Back to Journals » Local and Regional Anesthesia » Volume 9
Postoperative epidural analgesia for patients undergoing pectus excavatum corrective surgery: a 10-year retrospective analysis
Authors Siddiqui A, Tse A, Paul J, Fitzgerald P, Teh B
Received 10 January 2015
Accepted for publication 14 August 2015
Published 25 May 2016 Volume 2016:9 Pages 25—33
DOI https://doi.org/10.2147/LRA.S80710
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Stefan Wirz
Asad Siddiqui,1 Andrew Tse,2 James E Paul,3 Peter Fitzgerald,4 Bernice Teh,5
1Department of Anesthesia, University of Toronto, Toronto, 2Department of Anesthesia, University of Ottawa, Ottawa, 3Department of Anesthesia, 4Department of Surgery, McMaster University, Hamilton, Ontario, Canada; 5Canterbury Anesthetic Services, Victoria, Australia
Introduction: Managing postoperative pain in patients undergoing minimally invasive pectus excavatum repair (Nuss procedure) is challenging but essential in facilitating ambulation and minimizing the length of stay. Although multiple epidural regimens with varying opioids are presently used for pain management, there is currently no clinical consensus regarding which epidural regimen provides the best analgesia outcomes with the fewest side effects. This 10-year retrospective cohort study was performed to compare the quality of analgesia and the incidence of side effects associated with the three most common epidural regimens used at a tertiary care children's hospital, in patients undergoing the Nuss procedure.
Methods: Seventy-two pediatric patients were identified as having been treated with one of three epidural regimens for postoperative pain management following the Nuss procedure: Group A (n=12) received 0.125% bupivacaine and 5 µg/mL fentanyl, Group B (n=21) received 0.125% bupivacaine and 10 µg/mL hydromorphone, and Group C (n=39) received 0.1% ropivacaine and 20 µg/mL hydromorphone. Our primary outcome was maximal daily pain scores (numerical rating scale 0–10), with an analytical focus on postoperative day 1 scores. The primary outcome was analyzed using linear regression. The secondary outcomes included the length of stay, side-effect profiles as reflected by the number of treatments for nausea and pruritus, pain scores according to epidural site insertion, occurrence of breakthrough pain, and presence of severe pain throughout their hospital stay. Secondary outcomes were analyzed using linear or logistic regression adjusted for pain scores at baseline. The criterion for statistical significance was set a priori at alpha =0.05.
Results: Group A had significantly higher day-1 pain scores (score 5.42/10) than Group B (4.52/10; P=0.030) and Group C (4.49/10; P=0.015) after adjusting for baseline pain and age. No significant difference in maximum daily pain scores was found between groups during postoperative days 2–5. Among secondary outcomes, Group C had a significantly lower incidence of nausea/vomiting than Group B (P=0.003). There was also significantly more severe pain in Group A than in Group C (P=0.031). No significant difference was found between the three groups for the incidence of pruritus, critical events, breakthrough pain, or patient satisfaction.
Conclusion: There is no significant difference in managing postoperative pain overall between the three epidural regimens employed at our center. However, in managing day-1 postoperative pain and minimizing nausea/vomiting, our study suggests that a hydromorphone–ropivacaine epidural regimen appears to have more favorable results than a fentanyl–bupivacaine regimen or a hydromorphone–bupivacaine regimen.
