;61(Pt 12):1719–1726. | ||
Jin JC, Wu XJ, Xu J, Wang BB, Jiang FL, Liu Y. Ultrasmall silver nanoclusters: highly efficient antibacterial activity and their mechanisms. Biomater Sci. 2017;5(2):247–257. | ||
Lombardo PC, Poli AL, Castro LF, Perussi JR, Schmitt CC. Photochemical deposition of silver nanoparticles on clays and exploring their antibacterial activity. ACS Appl Mater Interfaces. 2016;8(33):21640–21647. | ||
Kim T, Braun GB, She ZG, Hussain S, Rouslahti E, Sailor MJ. Composite porous silicon-silver nanoparticles as theranostic antibacterial agents. ACS Appl Mater Interfaces. 2016;8(44):30449–30457. | ||
Zawadzka K, Kądzioła K, Felczak A, et al. Surface area or diameter – which factor really determines the antibacterial activity of silver nanoparticles grown on TiO 2 coatings? New J Chem. 2014;38(7):3275–3281. | ||
Klueh U, Wagner V, Kelly S, Johnson A, Bryers JD. Efficacy of silver-coated fabric to prevent bacterial colonization and subsequent device-based biofilm formation. J Biomed Mater Res. 2000;53(6):621–631. | ||
Holt KB, Bard AJ. Interaction of silver(I) ions with the respiratory chain of Escherichia coli: an electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+. Biochemistry. 2005;44(39):13214. | ||
Schreurs WJ, Rosenberg H. Effect of silver ions on transport and retention of phosphate by Escherichia coli. J Bacteriol. 1982;152(1):7–13. | ||
Hatchett DW, White HS. Electrochemistry of sulfur adlayers on the low-index faces of silver. J Phys Chem. 1996;100(23):9854–9859. | ||
Monteiro DR, Gorup LF, Takamiya AS, de Camargo ER, Filho AC, Barbosa DB. Silver distribution and release from an antimicrobial denture base resin containing silver colloidal nanoparticles. J Prosthodont. 2012;21(1):7–15. | ||
Long YM, Hu LG, Yan XT, et al. Surface ligand controls silver ion release of nanosilver and its antibacterial activity against Escherichia coli. Int J Nanomedicine. 2017;12:3193–3206. | ||
Gon S, Faulkner MJ, Beckwith J. In vivo requirement for glutaredoxins and thioredoxins in the reduction of the ribonucleotide reductases of Escherichia coli. Antioxid Redox Signal. 2006;8(5–6):735–742. | ||
Liao X, Yang F, Li H, et al. Targeting the thioredoxin reductase–thioredoxin system from Staphylococcus aureus by silver ions. Inorg Chem. 2017;56(24):14823–14830. | ||
Kaweeteerawat C, Na Ubol P, Sangmuang S, Aueviriyavit S, Maniratanachote R. Mechanisms of antibiotic resistance in bacteria mediated by silver nanoparticles. J Toxicol Environ Health A. 2017;80(23–24):1276–1289. | ||
Panáček A, Kvítek L, Smékalová M, et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat Nanotechnol. 2018;13(1):65. | ||
Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Ann Rev Microbiol. 2002;56(1):187–209. | ||
Franci G, Falanga A, Galdiero S, et al. Silver nanoparticles as potential antibacterial agents. Molecules. 2015;20(5):8856–8874. | ||
Du J, Singh H, Yi T-H. Antibacterial, anti-biofilm and anticancer potentials of green synthesized silver nanoparticles using benzoin gum (Styrax benzoin) extract. Bioprocess Biosyst Eng. 2016;39(12):1923–1931. | ||
Qin H, Cao H, Zhao Y, et al. In vitro and in vivo anti-biofilm effects of silver nanoparticles immobilized on titanium. Biomaterials. 2014;35(33):9114–9125. | ||
Van Hengel IAJ, Riool M, Fratila-Apachitei LE, et al. Selective laser melting porous metallic implants with immobilized silver nanoparticles kill and prevent biofilm formation by methicillin-resistant Staphylococcus aureus. Biomaterials. 2017;140:1–15. | ||
Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2002;5(1):32. | ||
Shapiro F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur Cell Mater. 2008;15(53):e76. | ||
