Back to Journals » Neuropsychiatric Disease and Treatment » Volume 13
Post-marketing observational program of the effectiveness of fluvoxamine for the treatment of depression in patients with neurological disorders: the FRIENDS study
Authors Yahno NN, Fedotova AV
Received 5 July 2017
Accepted for publication 10 October 2017
Published 2 November 2017 Volume 2017:13 Pages 2747—2756
DOI https://doi.org/10.2147/NDT.S145614
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Roger Pinder
Nikolay N Yahno,1 Anastasia V Fedotova2
1Neurology Department, I.M. Sechenov First Moscow State Medical University, 2Neurology Department, Additional Professional Education Faculty, Pirogov Russian National Research Medical University, Moscow, Russian Federation
Abstract: In a prospective, non-blinded, uncontrolled, multicenter, post-marketing, observational study (FRIENDS; NCT02043197), fluvoxamine (50–300 mg/day for 90 days) was effective for the treatment of depression in 299 adult patients (age ≥18 years) with neurological disorders at baseline. The therapeutic effect of fluvoxamine was measured by means of changes in the Hospital Anxiety and Depression Scale depression and anxiety scores (HADS-D and HADS-A, respectively), global severity of illness, and clinical condition (measured using the Clinical Global Improvement [CGI] scale). The mean HADS-D subscale score at baseline in the per-protocol cohort (n=296) was 11.7±3.1 points and the corresponding mean HADS-A score was 12.6±3.2. Significant (P<0.0001) improvements in both scores were recorded during fluvoxamine treatment and later follow-up. Most patients (>85%) recorded reductions versus baseline in both indices. In the CGI-based assessment, most evaluated patients (>200) experienced moderate to very substantial clinical improvement, with no or limited side effects. Significant improvements were also recorded in the exploratory outcomes of sleep quality, assessed using the Insomnia Severity Index, and cognitive function, assessed using the Montreal Cognitive Assessment (P<0.0001 vs baseline for both). No death or serious adverse drug reactions were reported during the study. The results of this observational study affirm that fluvoxamine is effective and well tolerated for the treatment of depression in the context of neurological disorders. The effects on the exploratory endpoints of this research merit evaluation in controlled trials.
Keywords: depression, anxiety, fluvoxamine, neurological disease, sleep, cognitive function
Introduction
Depression is frequently encountered in conjunction with neurological disorders such as epilepsy, multiple sclerosis, stroke, Parkinson’s disease, consequences of traumatic brain injury, and chronic pain syndromes.1,2 Exactly how, or even whether, neurological disease per se predisposes patients to depression or whether depression is either endogenous or a contextualized response to the effects of neurological disease is unclear; for example, depressive states appear to be infrequent in amyotrophic lateral sclerosis, which is arguably one of the worst neurological diagnoses. In many other areas of neurology, however, depression is a recurring theme that warrants medical attention.3–5
The impact of treating depression in the setting of neurological disorders is incompletely documented.6 Hence, it is unclear whether the response of depression to treatment in these patients is broadly consistent with responses in the general population or whether successful treatment of depression has an impact on the trajectory of the concomitant neurological condition. Depression is strongly implicated in outcomes among stroke survivors7 and correlates with worse health-related quality of life in patients with epilepsy,8 Parkinson’s disease,9 and multiple sclerosis,10 as well as in the general population of urban areas in the Russian Federation,11 and has been hypothesized to “amplify” pains associated with neurological pathologies.12 Nevertheless, the impact of depression in neurological patients appears to have been underappreciated, contributing to unsatisfactory management.13 This situation is reflected in the relative underdevelopment of online self-management tools for patients with neurological disease and concomitant depression.14,15
This deficit of information may arise in part from under-recognition and undertreatment of depression in the neurological setting. Data from the Epidemiology of Cardiovascular Diseases and their Risk Factors in the Russian Federation (ESSE-RF) study indicate that the prevalences of depression and anxiety in the Russian Federation are 25.6% and 46.3%, respectively,16 and other studies have shown that the prevalence of current depressive disorder in the Russian Federation is ≈20%.7 These estimates may be compared with data for high- and middle-income countries including Belgium (14.1%), France (21%), Germany (9.9%), Mexico (8.0%), the Netherlands (17.9%), New Zealand (17.8%), Spain (10.6%), and USA (19.2%).17 Despite this, rates of antidepressant prescription are low (≈20%), even in specialized mental health services.18 Limitations of rating scales that emphasize somatic symptoms may be a contributing factor to this lack of recognition of depression.3
We sought to raise awareness of the issue of depression in the neurological setting by conducting an observational study of the effects of the selective serotonin reuptake inhibitor (SSRI) fluvoxamine on depression and anxiety, as well as on indices of sleep quality and cognitive function, in neurology outpatients diagnosed with depression.