Keywords: Nuss procedure, pain, opioids, pediatric patients, epidural
Introduction
Pectus excavatum (PE) is a common congenital anomaly of the anterior thorax characterized by sternal depression that results in a concave precordium. Occurring in 1:400 births, PE is six times more common than pectus carinatum and is cited as the most frequent congenital deformity of the anterior chest wall.1 Clinically, symptoms of easy fatiguability, dyspnea on exertion, wheezing, anterior chest wall pain, palpitations, and recurrent lower respiratory tract infections may develop as a result of this, but these symptoms are not common; rather, most patients are healthy and asymptomatic. Despite this, the role of surgery in correcting PE is underscored by the findings that repair of PE (post bar removal) results in an increase in cardiopulmonary function.2 In addition, surgical intervention improves exercise tolerance, cosmesis, and self-image.3,4
In 1997, Nuss et al described a minimally invasive technique where they preserved costal cartilages and elevated the sternum with a convex internal steel bar. At that time, it had been performed in 42 patients older than 10 years.5 The more recent 21-year report on the Nuss procedure shows that the procedure had been performed in 1,215 patients with 95.8% good-to-excellent anatomic result.6 Naturally, it is now becoming widely accepted as the primary surgical intervention for the correction of PE deformity due to its shorter operating duration, better cosmesis, and less blood loss in comparison to the more invasive Ravitch costal resection procedure.
The Nuss procedure is performed as follows: 1) a small lateral subcostal incision is made on the right and left side for insertion of a convex steel bar under the sternum; 2) a separate, small lateral incision is made to allow for a thoracoscope for direct visualization as the bar is passed under the sternum; 3) thoracoscopy has been added to minimize the risk of mediastinal and liver injury during insertion of the substernal bar; 4) the convex bar is rotated to elevate the sternum, and is then fixed to the ribs on either side; 5) the incisions are closed and dressed; and 6) after a 2–4 year period, bar removal is performed as an outpatient procedure. In the past, bar displacement has been a major complication resulting in both recurrence of pectus excavatum and a need for reoperation. Since then, however, modifications to the procedure and restrictions on patient mobility have been made to minimize complications of bar displacement; bar stabilizers are now used and patients are restricted from flexion and rotation at the waist and hips during the perioperative period.7,8
Despite its minimally invasive approach, it is well recognized that the Nuss procedure has a more painful postoperative course than the Ravitch procedure for reasons not entirely elucidated. It has been hypothesized that forcing the sternum into normal alignment causes stress on the ribs and sternum, as well as strain on the costal cartilage. This can eventually result in small fractures visible on ultrasound and nuclear medicine scans.9,10 Postoperative pain is furthermore associated with an increase in complication rates, including bar displacement. Therefore, this emphasizes the importance of determining the most effective pain management technique for postoperative care.11
Studies have examined the difference in visual analog pain scores between patient-controlled analgesia (PCA) and epidural treatments for the postoperative care of patients undergoing the Nuss procedure. A study of 40 children randomized between morphine PCA and a fentanyl/ropicavaine epidural regimen demonstrated lower visual analog pain scores in the group receiving epidural pain management.12 Another study of 28 randomized patients showed that there was no difference in postoperative pain control using PCA with fentanyl in comparison to an epidural block with fentanyl/bupivacaine.13 The use of PCA versus epidural intervention for pain management is thus not yet definitive and has consequently been left to the discretion of the anesthesiologist.
Independently, the epidural technique has been found to be effective in postoperative pain management for PE repair. One study of 21 patients who underwent the Nuss procedure used one of two epidural regimens to relieve postoperative pain: fentanyl in bupivacaine and fentanyl in ropivacaine. Use of these regimens resulted in 57.1% of patients requiring no additional analgesic, 19.0% of patients required one additional dose of pain management with diclofenac sodium or pentazocine, and 23.9% of patients required two or more additional doses of pain management.11 This study illustrates the utility of epidural pain management postoperatively for the Nuss procedure but does not specifically compare pain outcomes between the two different epidural groups.
Similarly, in a study conducted by Densmore et al, different epidural regimens were tested and compared for postoperative pain management in the Nuss procedure. The study determined a success rate of 96.4% in terms of pain management in its patient’s post-Nuss procedure. The regimens utilized included an opioid (morphine, hydromorphone, or fentanyl), a local anesthetic (bupivicaine or ropivicaine), and in 20% of cases, clonidine. The study found that the type of opioid used did not change outcomes with regard to the pain scores.14 The study, however, did not report on the incidence of side effects that were associated with each epidural regimen used.