Zielinska E, Tukaj C, Radomski MW, Inkielewicz-Stepnaik I. Molecular mechanism of silver nanoparticles-induced human osteoblast cell death: protective effect of inducible nitric oxide synthase inhibitor. PLoS One. 2016;11(10):e0164137. | ||
Pauksch L, Hartmann S, Rohnke M, et al. Biocompatibility of silver nanoparticles and silver ions in primary human mesenchymal stem cells and osteoblasts. Acta Biomater. 2014;10(1):439–449. | ||
Flores CY, Miñán AG, Grillo CA, Salvarezza RC, Vericat C, Schilardi PL. Citrate-capped silver nanoparticles showing good bactericidal effect against both planktonic and sessile bacteria and a low cytotoxicity to osteoblastic cells. ACS Appl Mater Interfaces. 2013;5(8):3149–3159. | ||
Albers CE, Hofstetter W, Siebenrock KA, Landmann R, Klenke FM. In vitro cytotoxicity of silver nanoparticles on osteoblasts and osteoclasts at antibacterial concentrations. Nanotoxicology. 2013;7(1):30–36. | ||
Kim TH, Kim M, Park HS, Shin US, Gong MS, Kim HW. Size-dependent cellular toxicity of silver nanoparticles. J Biomed Mater Res A. 2012;100(4):1033–1043. | ||
Mahmood M, Li Z, Casciano D, et al. Nanostructural materials increase mineralization in bone cells and affect gene expression through miRNA regulation. J Cell Mol Med. 2011;15(11):2297–2306. | ||
Cao H, Liu X, Meng F, Chu PK. Biological actions of silver nanoparticles embedded in titanium controlled by micro-galvanic effects. Biomaterials. 2011;32(3):693–705. | ||
Stains JP, Civitelli R. Cell-cell interactions in regulating osteogenesis and osteoblast function. Birth Defects Res C Embryo Today. 2005;75(1):72–80. | ||
Gao A, Hang R, Huang X, et al. The effects of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts. Biomaterials. 2014;35(13):4223–4235. | ||
Cao H, Qiao Y, Liu X, et al. Electron storage mediated dark antibacterial action of bound silver nanoparticles: smaller is not always better. Acta Biomater. 2013;9(2):5100–5110. | ||
Zheng Y, Li J, Liu X, Sun J. Antimicrobial and osteogenic effect of Ag-implanted titanium with a nanostructured surface. Int J Nanomedicine. 2012;7:875–884. | ||
Greulich C, Kittler S, Epple M, Muhr G, Köller M. Studies on the biocompatibility and the interaction of silver nanoparticles with human mesenchymal stem cells (hMSCs). Langenbecks Arch Surg. 2009;394(3):495–502. | ||
Hackenberg S, Scherzed A, Kessler M, et al. Silver nanoparticles: evaluation of DNA damage, toxicity and functional impairment in human mesenchymal stem cells. Toxicol Lett. 2011;201(1):27–33. | ||
Jung SK, Kim JH, Kim HJ, Ji YH, Kim JH, Son SW. Silver nanoparticle-induced hMSC proliferation is associated with HIF-1 [alpha]-mediated upregulation of IL-8 expression. J Invest Dermatol. 2014;134(12):3003. | ||
Sengstock C, Diendorf J, Epple M, Schildhauer TA, Köller M. Effect of silver nanoparticles on human mesenchymal stem cell differentiation. Beilstein J Nanotechnol. 2014;5:2058–2069. | ||
Samberg ME, Loboa EG, Oldenburg SJ, Monteiro-Riviere NA. Silver nanoparticles do not influence stem cell differentiation but cause minimal toxicity. Nanomedicine. 2012;7(8):1197–1209. | ||
Qin H, Zhu C, An Z, et al. Silver nanoparticles promote osteogenic differentiation of human urine-derived stem cells at noncytotoxic concentrations. Int J Nanomedicine. 2014;9:2469–2478. | ||
Zhang R, Lee P, Lui VC, et al. Silver nanoparticles promote osteogenesis of mesenchymal stem cells and improve bone fracture healing in osteogenesis mechanism mouse model. Nanomedicine. 2015;11(8):1949–1959. | ||
Rajan R, Chandran K, Harper SL, Yun SI, Kalaichelvan PT. Plant extract synthesized silver nanoparticles: an ongoing source of novel biocompatible materials. Ind Crops Prod. 2015;70:356–373. | ||
