Back to Journals » Journal of Asthma and Allergy » Volume 10
Postpartum airway responsiveness and exacerbation of asthma during pregnancy – a pilot study
Authors Ali Z , Nilas L, Ulrik CS
Received 24 March 2017
Accepted for publication 17 June 2017
Published 3 October 2017 Volume 2017:10 Pages 261—267
DOI https://doi.org/10.2147/JAA.S137847
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Amrita Dosanjh
Zarqa Ali,1 Lisbeth Nilas,2,3 Charlotte Suppli Ulrik1,3
1Department of Pulmonary Medicine, 2Department of Gynaecology and Obstetrics, Hvidovre Hospital, Hvidovre, 3Institute of Clinical Medicine, University of Copenhagen, Copenhagen, Denmark
Background: Airway responsiveness and inflammation are associated with the clinical manifestations of asthma and the response to pharmacological therapy.
Objective: To investigate if airway responsiveness and inflammatory characteristics are related to asthma exacerbations during pregnancy.
Materials and methods: In women with asthma who were prescribed controller medication and monitored closely during pregnancy, the risk of exacerbations was analyzed in relation to postpartum measures of fractional exhaled nitric oxide (FENO), skin prick test reactivity, static and dynamic lung volumes, diffusing capacity for carbon monoxide, bronchial responsiveness to inhaled mannitol, and inflammatory characteristics in induced sputum. Obtained data were analyzed in relation to exacerbation status during pregnancy. The PD15 is defined as the cumulative administered dose causing a 15% decline in forced expiratory volume in the first second (FEV1).
Results: Fifty women (mean age ± standard deviation of 32±5 years) were enrolled over an 11-month period and examined on average 4 months postpartum. During pregnancy, 13 women had a total of 16 exacerbations (8 mild and 8 severe). Women with asthma exacerbation during pregnancy had more pronounced airway responsiveness to inhaled mannitol (geometric mean PD15 82 vs 171 mg, p=0.04) and were less likely to be atopic (62% vs 86%, respectively; p=0.04) than the non-exacerbators. No statistically significant difference was found between the 2 groups of women with regard to type of airway inflammation in sputum and fractional exhaled nitric oxide (FENO).
Conclusion: More pronounced airway hyperresponsiveness together with nonatopic status appears to characterize women at high risk of exacerbation of asthma during pregnancy.
Keywords: asthma, inflammation, pregnancy
Introduction
Asthma is characterized by variable airflow obstruction, airway hyperresponsiveness, and airway inflammation.1 Airway inflammation is a major factor determining the degree of airway responsiveness2 and may predict both the course of asthma and the response to anti-asthma therapy.3,4 Furthermore, it is generally accepted, also in international guidelines, that treating airway inflammation is a prerequisite for achieving sufficient symptom control in asthma.5
The prevalence of asthma is higher in women than in men.6,7 One-third of pregnant women with asthma will experience a worsening in their asthma,8 and an exacerbation of asthma during pregnancy is the most significant risk factor for fetal morbidity and mortality in asthmatic mothers.9 Despite abundant evidence of the occurrence of asthma exacerbations during pregnancy8 and its adverse effect on pregnancy outcomes,10,11 our knowledge of the mechanisms underlying an exacerbation remains limited, largely because investigations have been restricted to retrospective- and register-based studies. Furthermore, studies so far have primarily looked at asthma severity,9,12 smoking,13 adherence with asthma medication,14 and obesity15 as risk factors for exacerbations of asthma during pregnancy, whereas studies have not evaluated airway responsiveness, except the study by Juniper et al,16 or airway inflammation in pregnant women with asthma, either during pregnancy or postpartum. A prospective study by Juniper et al16 with 16 participants found no changes in airway hyperresponsiveness to methacholine inhalation test 1 month postpartum compared to preconception mean.
The aim of the present pilot study was to investigate potential differences in physiological and inflammatory characteristics between women with and without asthma exacerbation during pregnancy.