At McMaster University, patients undergoing the Nuss procedure have been followed closely by our Acute Pain Service (APS), and we have been collecting analgesia outcomes since we launched a quality assurance database (APS Manager; Adjuvant Informatics) in 2002. Over the course of 10 years, we have tried a number of epidural analgesia regimens for these patients, and it is not clear which is optimal and which has the fewest side effects. The purpose of this retrospective quality assurance study was to compare the quality of analgesia and the incidence of side effects associated with the three most common epidural regimens, over a 10-year period, used at our center for the treatment of patients undergoing the Nuss procedure.
Methods
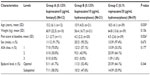
This was a single-center, retrospective cohort study comparing postoperative pain management in patients undergoing the Nuss procedure between 2002 and 2012 at the McMaster University Medical Centre in Hamilton, Ontario. After obtaining Research Ethics Board approval from Hamilton Health Sciences Research Ethics Board, one surgeon (Peter Fitzgerald) provided a list of 153 patients originally referred for an assessment for the Nuss procedure. Written informed patient consent was not obtained as this was a retrospective chart review. Of these patients, 112 underwent the Nuss procedure and were identified for review. Eighteen patients were immediately excluded from the review based on the criteria that medical records could not be found (14 patients), redo pectus repair was necessary (two patients), or they were the sole recipients of a unique type of epidural regimen (two patients). Original medical files, microfiche, and computer-based copies of medical files were thus extracted for 94 patients for retrospective chart analysis. From this, 72 patients were identified as having received treatment with the three most common epidural regimens employed at our center for postoperative pain management of the Nuss procedure: Group A (n=12) received 0.125% bupivacaine and 5 μg/mL fentanyl, Group B (n=21) received 0.125% bupivacaine and 10 μg/mL of hydromorphone, and Group C (n=39) received 0.1% ropivacaine and 20 μg/mL hydromorphone (Table 1).
The anesthetic records, pain management flow sheets, medication charts, and admission data were extracted for these 72 patients. Patients’ sex, age, weight, American Society of Anesthesiologists (ASA) classification, duration of hospital stay, satisfaction, site of insertion of epidural catheter, and time of starting the epidural infusion were recorded. Maximal daily pain scores (numerical rating scale: 0–10), breakthrough analgesia requirements, and the presence of severe pain, critical events, and side effects (nausea and pruritus) were extracted from a computerized acute pain database from the APS. We considered the maximum pain score on postoperative day 0 to be considered the patient’s baseline pain score. A critical event was defined as a medication error, respiratory depression, respiratory or cardiac arrest, severe hypotension, prolonged motor blockade, or epidural hematoma or abscess.
All patients except one had a thoracic epidural catheter inserted preoperatively and epidural infusion either commenced or continued postoperatively. Epidural catheter insertions were considered optimal if inserted at or higher than T6/7 and were considered suboptimal if inserted below T6/7. Patients were discharged from the postanesthesia care unit when their analgesia was adequate, and epidural infusions were maintained between 0.1 mL/kg/h and 0.4 mL/kg/h. The epidural regimen employed was left to the APS team and the regimen used for these cases changed over time that provided us with the three treatment groups for comparison. The epidural regimen employed was up to the case anesthesiologist. Patients were placed on regular acetaminophen and a nonsteroidal anti-inflammatory drug postoperatively. For breakthrough analgesia, patients received either an epidural solution bolus or epidural narcotic (fentanyl or meperidine). Patients sat out of bed on day 2 postoperative and ambulated from day 3 onward with regular incentive spirometry for every hour.