Mittal AK, Bhaumik J, Kumar S, Banerjee UC. Biosynthesis of silver nanoparticles: elucidation of prospective mechanism and therapeutic potential. J Colloid Interface Sci. 2014;415:39–47. | ||
Ovais M, Khalil AT, Raza A, et al. Green synthesis of silver nanoparticles via plant extracts: beginning a new era in cancer theranostics. Nanomedicine (Lond). 2016;12(23):3157–3177. | ||
Pérez ZEJ, Mathiyalagan R, Markus J, et al. Ginseng-berry-mediated gold and silver nanoparticle synthesis and evaluation of their in vitro antioxidant, antimicrobial, and cytotoxicity effects on human dermal fibroblast and murine melanoma skin cell lines. Int J Nanomedicine. 2017;12:709–723. | ||
Baghbani-Arani F, Movagharnia R, Sharifian A, Salehi S, Shandiz SAS. Photo-catalytic, anti-bacterial, and anti-cancer properties of phyto-mediated synthesis of silver nanoparticles from Artemisia tournefortiana Rchb extract. J Photochem Photobiol B. 2017;173:640–649. | ||
Qayyum S, Oves M, Khan AU. Obliteration of bacterial growth and biofilm through ROS generation by facilely synthesized green silver nanoparticles. PLoS One. 2017;12(8):e0181363. | ||
Kasithevar M, Saravanan M, Prakash P, et al. Green synthesis of silver nanoparticles using Alysicarpus monilifer leaf extract and its antibacterial activity against MRSA and CoNS isolates in HIV patients. J Interdiscip Nanomed. 2017;2(2):131–141. | ||
Li L, Sun J, Li X, et al. Controllable synthesis of monodispersed silver nanoparticles as standards for quantitative assessment of their cytotoxicity. Biomaterials. 2012;33(6):1714–1721. | ||
Carlson C, Hussain SM, Schrand AM, et al. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B. 2008;112(43):13608–13619. | ||
Tak YK, Pal S, Naoghare PK, Rangasamy S, Song JM. Shape-dependent skin penetration of silver nanoparticles: does it really matter? Sci Rep. 2015;5:16908. | ||
Kumari M, Pandey S, Giri VP, et al. Tailoring shape and size of biogenic silver nanoparticles to enhance antimicrobial efficacy against MDR bacteria. Microb Pathog. 2017;105:346–355. | ||
Sun B, Barnard AS. The impact of size and shape distributions on the electron charge transfer properties of silver nanoparticles. Nanoscale. 2017;9(34):12698–12708. | ||
Labhasetwar V, Leslie-Pelecky DL. Biomedical Applications of Nanotechnology [M]. Hoboken, NJ: John Wiley & Sons; 2007. | ||
Ravindran A, Chandran P, Khan SS. Biofunctionalized silver nanoparticles: advances and prospects. Colloids Surf B Biointerfaces. 2013;10:342–352. | ||
Divakar DD, Jastaniyah NT, Altamimi HG, et al. Enhanced antimicrobial activity of naturally derived bioactive molecule chitosan conjugated silver nanoparticle against dental implant pathogens. Int J Biol Macromol. 2018;108:790–797. | ||
Luna-Hernández E, Cruz-Soto M, Padilla-Vaca F, et al. Combined antibacterial/tissue regeneration response in thermal burns promoted by functional chitosan/silver nanocomposites. Int J Biol Macromol. 2017;105:1241–1249. | ||
Tyliszczak B, Drabczyk A, Kudłacik-Kramarczyk S, Bialik-Wąs K, Kijkowska R, Sobczak-Kupiec A. Preparation and cytotoxicity of chitosan-based hydrogels modified with silver nanoparticles. Colloids Surf B Biointerfaces. 2017;160:325–330. | ||
Peng Y, Song C, Yang C, Guo Q, Yao M. Low molecular weight chitosan-coated silver nanoparticles are effective for the treatment of MRSA-infected wounds. Gerodontology. 2017;12:295–304. | ||
Kwon T, Woo HJ, Kim YH, et al. Optimizing hemocompatibility of surfactant-coated silver nanoparticles in human erythrocytes. J Nanosci Nanotechnol. 2012;12(8):6168–6175. | ||
Zhou W, Jia Z, Xiong P, et al. Bioinspired and biomimetic AgNPs/gentamicin-embedded silk fibroin coatings for robust antibacterial and osteogenetic applications. ACS Appl Mater Interfaces. 2017;9(31):25830–25846. | ||