Materials and methods
Materials
Pregnant women with asthma referred to give birth at Hvidovre Hospital have since 2007 been offered participation in the Management of Asthma during Pregnancy (MAP) program at the outpatient clinic at the Department of Pulmonary Medicine, Hvidovre Hospital. Scheduled follow-up visits were planned every 4 weeks during pregnancy with adjustment, if necessary, of asthma medication based on symptoms, level of fractional exhaled nitric oxide (FENO), and spirometry in accordance with the algorithm subsequently described by Powell et al.17 The MAP study has been described in detail previously.18–21
Inclusion criteria for the present study were:
- Enrolled in the MAP program during 2015.
- Prescribed controller medication, ie, inhaled corticosteroid.
- Delivery within the past 9 months.
One hundred and four women fulfilled the inclusion criteria and were invited consecutively from November 2015 to September 2016 to a postpartum examination.
Definitions
Smoking status was classified as current smokers, ex-smokers, or lifelong non-smokers. Pack-years (number of cigarettes per day/20× duration of smoking [years]) were used as an estimate of life-time tobacco exposure. Exposure to environmental tobacco smoke (ETS) was defined as living with someone smoking at home (as smoking in work places, restaurants etc. is currently not allowed in Denmark).
Self-reported adherence with controller medication was recorded (at each visit to the out-patient clinic) as good, acceptable, or poor.
Exacerbations were defined as mild (managed by an increase in therapy, but not requiring oral corticosteroids) or severe (requiring hospital admission, emergency department treatment, and/or a rescue course of systemic corticosteroids).
Methods
The postpartum examination program included the following procedures: measurement of FENO, skin prick test (SPT), body plethysmography, spirometry, diffusing capacity for carbon monoxide (DLCO), bronchial provocation test with mannitol, and induction of sputum.
Exhaled nitric oxide
FENO was measured with Eco Medics CLD 88sp analyzer and DENOX 88 (Eco Medics, Duernten, Switzerland) at a controlled flow rate of 50 mL/s according to American Thoracic Society (ATS) guidelines.22 The mean value of 2 measurements in parts per billion (ppb) was recorded.
SPT
The SPT was performed according to European Academy of Allergy and Clinical immunology standards23,24 and a wheal diameter ≥3 mm was defined positive. SPT was performed with a panel of aeroallergen extracts, including birch (Betula verrucosa), grass (Phleum pratense), mugworts (Artemisia vulgaris and Ambrosia artemisiifolia), cat (Felix domesticus), dog (Canis familiaris), horse (Equus caballus), house dust mite (Dermatophagoides pteronyssinus and Dermatophagoides farinae), and molds (Alternaria alternata, Cladosporium herbarum, and Aspergillus fumigatus). Positive (10 mg/mL histamine hydrochloride solution) and negative (diluent) controls were used. Subjects were considered atopic if they had a positive reaction to at least one of the aeroallergens.
Spirometry, plethysmography, and diffusing capacity
Forced expiratory volume in the first second (FEV1) from a maximum inspiration, forced vital capacity (FVC), along with vital capacity (VC), residual volume (RV), total lung capacity (TLC), forced residual capacity, specific airway conductance, and functional residual capacity (FRC) were measured using a whole body plethysmograph25 and were performed according to the ATS/European Respiratory Society guidelines26 using standardized equipment (Jaeger MasterScreen Body; IntraMedic, Sollentuna, Sweden). Prior to each test calibration was conducted according to the manufacturer’s instructions. The procedure was explained in detail, and the maneuver was demonstrated to the patient.
DLCO was measured by the single-breath carbon monoxide (CO) gas transfer method, and the predicted DLCO was adjusted for hemoglobin.27 The patient inhaled a gas mixture containing 0.3% CO, 9.3% helium, 21% oxygen, and nitrogen to make up the balance. All results are expressed as percentage predicted.
Mannitol bronchial provocation test
The mannitol bronchial provocation test was performed using the commercial mannitol kit Osmohale© Pharmaxis (Pharmaxis Ltd, Sydney, NSW, Australia) according to the method described by Anderson et al.28 The mannitol capsule dose started at 0 mg (placebo) and increased (5, 10, 20, 40, 80, 160, 160, and 160 mg) to a total cumulative dose of 635 mg of mannitol. A positive response was defined as a fall in FEV1 ≥15% with a cumulative dose of mannitol ≤635 mg (PD15). The PD15 was calculated by linear interpolation of the individual dose–response relationship between the percentage decrease in FEV1 and the cumulative dose of mannitol required to provoke this decrease. Rescue bronchodilator was administered at the end of the challenge test to aid recovery.