The primary outcome was maximal daily pain scores (numerical rating scale 0–10) as determined by the ward nurse for each postoperative day. Our primary time period of focus was day 1 pain scores as pain is likely most severe immediately postoperatively. Our secondary time period of focus was the pain scores for the remaining days. Daily morning pain scores assessed by the APS nurses were also extracted, and any pain problems found on follow-up outpatient assessments were recorded. The secondary outcomes included the length of stay (LOS) of the patients, the side-effect profiles as reflected by the number of treatments for nausea and pruritus, the occurrence of breakthrough pain, and the presence of severe pain throughout the course of their hospital stay. Another secondary outcome also included daily pain scores according to epidural site insertion point (optimal vs suboptimal).
Statistical analysis
The demographic and anesthetic factors of the patients were analyzed by group using descriptive statistics reported as a mean (SD) for continuous variables and count (percentage) for categorical variables. The primary outcome (daily pain scores after treatment) is illustrated by box plot and analyzed using linear regression. They were adjusted for age and baseline pain score. A generalized estimating equation (GEE) model was also used to analyze the pain scores and provide an overall pain score for each group, adjusting for repeated measurements acquired for each patient on a day-to-day basis. Daily pain scores were also analyzed by epidural catheter location (optimal vs suboptimal) using linear regression. They are illustrated by a box plot.
The secondary outcomes include LOS in hospital, requirement of breakthrough analgesia, severe pain, critical events, pruritus, nausea/vomiting, and satisfaction. The LOS is analyzed using linear regression. Other secondary outcomes are illustrated by bar plots and analyzed using logistic regression. All linear and logistic regression analyses are adjusted for pain scores at baseline and for age. The results from the linear and mixed-effects models are reported as adjusted difference between the treatment groups, corresponding two-sided 95% confidence intervals (CIs), and associated P-values. Results from logistic regression are reported as odds ratio, 95% CI, and associated P-values. All statistical tests are performed using two-sided tests at the 0.05 level of significance. P-values are reported to three decimal places with values <0.001 reported as <0.001. All the analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
Of the 72 patients, the most common epidural regimen was 0.1% ropivacaine/20 μg/mL hydromorphone (Group C, N=39), the next most frequent regimen was 0.125% bupivacaine/10 μg/mL hydromorphone (Group B, N=21), followed by the 0.125% bupivacaine/5 μg/mL fentanyl (Group A, N=12). The groups were similar with respect to sex, ASA status, weight, LOS, and site of epidural catheter insertion (Table 1). Group C had a significantly higher mean age (18.5) than did Group A (15.2) and Group B (15.9, P=0.030). One patient was considered a fail of epidural technique and was switched to PCA on postoperative day 2, when his pain control was considered inadequate. With respect to pain scores at baseline, Group A (2.1/10) was found to have a statistically significant (but clinically modest) lower pain score than both Group B (4.2/10) and Group C (4.5/10, P=0.026).
Primary outcome – maximal daily pain scores
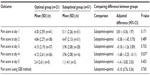
On postoperative day 1, Group A was found to have a significantly higher amount of pain (score 5.42/10) than both Group B (4.52/10, P=0.030, 95% CI [−3.81, −0.21]) and Group C (4.49/10; P=0.015, 95% CI [−3.91, −0.43]) after adjusting for baseline pain between the groups. No significant difference in maximum daily pain scores was found between groups during the postoperative days 2, 3, 4, or 5 (Table 2 and Figure 1). When using the GEE method, no significant differences were found in overall pain scores between any of the groups.
Secondary outcomes
With respect to side effects, 67% of patients from Group A, 81% from Group B, and 34% from Group C experienced nausea/vomiting, requiring treatment throughout their stay. A significant difference was found between Group B and Group C (P=0.003, 95% CI [0.04, 0.52]). No significant difference was found for nausea/vomiting between the other groups. In addition, 67% of patients from Group A, 52% of patients from Group B, and 49% of patients from Group C experienced severe pain throughout their stay. Among these, there was a significant difference between Group C and Group A (P=0.031, 95% CI [0.03, 0.84]). No significant difference was found between the three groups for the incidence of pruritus, incidence of critical events, occurrence of breakthrough pain, or satisfaction with their pain management throughout their stay (Table 3 and Figure 2).
