Back to Journals » OncoTargets and Therapy » Volume 11
Postoperative hepatic arterial infusion chemotherapy improved survival of pancreatic cancer after radical pancreatectomy: a retrospective study
Authors Wang Y, Xu Y, Zheng Y, Bao Y, Wang P
Received 12 October 2017
Accepted for publication 5 January 2018
Published 21 February 2018 Volume 2018:11 Pages 903—907
DOI https://doi.org/10.2147/OTT.S153886
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tohru Yamada
Yongchun Wang,1 Yongqiang Xu,1 Yinyuan Zheng,2 Ying Bao,1 Ping Wang1
1Department of General Surgery, First People’s Hospital affiliated to Huzhou Normal College, Huzhou, Zhejiang Province, People’s Republic of China; 2Department of Radiology, First People’s Hospital affiliated to Huzhou Normal College, Huzhou, Zhejiang Province, People’s Republic of China
Objective: To determine the effect of postoperative hepatic arterial infusion chemotherapy (HAIC) on long-term survival of patients with pancreatic cancer (PC) after radical pancreatectomy.
Methods: A total of 87 patients with PC underwent radical pancreatectomy in the First People’s Hospital affiliated to Huzhou Normal College between June 2008 and May 2013. Among these patients, after surgery, 43 received two sessions of HAIC followed by four sessions of systemic chemotherapy (HAIC group), while 44 received six sessions of systemic chemotherapy alone (control group). Both the HAIC and systemic chemotherapy regimen included 5-fluorouracil (1,000 mg/m2) as a 5-h infusion on day 1, and gemcitabine (800 mg/m2) as an over 30-min infusion on days 1 and 8. The toxicity, complication, and long-term survival were retrospectively compared.
Results: No significant difference in patient characteristics between the two groups was found. No chemotherapy-related deaths were recorded, and no significant difference in toxicities was observed between the two groups. The 5-year disease-free survival probability did not differ between the two groups (P=0.2029, hazard ratio for recurrence=0.7561; 95% CI=0.4768–1.1989, by the log-rank test). The HAIC group had significantly higher 5-year overall survival probability (P=0.0288, hazard ratio for death=0.6059; 95% CI=0.3734–0.9832, by the log-rank test) and higher 5-year hepatic metastases-free survival probability (P=0.0321, hazard ratio for hepatic metastases=0.5006; 95% CI=0.2546–0.9843, by the log-rank test) than the control group.
Conclusions: Postoperative HAIC has the potential to prevent hepatic metastases and increase long-term survival probability of patients with PC after radical pancreatectomy.
Keywords: pancreatic cancer, hepatic arterial infusion chemotherapy, hepatic metastases, survival
Introduction
Pancreatic cancer (PC) remains a lethal disease, with an overall 5-year survival rate of ~5%.1,2 Even after radical pancreatectomy, there is a high probability of systemic and/or local recurrence, and the median survival is only ~1 year, suggesting that surgery alone is generally inadequate.3,4
Hepatic metastasis is a common cause of treatment failure after curative resection of PC.5 Accordingly, chemotherapy represents one of the most important postresection therapeutic strategies in the treatment of PC. However, currently, the commonly used chemotherapy still cannot achieve a satisfactory clinical curative effect and has serious adverse reactions. Consequently, it is of great value to develop alternative treatment regimens. Our previous study revealed that hepatic arterial infusion chemotherapy (HAIC) significantly prevented metachronous hepatic metastases from colorectal cancer and improved the prognosis.6 Therefore, this retrospective study aimed to determine whether HAIC has the potential to prevent hepatic metastasis and improve the prognosis of patients with PC after radical pancreatectomy.
Methods
Patients
During June 2008 and May 2013, 87 patients with PC underwent radical pancreatectomy in the First People’s Hospital affiliated to Huzhou Normal College. Among these patients, after surgery, 43 received two sessions of HAIC followed by four sessions of systemic chemotherapy (HAIC group), while 44 received six sessions of systemic chemotherapy alone (control group). The main inclusion criteria were as follows: histologically confirmed as ductal adenocarcinoma of the pancreas, no prior cancer therapy, Karnofsky performance score (KPS) ≥70, postresection survival time >6 months, Child–Pugh Classes A–B, adequate bone marrow and renal function, and aged from 18 to 75 years. The medical records of these patients were retrospectively reviewed. The baseline characteristics, complications, toxicities, and long-term survival were collected and compared. Patients chose treatment modality by themselves after being told by the doctors about advantages and disadvantages of each treatment modality. After choosing the treatment modality, all patients signed a written informed consent form for the treatment. This research was approved by the ethics committee of the First People’s Hospital affiliated to Huzhou Normal College.