Induced sputum
Hypertonic (3.5%) saline was nebulized with an ultrasonic nebulizer (Ultrasonic inhalation device USC, [Medisana, Neuss, Germany]) with an output of ~1 mL/min at room temperature. The induction was performed at 5-min intervals for ≤15 min. The women were instructed to cough and spit after 5, 10, and 15 min of induction or whenever they got the urge to do so.
Sputum plugs were selected from the expectorate and analyzed according to the method described by Pizzichini et al.29 Cytospins were prepared and stained with May-Grünwald and Giemsa stain, and differential cell count of 400 non-squamous cells was performed.
Squamous cells were counted to determine sample quality. A sputum sample of inadequate quality was defined as a squamous cell percentage of >80%.30
Sputum samples were categorized into 4 subgroups based on the prevailing sputum cell count: eosinophilic (>3% eosinophils), neutrophilic (>60% neutrophils), mixed granulocytic (>3% eosinophils and >60% neutrophils), and paucigranulocytic (<3% eosinophils and <60% neutrophils).31
Outcome
The primary outcome variable was airway responsiveness to mannitol (PD15) at the postpartum examination in women with and without asthma exacerbation during pregnancy.
The secondary outcome variables were:
- eosinophilic inflammation vs neutrophilic inflammation in induced sputum,
- SPT reactivity,
- FENO level (ppb),
- FEV1% of predicted, and
- static lung volumes and diffusion capacity.
Statistical analysis
Data were analyzed using SAS Enterprise Guide 7.1. Continuous data were analyzed by the 2-sample t-test and binary outcomes by the Chi square test or Fisher’s exact test. Geometric mean was given for PD15 along with 95% confidence interval (CI). Mann–Whitney test was used to analyze the association between asthma exacerbations and PD15 because the PD15 data were not normally distributed. For each association a crude model was first analyzed and then adjusted for potential confounders: age, smoking status (never smoker vs ever smoker), inhaled corticosteroids (ICS) treatment (low dose vs middle and high dose), and self-reported adherence with ICS (good/acceptable vs poor).
The response–dose ratio (RDR) was calculated according to the method described by O’Connor et al,32 as the percentage decline in FEV1 after the last mannitol dose divided by the cumulative mannitol dose in milligrams. To meet normal distribution, log-transformed values of RDR were used, and an additional 1% was added to the percentage decrease in FEV1 to eliminate zero values.32 The relationship between airway responsiveness to mannitol expressed as the RDR and the airway inflammation in sputum was analyzed using Pearson’s correlation coefficient (r), with -1 indicating a perfect negative correlation, +1 indicating a perfect positive correlation, and 0 indicating no correlation at all.
A p-value ≤ 0.05 was considered significant.
Ethics statement
This study was performed in accordance with the Declaration of Helsinki II and according to Danish legislation. The study was approved by the Research Ethics Committee of the Capital Region of Denmark (H-2-2014-051) and by the Danish data protection agency (2007-58-0015). All patients provided written informed consent.
Results
During the 11-month study period, 104 women fulfilled the inclusion criteria, of whom 50 (48%) women accepted the invitation and were examined 1–7 months after delivery. In 13 women, a total of 16 exacerbations (8 mild and 8 severe) were observed during pregnancy. The general characteristics of the participants are presented in Table 1.
The result of the SPT had to be omitted in 1 patient due to a reaction to the negative control.
The mannitol test was positive in 78% (n=39) with a geometric mean PD15 of 137 mg (95% CI 99–189). There were no significant differences between women with a positive and a negative mannitol provocation test with regard to age, lung function parameters, smoking status, allergy, and FENO.
Sputum induction was successful in 40 out of 50 women (success rate 80%). The cell viability in the sputum samples was 89.9±7.9%, and the median squamous cell contamination was 0.3±0.8 (range 0–4%). There were no significant differences between patients with and without successful induction of sputum according to age, lung function parameters, smoking status, and allergy. However, women with successful sputum induction had significantly higher level of FENO compared to those with unsuccessful sputum induction (mean FENO level 21 ppb and 12 ppb, respectively, p=0.04).