In our study, two critical events occurred: one patient in Group B had a medication error wherein inappropriate sedatives were prescribed by a non-APS physician, and another patient in Group C was found to have a respiratory rate of eight with no sequelae.
Pain scores based on epidural catheter location
In total, 41 patients (57%) were found to have optimal epidural catheter location (inserted above T6/7), and 31 patients (43%) had suboptimal epidural catheter placement (insertion below T6/7). The groups were similar with regard to all baseline characteristics, including age, weight, pain scores at baseline, sex, and ASA class (Table 4).
A significant difference in pain scores between groups was found only on the fourth postoperative day (P=0.037, CI −3.25, −0.11). No significant differences were found on any of the remaining daily pain scores or using the GEE method for analysis (Table 5).
Pain after discharge from hospital
All patients were discharged with oral analgesia – acetaminophen and ibuprofen plus codeine or oxycodone or MS Contin (morphine sulfate). Only one patient did not require opioid discharge medication. All patients were seen by the surgeon in a clinic postoperatively, and none of the patients were using the analgesics at the follow-up appointment or experienced any pain problems.
Discussion
This study found no significant differences in pain scores between the three epidural regimens overall. However, this study demonstrated that patients who received fentanyl in their epidural regimen (Group A) had significantly higher pain scores on day 1 than did those who received hydromorphone (Groups B and C) when adjusted for age and baseline pain score. This finding, better analgesia with epidural hydromorphone than fentanyl, has not yet been reported in the literature.
It was important to determine whether or not the significance of our results was confounded by the variable sites of insertion of the epidural catheters between patients. It should be noted that there was no significant difference in the site of epidural catheter insertion between the three groups. Based on our analysis (Tables 4 and 5), the only significant difference in pain was found on the fourth postoperative day (P=0.037, CI −3.25, −0.11). Since the first postoperative day was our main focus for analyzing postoperative pain, we are confident that the catheter site did not impact our primary outcome. The significance of the results on day 4 must also be interpreted with caution due to the fact that the sample size of patients for that day was considerably lower (n=26 in the optimal group, n=16 in the suboptimal group).
It should be noted that >90% of patients were satisfied with the management of their postoperative pain, and none of the patients had ongoing pain problems after discharge from the hospital. This is consistent with the evidence in the literature demonstrating the efficacy of epidural regimens for postoperative pain control in patients undergoing the Nuss procedure.
Analgesia results of this study are consistent with the mixed results of previous studies examining epidural analgesia alone for the Nuss procedure. Several studies15,16 have reported the need for supplemental intravenous morphine in order to achieve adequate pain control in their patients; of these, one study15 did not report pain scores, and the other16 found that IV (intravenous) morphine was needed in 47% of their patients. Another case series reported excellent epidural analgesia without IV opioids, but they required regular, large doses of epidural opioid in order to achieve this – 40 μg/kg morphine Q8H.17 In either case, both of these studies corroborate with our finding that severe pain occurs in patients despite using a multimodal analgesic approach. A larger retrospective chart review using morphine, hydromorphone, or fentanyl as the opioid accompanying the local anesthetic in the epidural catheter found that IV ketorolac was used in 69% of epidural patients to provide further pain relief.14 Further statistical analysis failed to find a significant difference between those who received it and those who did not.14 When actually comparing the types of opioids used in the epidural catheters, their study’s findings were similar to our own in that no significant difference in pain scores was found between groups.14 Thus, our paper supports the current evidence that the choice of epidural opioid does not have a significant impact on postoperative pain scores in patients undergoing the Nuss procedure.