Chemotherapy administration
Chemotherapy was begun within 28 days postsurgery and administered every 4 weeks. Both the HAIC and systemic chemotherapy regimen included 5-fluorouracil and gemcitabine.7 In this study, 5-fluorouracil (1,000 mg/m2) was administered as a 5-h infusion on day 1 and gemcitabine (800 mg/m2) was administered as an over 30-min infusion on days 1 and 8.
In the HAIC group, chemotherapy agents were infused through a catheter, with one end inserted into the common hepatic artery or proper hepatic artery, and the other end linked to the infusion pump.8 After infusion, the infusion catheter was withdrawn. Toxicities and complications were observed and managed by symptomatic therapies. Chemotherapy would be discontinued once life-threatening toxicities or complications occurred or according to the patients’ request. The National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0,17 was used to evaluate toxicities.
Follow-up
All patients had a follow-up visit every month during the first year after surgery and every 3 months thereafter. The follow-up visit included physical examination, biochemical tests, measurement of tumor markers, and thoracic and abdominal imaging examination. Relapse or metastasis was detected by imaging and, if necessary, biopsy. Repeat chemotherapy with the same regime was administered as soon as relapse or metastasis was diagnosed. Alternative regimens consisting of combinations of gemcitabine, oxaliplatin, or capecitabine were administered if the disease was not controlled.
Statistical analysis
All measurements were expressed as mean ± SD. Student’s t-, chi-square, or Fisher’s exact test was appropriately applied to analyze the clinical data. Survival curves were obtained using the Kaplan–Meier method and compared using the log-rank test. For all tests, P<0.05 was considered statistically significant.
Results
Patient characteristics
There was no significant difference between the two groups in patient characteristics, such as gender, age, operating time, tumor stage, tumor location, lymph nodal involvement, portal vein invasion, blood loss during surgery, tumor differentiation, tumor size, or serum carbohydrate antigen 19-9 (CA19-9) level (Table 1).
  | Table 1 Patient characteristics |
Disease-free survival
During the first 5 postresection years, 35 patients from the HAIC group and 38 patients from the control group developed relapse or metastasis. Details of recurrent sites are shown in Table 2. No significant difference was found in the 5-year disease-free survival probability between the two groups (P=0.2029, hazard ratio for recurrence=0.7561; 95% CI=0.4768–1.1989, by the log-rank test) (Figure 1).
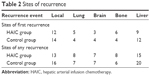  | Table 2 Sites of recurrence |
Overall survival
During the first 5 postresection years, 32 patients from the HAIC group and 36 patients from the control group died. The HAIC group had a significantly higher 5-year overall survival probability than the control group (P=0.0288, hazard ratio for death=0.6059; 95% CI=0.3734–0.9832, by the log-rank test) (Figure 2).
Hepatic metastases-free survival
During the first 5 postresection years, hepatic metastases were reported in 15 patients from the HAIC group and 20 patients from the control group. Survival analysis (Figure 3) showed a significantly higher 5-year hepatic metastases-free survival probability in the HAIC group compared with the control group (P=0.0321, hazard ratio for hepatic metastases=0.5006; 95% CI=0.2546–0.9843, by the log-rank test).
Toxicity and complication
Chemotherapy-related toxicities are shown in Table 3, and no significant difference was observed between the two groups. No chemotherapy-related deaths were recorded. No life-threatening toxicity or complication was reported. All patients completed six sessions of chemotherapy. All side effects and complications were ameliorated by symptomatic therapies. Hematoma of the femoral artery puncture site was reported in three patients after the HAIC procedure and was controlled by pressure bandage.
  | Table 3 Toxicities |
Discussion
In order to improve the long-term survival of patients with PC, surgeons usually conduct radical pancreatectomy, including extensive lymph node dissection and complete resection of the extra pancreatic nerve plexus of the superior mesenteric artery or celiac axis.9,10 However, postresection recurrence rate remains high, even in patients who received margin-negative (R0) resections. Hepatic metastasis is one of the major causes of treatment failure after radical pancreatectomy. HAIC is a well-established therapeutic and prophylactic option for hepatic metastases from colorectal cancer.6 Some studies have shown that HAIC has beneficial effects on unresectable PC and postresection hepatic metastases, and can assist in the prevention of postresection hepatic cancer recurrence.11,12 Accordingly, HAIC might be an attractive treatment option, because infusion of chemotherapy agents through the hepatic arterial circulation can achieve high local concentrations with minimal systemic side effects.13–15
It was demonstrated that gemcitabine combined with fluorouracil drugs significantly improved overall survival and increased 1-year survival probability and objective response rate compared to gemcitabine alone in patients with PC.16 Therefore, gemcitabine combined with fluorouracil drugs may be considered as an acceptable alternative treatment for PCs. Both the HAIC and systemic chemotherapy regimen in our study included 5-fluorouracil and gemcitabine.