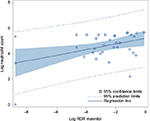
No significant difference was found in prevalence of airway responsiveness to mannitol between women with and without an asthma exacerbation during pregnancy, however, women with an exacerbation were more responsive than women without an exacerbation (geometric mean PD15 82 vs 178 mg, p=0.04). Furthermore, there was a significant positive correlation between the degree of airway responsiveness to mannitol (RDR mannitol) and neutrophil count in sputum (r=0.44, p=0.01) (Figure 1), but not to the eosinophil count (r=0.00, p=0.97).
Women without an asthma exacerbation during pregnancy were more likely to have a positive SPT compared to women with exacerbation (86% vs 62%, odds ratio [OR] 0.20, 95% CI 0.05–0.92, p=0.04) (Table 2).
There were no significant changes in lung function parameters, the level of FENO, and inflammatory phenotypes after adjusting for age, smoking status, ICS treatment, and self-reported adherence with ICS.
There were no significant differences in women with and without an exacerbation during pregnancy regarding the inflammatory phenotype in sputum, ie, eosinophilia or neutrophilia.
Discussion
In the present study of pregnant women with asthma, we found that women with an exacerbation during pregnancy were more responsive to inhaled mannitol and more likely to be nonatopic compared to women without asthma exacerbations.
Our results are in line with Leuppi et al,33 who have reported that increased responsiveness to mannitol is a better indicator for loss of asthma control than symptoms, spirometry, and/or FENO. Furthermore, we found that the level of airway responsiveness to mannitol was associated with sputum neutrophilia, which supports findings from another study by Leuppi et al.34 The lack of an association between sputum eosinophilia and airway responsiveness in our population is similar to the results in a study of Simpson et al.31 In contrast, Porsbjerg et al35 have observed a higher level of airway responsiveness to mannitol in eosinophilic asthma, which may be explained by the difference in the population. In the study by Porsbjerg et al,35 the cohort of patients studied were not on controller therapy, ie, ICS, whereas all the women in our cohort were treated with ICS. Eosinophilia in sputum is effectively reduced by ICS treatment,36 while neutrophilic inflammation in sputum shows little or no response to ICS.36 It is likely that treatment with ICS has reduced the eosinophilic airway inflammation and by that may have prevented some exacerbations in our study.
Additionally, women with an asthma exacerbation during pregnancy were more likely to be nonatopic. This is in line with a study by Stenius-Aarniala et al,37 who found a higher rate of exacerbation and hospital admission in pregnant women with nonatopic asthma than atopic asthma.
There are several strengths and limitations in our study that need to be addressed. First of all, the study is the first of its kind to investigate the differences in asthma phenotypes in women with and without asthma exacerbation during pregnancy. We have used hypertonic saline-induced sputum to evaluate the airway inflammation in patients with asthma. This is a non-invasive test, and the results correlate well with inflammation assessed by bronchoalveolar lavage (BAL).38 Furthermore, Simpson et al.31 have demonstrated that the inflammatory subtypes in sputum are stable over a longer term (5 years), and an important strength of our study is, therefore, that the examinations were performed within the first half year after birth and thereby may be likely to reflect the conditions prior to pregnancy. Additionally, Juniper et al16 have demonstrated, in a very limited cohort of 16 women, no changes in mean concentration of methacholine needed to produce a 20% fall in FEV1 from baseline (PC20) of methacholine inhalation test 1 month postpartum compared to preconception mean. There are no such studies for mannitol challenge test.
Finally, there was no significant difference in spirometry and level of FENO measured during pregnancy and postpartum.
However, there are also some limitations. The study is limited by the fact that the tests are done postpartum and not during pregnancy. We found no significant association between asthma phenotype and the risk of asthma exacerbation during pregnancy, which may be explained by the modest number of subjects. In addition, sputum induction was possible in only 80%, and failure of induction was associated with low FENO. Women with neutrophilic airway inflammation not being able to produce sputum could explain the lack of a relationship; however, the average FENO level in both groups was below 25 ppb.