Lu T, Qiao Y, Liu X. Surface modification of biomaterials using plasma immersion ion implantation and deposition. Interface Focus. 2012;2(3):325–336. | ||
Anders A. From plasma immersion ion implantation to deposition: a historical perspective on principles and trends. Surf Coat Technol. 2002;156(1):3–12. | ||
Rautray TR, Narayanan R, Kwon TY, Kim KH. Surface modification of titanium and titanium alloys by ion implantation. J Biomed Mater Res B Appl Biomater. 2010;93(2):581–591. | ||
Qiao S, Cao H, Zhao X, et al. Ag-plasma modification enhances bone apposition around titanium dental implants: an animal study in Labrador dogs. Int J Nanomedicine. 2015;10:653–664. | ||
Qin H, Cao H, Zhao Y, et al. Antimicrobial and osteogenic properties of silver-ion-implanted stainless steel. ACS Appl Mater Interfaces. 2015;7(20):10785–10794. | ||
Cao H, Zhang W, Meng F, et al. Osteogenesis catalyzed by titanium-supported silver nanoparticles. ACS Appl Mater Interfaces. 2017;9(6):5149–5157. | ||
Wang G, Jin W, Qasim AM, et al. Antibacterial effects of titanium embedded with silver nanoparticles based on electron-transfer-induced reactive oxygen species. Biomaterials. 2017;124:25–34. | ||
Zhao Y, Cao H, Qin H, et al. Balancing the osteogenic and antibacterial properties of titanium by codoping of Mg and Ag: an in vitro and in vivo study. ACS Appl Mater Interfaces. 2015;7(32):17826–17836. | ||
Jin G, Qin H, Cao H, et al. Zn/Ag micro-galvanic couples formed on titanium and osseointegration effects in the presence of S-aureus. Biomaterials. 2015;65:22–31. | ||
Wan T, Aoki H, Hikawa J, Lee JH. RF-magnetron sputtering technique for producing hydroxyapatite coating film on various substrates. Biomed Mater Eng. 2007;17(5):291–297. | ||
Li R, Qin Y, Liu G, et al. Tantalum nitride coatings prepared by magnetron sputtering to improve the bioactivity and osteogenic activity for titanium alloy implants. RSC Adv. 2017;7(87):55408–55417. | ||
Socol G, Macovei A, Miroiu F, et al. Hydroxyapatite thin films synthesized by pulsed laser deposition and magnetron sputtering on PMMA substrates for medical applications. Mater Sci Eng B. 2010;169(1):159–168. | ||
Hsieh J, Tseng C, Chang Y, Chang SY, Wu W. Antibacterial behavior of TaN–Ag nanocomposite thin films with and without annealing. Surf Coat Technol. 2008;202(22):5586–5589. | ||
Hsieh JH, Yeh TH, Hung SY, Chang SY, Wu W, Li C. Antibacterial and tribological properties of TaN–Cu, TaN–Ag, and TaN–(Ag, Cu) nanocomposite thin films. Mater Res Bull. 2012;47(10):2999–3003. | ||
Zawadzka K, Kisielewska A, Piwonski I, et al. Mechanisms of antibacterial activity and stability of silver nanoparticles grown on magnetron sputtered TiO2 coatings. Bull Mater Sci. 2016;39(1):57–68. | ||
Huang H-L, Chang Y-Y, Chen H-J, Chou Y, Lai C, Chen MYC. Antibacterial properties and cytocompatibility of tantalum oxide coatings with different silver content. J Vac Sci Technol. 2014;32(2):02B117. | ||
Uhm SH, Song DH, Kwon JS, Lee SB, Han JG, Kim KN. Tailoring of antibacterial Ag nanostructures on TiO2 nanotube layers by magnetron sputtering. J Biomed Mater Res B Appl Biomater. 2014;102(3):592–603. | ||
Liu X, Gan K, Liu H, Song X, Chen T, Liu C. Antibacterial properties of nano-silver coated PEEK preparedac through magnetron sputtering. Dent Mater. 2017;33(9):E348–E360. | ||
Kedziora A, Korzekwa K, Strek W, Pawlak A, Doroszkiewicz W, Bugla-Ploskonska G. Silver nanoforms as a therapeutic agent for killing escherichia coli and certain ESKAPE pathogens. Curr Microbiol. 2016;73(1):139–147. | ||
Chen Y, Deng Y, Pu Y, Tang B, Su Y, Tang J. One pot preparation of silver nanoparticles decorated TiO2 mesoporous microspheres with enhanced antibacterial activity. Mater Sci Eng C Mater Biol Appl. 2016;65:27–32. | ||