In our study, patients in the HAIC group had significantly higher overall survival and hepatic metastases-free survival than the control group. However, the disease-free survival showed no difference between the two groups, suggesting that the survival benefit of HAIC could be attributable to the decreased hepatic recurrence.
Our study also confirmed the safety of the HAIC procedure. The toxicity and complication of the HAIC group were similar to the control group. None of the symptoms was severe, and all were controlled by the conservative therapies in both groups.
Conclusion
HAIC has the potential to prevent hepatic metastases and increase the long-term survival probability of patients with PC after radical pancreatectomy. Due to the retrospective nature and the small sample number of the present study, a further prospective study with a larger sample number is needed to confirm the results of our study.
Disclosure
The authors report no conflicts of interest in this work.
References
Shaib YH, Davila JA, El-Serag HB. The epidemiology of pancreatic cancer in the United States: changes below the surface. Aliment Pharmacol Ther. 2006;24(1):87–94. | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. | ||
Malleo G, Maggino L, Marchegiani G, et al. Pancreatectomy with venous resection for pT3 head adenocarcinoma: Perioperative outcomes, recurrence pattern and prognostic implications of histologically confirmed vascular infiltration. Pancreatology. 2017;17(5):847–857. | ||
Takamori H, Hiraoka T, Kanemitsu K, Tsuji T. Pancreatic liver metastases after curative resection combined with intraoperative radiation for pancreatic cancer. Hepatogastroenterology. 2004;51(59):1500–1503. | ||
Tajima H, Ohta T, Kitagawa H, et al. Pilot study of hepatic arterial infusion chemotherapy with gemcitabine and 5-fluorouracil for patients with postoperative liver metastases from pancreatic cancer. Exp Ther Med. 2011;2(2):265–269. | ||
Wang Y, Sun XR, Feng WM, Bao Y, Zheng YY. Postoperative prophylactic hepatic arterial infusion chemotherapy for stage III colorectal cancer: a retrospective study. Onco Targets Ther. 2016;9:5897–5902. | ||
Kurtz JE, Kohser F, Negrier S, et al. Gemcitabine and protracted 5-FU for advanced pancreatic cancer. A phase II study. Hepatogastroenterology. 2000;47(35):1450–1453. | ||
Kasugai H, Kojima J, Tatsuta M, et al. Treatment of hepatocellular carcinoma by transcatheter arterial embolization combined with intraarterial infusion of a mixture of cisplatin and ethiodized oil. Gastroenterology. 1989;97(4):965–971. | ||
Spitz FR, Abbruzzese JL, Lee JE, et al. Preoperative and postoperative chemoradiation strategies in patients treated with pancreaticoduodenectomy for adenocarcinoma of the pancreas. J Clin Oncol. 1997;15(3):928–937. | ||
Palmer DH, Stocken DD, Hewitt H, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol. 2007;14(7):2088–2096. | ||
Hayashibe A, Kameyama M, Shinbo M, Makimoto S. Clinical results on intra-arterial adjuvant chemotherapy for prevention of liver metastasis following curative resection of pancreatic cancer. Ann Surg Oncol. 2007;14(1):190–194. | ||
Kurosaki I, Kawachi Y, Nihei K, et al. Liver perfusion chemotherapy with 5-Fluorouracil followed by systemic gemcitabine administration for resected pancreatic cancer: preliminary results of a prospective phase 2 study. Pancreas. 2009;38(2):161–167. | ||
Ackerman NB, Lien WM, Kondi ES, Silverman NA. The blood supply of experimental liver metastases. I. The distribution of hepatic artery and portal vein blood to “small” and “large” tumors. Surgery. 1969;66(6):1067–1072. | ||
Conway JG, Popp JA, Ji S, Thurman RG. Effect of size on portal circulation of hepatic nodules from carcinogen-treated rats. Cancer Res. 1983;43(7):3374–3378. | ||
Archer SG, Gray BN. Vascularization of small liver metastases. Br J Surgery. 1989;76(6):545–548. | ||
Li Q, Yan H, Liu W, Zhen H, Yang Y, Cao B. Efficacy and safety of gemcitabine-fluorouracil combination therapy in the management of advanced pancreatic cancer: a meta-analysis of randomized controlled trials. PLoS One. 2014;9(8):e104346. | ||
National Cancer Institute. 2009. Common Terminology Criteria for Adverse Events (CTCAE) (version 4.0). |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