Methods
General
This was a prospective, non-blinded, uncontrolled, multicenter, post-marketing observational study in patients for whom fluvoxamine was prescribed for the treatment of depression in accordance with the locally approved label/prescribing information. The study was registered with www.ClinicalTrials.gov (NCT02043197).
The primary purpose of the study was to identify neurological comorbidities associated with depression treated with fluvoxamine. Secondary aims included documenting changes in depression symptoms score (as measured by the Hospital Anxiety and Depression Scale depression score [HADS-D]), anxiety symptoms score (as measured by the Hospital Anxiety and Depression Scale anxiety score [HADS-A]), global severity of illness, and clinical condition (as measured by the Clinical Global Improvement [CGI] scale) during treatment with fluvoxamine and during subsequent follow-up. Exploratory assessments of sleep quality and cognitive function were also undertaken using the Insomnia Severity Index (ISI) and the Montreal Cognitive Assessment (MoCA), respectively.
Patients
The study aimed to include patients with depression and neurological disorders. Eligibility criteria comprised age ≥18 years, mild or moderate symptoms of depression with HADS-D and HADS-A scale scores both ≥8, outpatients with neurological disorders, prescription of fluvoxamine no earlier than 7 days before the baseline visit, and written informed consent.
Exclusion criteria included labeled contraindications to fluvoxamine; psychotic symptoms and/or suicidal ideation, schizophrenia, bipolar disorder, schizoaffective disorder, severe dementia, alcohol or drug abuse or psychiatric disorders requiring hospitalization; acute or rapidly deteriorating neurological disorders; use of other medications for depression/monoamine oxidase (MAO) inhibitors; pregnancy, anticipated pregnancy or breastfeeding; and participation in another clinical trial within the previous 30 days.
Study design
Eligible patients were enrolled after 0–14 days of screening. Activities during the screening period comprised acquisition of demographic data and neurological diagnosis and conducting HADS assessment, MoCA assessment, and CGI assessment; the quality of sleep scale was also administered. Adverse events data were collected. Thereafter, patients were prescribed oral fluvoxamine for ≈3 months. The daily dose range was specified as 50–300 mg which could be varied at the sole discretion of participating physicians according to the needs and circumstances of individual patients. Doses of 50–100 mg/day were to be taken as a single dose but doses of 150–300 mg/day were to be taken as two or three divided doses. Responses to treatment were assessed ≈30 and 90 days after the first dose of fluvoxamine. After completion of the treatment phase, patients were followed up for a further period of ≈3 months, during which safety and psychiatric status were assessed. The total duration of the study was thus ≤6.5 months.
Patients received standard therapy according to the primary diagnosis of the patient. Medications forbidden during the study included any other medicaments for depression and MAO inhibitors. We did not investigate or monitor the use of non-pharmaceutical interventions.
Statistical considerations
A comprehensive statistical analysis plan was prepared and approved prior to performing the statistical analyses. Statistical analyses were performed after all patients had completed their participation in the study and the database had been locked.
As the primary purpose of this study was exploratory and no hypothesis was being tested, no formal sample size calculation was performed. A recruitment target of 300 patients at 20–30 sites was projected and was considered sufficient to allow a scientifically relevant evaluation of the results. Missing values for effectiveness or safety data were not imputed.
Two patient sets were identified for analysis. The safety set included those patients who attended the baseline visit and consumed at least one dose of fluvoxamine. The effectiveness set comprised those patients who completed ≥30 days of fluvoxamine treatment. The safety set was used to examine the safety findings; the effectiveness set was used to examine changes in depression, anxiety, illness, sleep quality, and cognitive function scores. Changes from baseline were tested for statistical significance using the Wilcoxon signed-rank test.
All statistical analyses were performed using SAS®, version 9.2 (SAS Institute Inc, Cary, NC, USA) on a Microsoft Windows platform.
Ethics
The study protocol and the Russian Patient Authorization for Use/Disclosure of Data form (PAF; dated October 4, 2013) were reviewed and approved by the central ethics committee (Interacademic Ethics Committee, Gagarinsky per, 37, 119002, Moscow, Russian Federation; www.ethicmke.ru) prior to enrollment of any patients in the study. The investigation itself was conducted in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonization (ICH) recommendation on Good Clinical Practice (ICH/135/95; July 2002), and all applicable local regulations. Patient enrollment started only after written approval was issued by the ethics committee and the Russian Ministry of Health and after each patient had provided written informed consent.