The mannitol bronchial provocation test and induction of sputum with hypertonic saline were done on the same day. Nevertheless, Wood et al39 have demonstrated that mannitol bronchial provocation test does not enhance airway responsiveness, the quality of sputum cells collected, or the concentration of inflammatory mediators in sputum. A total of 104 women fulfilled the inclusion criteria and were invited to participate in the present study, of whom 50 women agreed to participate. The examination procedures themselves took about 3 h and calculating the transport time and considering that they also have to bring a newborn with them it was expected that a high percentage would refuse to participate. Our cohort is representative of the entire MAP cohort; however, the results are not generalizable to the general population of pregnant women with asthma due to the close monitoring of asthma during pregnancy.
Future studies are needed to investigate the link between asthma phenotypes and exacerbations during pregnancy by evaluating the airway inflammation early in pregnancy in a prospective design. Furthermore, there is need for studies investigating the stability of airway inflammatory characteristics early in pregnancy and postpartum.
Conclusion
We found that women experiencing an asthma exacerbation during pregnancy are more responsive to mannitol and less likely to be atopic compared to women without an asthma exacerbation during pregnancy, whereas the present pilot study did not reveal a difference in inflammatory phenotype.
Disclosure
The authors report no other conflicts of interest in this work.
References
Wardlaw AJ, Brightling C, Green R, Woltmann G, Pavord I. Eosinophils in asthma and other allergic diseases. Br Med Bull. 2000;56(4):985–1003. | ||
National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl): S94–S138. | ||
Fahy JV. Eosinophilic and neutrophilic inflammation in asthma: insights from clinical studies. Proc Am Thorac Soc. 2009;6(3):256–259. | ||
Haldar P, Pavord ID, Shaw DE, et al. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178(3):218–224. | ||
Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS board of directors, November 1986. Am Rev Respir Dis. 1987;136(1):225–244. | ||
Leynaert B, Sunyer J, Garcia-Esteban R, et al. Gender differences in prevalence, diagnosis and incidence of allergic and non-allergic asthma: a population-based cohort. Thorax. 2012;67(7):625–631. | ||
Schatz M, Camargo CA Jr. The relationship of sex to asthma prevalence, health care utilization, and medications in a large managed care organization. Ann Allergy Asthma Immunol. 2003;91(6):553–558. | ||
Schatz M, Harden K, Forsythe A, et al. The course of asthma during pregnancy, post partum, and with successive pregnancies: a prospective analysis. J Allergy Clin Immunol. 1988;81(3):509–517. | ||
Murphy VE, Gibson P, Talbot PI, Clifton VL. Severe asthma exacerbations during pregnancy. Obstet Gynecol. 2005;106(5 pt 1):1046–1054. | ||
Murphy VE, Namazy JA, Powell H, et al. A meta-analysis of adverse perinatal outcomes in women with asthma. BJOG. 2011;118(11):1314–1323. | ||
Wang G, Murphy VE, Namazy J, et al. The risk of maternal and placental complications in pregnant women with asthma: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2014;27(9):934–942. | ||
Schatz M, Dombrowski MP, Wise R, et al. Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol. 2003;112(2):283–288. | ||
Murphy VE, Clifton VL, Gibson PG. The effect of cigarette smoking on asthma control during exacerbations in pregnant women. Thorax. 2010;65(8):739–744. | ||
Apter AJ, Greenberger PA, Patterson R. Outcomes of pregnancy in adolescents with severe asthma. Arch Intern Med. 1989;149(11):2571–2575. | ||
Hendler I, Schatz M, Momirova V, et al; National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Association of obesity with pulmonary and nonpulmonary complications of pregnancy in asthmatic women. Obstet Gynecol. 2006;108(1):77–82. | ||