The use of postoperative daily maximal pain scores may not reflect epidural efficacy if a brief moment of severe pain (commonly seen during ambulation) occurred for only a short duration of time. However, it still reflects the lack of adequate analgesia. Although a record of the duration of maximal pain score may better reflect the efficacy of the epidural, this was not possible at our center given the variations of the recording process on the ward in the 10-year period and also given that the pain nurse only visited the patient to record the pain scores once daily. Thus, a prospective randomized trial with hourly pain score recordings might better reflect the ability of the epidural regimen to control pain.
The chronological progression from Group A to B to C at our center occurred due to several perceived benefits as per the anesthesiology team. Switching from a fentanyl-based regimen (Group A, 2002–2003) to a hydromorphone-based regimen (Group B, 2004–2005) was thought to decrease the opioid-related side effects associated with fentanyl. Furthermore, the rationale from switching the local anesthetic from bupivacaine (Group B) to ropivacaine (Group C, 2005–2012) was to allow for an increase in the amount of hydromorphone that could be administered in order to improve overall analgesia. Severe pain was found to be improved in Group C (49%) when compared to Group A (67%), which may suggest that a higher dose of hydromorphone in a ropivacaine regimen may better control severe pain than a bupivacaine–fentanyl-based regimen. With regard to side effects, no significant difference was seen in nausea/vomiting once the opioid was changed from fentanyl to hydromorphone. This was contrary to the expectations of the anesthesia team. Surprisingly, however, a decrease in nausea/vomiting was seen when the hydromorphone dose was doubled and the local anesthetic was changed from bupivacaine to ropivacaine. Although there was not a statistically significant difference in the proportion of optimal epidural insertions between the treatment groups, there was a trend for more optimal placement over time (from 42% in Group A to 64% in Group C) and this may reflect an improvement in anesthesia practice and regional anesthesia training in recent years. There was no significant difference in pruritus between any of these groups. We cannot corroborate these findings with the available literature, as this is the first study to evaluate the side-effect profiles of various epidural regimens for postoperative pain control for the Nuss procedure.
One of the biggest strengths of this review is that it is the first retrospective cohort study that evaluates epidural regimens for both their efficacy in postoperative pain control and side-effect profiles for patients undergoing the Nuss procedure. Its sample size also reflects all the patients who underwent the Nuss procedure at a large tertiary center over a significant period of time (10 years). The current literature does not have similar studies examining patients over this period of time. Finally, the pain measurements and data were collected systematically and prospectively using dedicated APS nurses and a quality assurance database for the duration of the review.
The weaknesses of the study include its retrospective nature, lack of randomization, and limited ability to detect significant differences between treatment groups due to its seemingly small sample size. Other confounders not controlled for in this retrospective design include the era of the surgery, the severity of the deformity, length of surgery, and the degree of surgical manipulation required. However, given that the Nuss procedure is relatively uncommon and given that our data comprise all the patients who underwent minimally invasive repair over an entire decade, we argue that our sample size is acceptable. It should also be noted that our statistical analysis adjusted for the difference in age and baseline pain scores between the three groups in order to prevent confounding from these variables.
Conclusion
Our study demonstrates that there is no significant difference between the three epidural regimens in managing overall postoperative pain in patients undergoing the Nuss procedure. However, in managing day-1 postoperative pain and minimizing nausea/vomiting, our study suggests that a hydromorphone–ropivacaine epidural regimen appears to have more favorable results than a fentanyl–bupivacaine regimen or a hydromorphone–bupivacaine regimen. Although pain is problematic for several weeks to months after surgery, the incidence of chronic pain following the Nuss procedure has yet to be reported and could be an area of future research. A prospective randomized trial with a larger sample size that recorded the duration of maximal pain scores may better reflect the ability of the regimen to control pain, along with the prevalence of side effects.