Kamaraj K, George RP, Anandkumar B, Parvathavarthini N, Kamachi Mudali U. A silver nanoparticle loaded TiO2 nanoporous layer for visible light induced antimicrobial applications. Bioelectrochemistry. 2015;106(Pt B):290–297. | ||
Jia Z, Xiu P, Li M, et al. Bioinspired anchoring AgNPs onto micro-nanoporous TiO2 orthopedic coatings: trap-killing of bacteria, surface-regulated osteoblast functions and host responses. Biomaterials. 2016;75:203–222. | ||
Xu Z, Li M, Li X, et al. Antibacterial activity of silver doped titanate nanowires on Ti implants. ACS Appl Mater Interfaces. 2016;8(26):16584–16594. | ||
Ma L, Liu M, Liu H, Chen J, Cui D. In vitro cytotoxicity and drug release properties of pH-and temperature-sensitive core–shell hydrogel microspheres. Int J Pharm. 2010;385(1):86–91. | ||
Dong Y, Ye H, Liu Y, et al. pH dependent silver nanoparticles releasing titanium implant: A novel therapeutic approach to control peri-implant infection. Colloids Surf B Biointerfaces. 2017;158:127–136. | ||
Echeverry-Rendón M, Galvis O, Aguirre R, Robledo S, Castaño JG, Echeverría F. Modification of titanium alloys surface properties by plasma electrolytic oxidation (PEO) and influence on biological response. J Mater Sci Mater Med. 2017;28(11):169. | ||
Rizwan M, Alias R, Zaidi UZ, Mahmoodian R, Hamdi M. Surface modification of valve metals using plasma electrolytic oxidation (PEO) for antibacterial applications: a review. J Biomed Mater Res A. 2018;106(2):590–605. | ||
Necula BS, Fratila-Apachitei LE, Zaat SA, Apachitei I, Duszczyk J. In vitro antibacterial activity of porous TiO 2–Ag composite layers against methicillin-resistant Staphylococcus aureus. Acta Biomater. 2009;5(9):3573–3580. | ||
Besinis A, Hadi SD, Le HR, Tredwin C, Handy RD. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. ACS Appl Mater Interfaces. 2017;11(3):327–338. | ||
Park SH, Choi YJ, Moon SW, et al. Three-dimensional bio-printed scaffold sleeves with mesenchymal stem cells for enhancement of tendon-to-bone healing in anterior cruciate ligament reconstruction using soft-tissue tendon graft. Arthroscopy. 2018;34(1):166–179. | ||
Holzapfel BM, Rudert M, Hutmacher DW. Gerüstträgerbasiertes knochen-tissue-engineering [Scaffold-based bone tissue engineering]. Orthopade. 2017;46(8):701–710. German. | ||
Yusa K, Yamanochi H, Takagi A, Lino M. Three-dimensional printing model as a tool to assist in surgery for large mandibular tumour: a case report. J Oral Maxillofac Res. 2017;8(2):e4. | ||
Ritz U, Gerke R, Götz H, Stein S, Rommens PM. A new bone substitute developed from 3D-prints of polylactide (PLA) loaded with collagen I: an in vitro study. Int J Mol Sci. 2017;18(12):2569. | ||
Correia TR, Figueira DR, De Sa KD, et al. 3D printed scaffolds with bactericidal activity aimed for bone tissue regeneration. Int J Biol Macromol. 2016;93:1432–1445. | ||
Zhang Y, Zhai D, Xu M, et al. 3D-printed bioceramic scaffolds with antibacterial and osteogenic activity. Biofabrication. 2017;9(2):025037. | ||
García-Alvarez R, Izquierdo-Barba I, Vallet-Regí M. 3D scaffold with effective multidrug sequential release against bacteria biofilm. Acta Biomater. 2017;49:113–126. | ||
Jessop ZM, Al-Sabah A, Gardiner MD, Combellack E, Hawkins K, Whitaker IS. 3D bioprinting for reconstructive surgery: principles, applications and challenges. J Plast Reconstr Aesthet Surg. 2017;70(9):1155–1170. | ||
Li Y-C, Zhang YS, Akpek A, Shin SR, Khademhosseini A. 4D bioprinting: the next-generation technology for biofabrication enabled by stimuli-responsive materials. Biofabrication. 2016;9(1):012001. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.