Juniper EF, Daniel EE, Roberts RS, Kline PA, Hargreave FE, Newhouse MT. Improvement in airway responsiveness and asthma severity during pregnancy. A prospective study. Am Rev Respir Dis. 1989; 140(4):924–931. | ||
Powell H, Murphy VE, Taylor DR, et al. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet. 2011; 378(9795):983–990. | ||
Ali Z, Nilas L, Ulrik CS. Low risk of adverse obstetrical and perinatal outcome in pregnancies complicated by asthma: a case control study. Respir Med. 2016;120:124–130. | ||
Grarup PA, Janner JH, Ulrik CS. Passive smoking is associated with poor asthma control during pregnancy: a prospective study of 500 pregnancies. PLoS One. 2014;9(11):e112435. | ||
Baarnes CB, Hansen AV, Ulrik CS. Enrolment in an asthma management program during pregnancy and adherence with inhaled corticosteroids: the ‘management of asthma during pregnancy’ program. Respiration. 2016;92(1):9–15. | ||
Ali Z, Nilas L, Ulrik CS. Excessive gestational weight gain in first trimester is a risk factor for exacerbation of asthma during pregnancy: a prospective study of 1283 pregnancies. J Allergy Clin Immunol. Epub May 24, 2017. | ||
Reddel HK, Taylor DR, Bateman ED, et al; American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99. | ||
Heinzerling L, Mari A, Bergmann KC, et al. The skin prick test – European standards. Clin Transl Allergy. 2013;3(1):3. | ||
Position paper: allergen standardization and skin tests. The European Academy of Allergology and Clinical Immunology. Allergy. 1993;48(14 suppl):48–82. | ||
Coates AL, Peslin R, Rodenstein D, Stocks J. Measurement of lung volumes by plethysmography. Eur Respir J. 1997;10(6):1415–1427. | ||
Miller MR, Hankinson J, Brusasco V, et al; ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. | ||
Miller A, Thornton JC, Warshaw R, Anderson H, Teirstein AS, Selikoff IJ. Single breath diffusing capacity in a representative sample of the population of Michigan, a large industrial state. Predicted values, lower limits of normal, and frequencies of abnormality by smoking history. Am Rev Respir Dis. 1983;127(3):270–277. | ||
Anderson SD, Brannan J, Spring J, et al. A new method for bronchial-provocation testing in asthmatic subjects using a dry powder of mannitol. Am J Respir Crit Care Med. 1997;156(3 pt 1):758–765. | ||
Pizzichini E, Pizzichini MM, Efthimiadis A, et al. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med. 1996;154(2 pt 1):308–317. | ||
Fahy JV, Liu J, Wong H, Boushey HA. Cellular and biochemical analysis of induced sputum from asthmatic and from healthy subjects. Am Rev Respir Dis. 1993;147(5):1126–1131. | ||
Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11(1):54–61. | ||
O’Connor G, Sparrow D, Taylor D, Segal M, Weiss S. Analysis of dose-response curves to methacholine. An approach suitable for population studies. Am Rev Respir Dis. 1987;136(6):1412–1417. | ||
Leuppi JD, Salome CM, Jenkins CR, et al. Predictive markers of asthma exacerbation during stepwise dose reduction of inhaled corticosteroids. Am J Respir Crit Care Med. 2001;163(2):406–412. | ||
Leuppi JD, Salome CM, Jenkins CR, et al. Markers of airway inflammation and airway hyperresponsiveness in patients with well-controlled asthma. Eur Respir J. 2001;18(3):444–450. | ||
Porsbjerg C, Lund TK, Pedersen L, Backer V. Inflammatory subtypes in asthma are related to airway hyperresponsiveness to mannitol and exhaled NO. J Asthma. 2009;46(6):606–612. | ||
Pavord ID, Brightling CE, Woltmann G, Wardlaw AJ. Non-eosinophilic corticosteroid unresponsive asthma. Lancet. 1999;353(9171):2213–2214. | ||
Stenius-Aarniala B, Piirila P, Teramo K. Asthma and pregnancy: a prospective study of 198 pregnancies. Thorax. 1988;43(1):12–18. | ||
Grootendorst DC, Sont JK, Willems LN, et al. Comparison of inflammatory cell counts in asthma: induced sputum vs bronchoalveolar lavage and bronchial biopsies. Clin Exp Allergy. 1997;27(7):769–779. | ||
Wood LG, Powell H, Gibson PG. Mannitol challenge for assessment of airway responsiveness, airway inflammation and inflammatory phenotype in asthma. Clin Exp Allergy. 2010;40(2):232–241. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.