Author contributions
Asad Siddiqui, Andrew Tse, and James E Paul, helped design the study, conduct the study, reviewed the analysis of the data, wrote and approved the final manuscript; Peter Fitzgerald, helped to design the study and analyze the data; Bernice Teh, helped to design the study and approved the final manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Fokin AA, Steuerwald NM, Ahrens WA, Allen KE. Anatomical, histologic, and genetic characteristics of congenital chest wall deformities. Semin Thorac Cardiovasc Surg. 2009;21(1):44–57. | |
Sigalet DL, Montgomery M, Harder J, Wong V, Kravarusic D, Alassiri A. Long term cardiopulmonary effects of closed repair of pectus excavatum. Pediatr Surg Int. 2007;23(5):493–497. | |
Lawson ML, Cash TF, Akers R, et al. A pilot study of the impact of surgical repair on disease-specific quality of life among patients with pectus excavatum. J Pediatr Surg. 2003;38(6):916–918. | |
Roberts J, Hayashi A, Anderson JO, Martin JM, Maxwell LL. Quality of life of patients who have undergone the Nuss procedure for pectus excavatum: preliminary findings. J Pediatr Surg. 2003;38(5):779–783. | |
Nuss D, Kelly RE Jr, Croitoru DP, Katz ME. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg. 1998;33(4):545–552. | |
Kelly RE, Goretsky MJ, Obermeyer R, et al. Twenty-one years of experience with minimally invasive repair of pectus excavatum by the Nuss procedure in 1215 patients. Ann Surg. 2010;252(6):1072–1081. | |
Croitoru DP, Kelly RE Jr, Goretsky MJ, Lawson ML, Swoveland B, Nuss D. Experience and modification update for the minimally invasive Nuss technique for pectus excavatum repair in 303 patients. J Pediatr Sur. 2002;37(3):437–445. | |
Hebra A, Swoveland B, Egbert M, Tagge EP, Georgeson K, Othersen HB Jr, Nuss D. Outcome analysis of minimally invasive repair of pectus excavatum: review of 251 cases. J Pediatr Surg. 2000;35(2):252–257; discussion 257–258. | |
Zeng Q, Lai JY, Wang XM, et al. Costochondral changes in the chest wall after the Nuss procedure: ultrasonographic findings. J Pediatr Surg. 2008;43(12):2147–2150. | |
Ohno K, Morotomi Y, Harumoto K, et al. Preliminary study on the effects of bar placement on the thorax after the Nuss procedure for pectus excavatum using bone scintigraphy. Eur J Pediatr Surg. 2006; 16(3):155–159. | |
Futagawa K, Suwa I, Okuda T, et al. Anesthetic management for the minimally invasive Nuss procedure in 21 patients with pectus excavatum. J Anesth. 2006;20(1):48–50. | |
Weber T, Matzl J, Rokitansky A, Klimscha W, Neumann K, Deusch E. Superior postoperative pain relief with thoracic epidural analgesia versus intravenous patient-controlled analgesia after minimally invasive pectus excavatum repair. J Thorac Cardiovasc Surg. 2007;134(4):865–870. | |
Butkovic D, Kralik S, Matolic M, Kralik M, Toljan S, Radesic L. Postoperative analgesia with intravenous fentanyl PCA vs epidural block after thoracoscopic pectus excavatum repair in children. Br J Anaesth. 2007;98(5):677–681. | |
Densmore JC, Peterson DB, Stahovic LL, et al. Initial surgical and pain management outcomes after Nuss procedure. J Pediatr Surg. 2010; 45(9):1767–1771. | |
Ong CC, Choo K, Morreau P, Auldist A. The learning curve in learning the curve: a review of Nuss procedure in teenagers. ANZ J Surg. 2005; 75(6):421–424. | |
McBride WJ, Dicker R, Abajian JC, Vane DW. Continuous thoracic epidural infusions for postoperative analgesia after pectus deformity repair. J Pediatr Surg. 1996;31(1):105–107. [discussion 107–108]. | |
Barros F. Continuous thoracic epidural analgesia with 0.2% ropivacaine for pectus excavatum repair in children. Paediatr Anaesth. 2004;14(2):192–194. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.