Individual investigators were responsible for obtaining the signed and dated PAF from every patient before any study-specific procedures were performed. The PAF itself was available in Russian and in a validated English translation. The patients were given adequate oral and written information about the nature, purpose, and possible risks and benefits of the study, were given an opportunity to ask questions prior to enrollment, and were assured that declining to participate would not prejudice their later treatment.
Results
Three hundred patients were enrolled from 23 centers in the Russian Federation between January 31, 2014 and November 20, 2015 (see Table S1 for investigator/center details). Of those, 299 met the conditions for inclusion in the intention-to-treat (ITT) population and 296 met the criteria for inclusion in the per-protocol (PP) population. In both study populations, ≈99% of patients attended all clinic visits during the period of active treatment and the first follow-up visit. Attendance at the last follow-up visit was ≈80%. Twenty-one of the 23 sites recruited ≥10 patients.
Identification of concomitant therapy taken by patients was limited to agents associated with adverse drug reactions (ADRs). In that context, one patient was recorded as taking amlodipine for blood pressure control and another was recorded as taking enalapril, also for hypertension.
The mean age of patients in the PP cohort was 50.41±14.1 years (range 21–85 years). Most patients were female (n=217). More than 99% of patients were classified as Caucasian. Between 62% and 66% were married, employed, and had university-level education. Data for the ITT contingent were very similar.
In the PP cohort, the mean prescribed dose of fluvoxamine was 73.0±24.89 mg/day and the average duration of treatment was 97.2±15.81 days. Use of 50 mg/day fluvoxamine was recorded in 251 patients (84.8%) and use of 100 mg/day in 164 (55.4%), reflecting widespread dose adjustment by physicians: >40% of patients were prescribed both 50 and 100 mg/day during the study. ITT data revealed a closely comparable profile. Fifty-four patients (18.1%) in the ITT cohort continued fluvoxamine therapy beyond the planned 3 months.
All classes of disorders for patients in the PP cohort are summarized in Table 1. The most common neurologic disorders relating to International Classification of Diseases (ICD) class G00–G99 include other disorders of the autonomic nervous system (n=28 [9.5%]), tension-type headache (n=21 [7%]), unspecified encephalopathy (n=14 [4.7%]), and multiple sclerosis (n=12 [4.1%]). Class I00–I99 comprises different disorders relating to chronic ischemia of the brain. Class M00–M99 is characterized by diseases that accompany dorsopathy. Class T00–T99 includes sequelae of intracranial injury.
  | Table 1 Summary of patients with different classes of disorders associated with depression according to ICD code |
Response-to-treatment endpoints
The mean HADS-D subscale score at baseline in the PP cohort was 11.7±3.1 points and the corresponding mean HADS-A score was 12.6±3.2 points. Significant reductions in both scores, signifying improvement in depression or anxiety status, were recorded during fluvoxamine treatment and later follow-up (Figure 1).
Improvement in HADS-D score from baseline to day 30, day 90, and end of follow-up was recorded in 261 (88.2%), 287 (97.6%), and 233 patients (97.5%), respectively. Corresponding improvements in the HADS-A score were recorded in 266 (89.9%), 285 (96.9%), and 237 patients (99.2%), respectively.
The HADS-D and HADS-A scores worsened in eight (2.7%) and four patients (1.4%), respectively, between baseline and days 30 and 90. At the end of the follow-up period, five (2.1%) and two patients (0.8%) experienced worsening of depression and anxiety symptoms, respectively.
No change from baseline to day 30, day 90, and end of follow-up was observed for the HADS-D subscale score in 27 (9.1%), three (1%), and one patient (0.4%), respectively.
The mean baseline CGI-severity score was 2.7±1.08 in the 291 patients assessed. At visit 3 (day 90), 293 patients had a mean score of 1.6±0.75 (P<0.0001). Median scores at baseline and day 90 were 3 and 1, respectively. Figure 2 depicts the distribution of CGI scores at both time-points and at the end of follow-up.
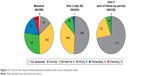  | Figure 2 Trends in the Clinical Global Impression-severity scale scores during the study. |
Examination of the CGI-efficacy index, which took into account both therapeutic effects and side effects of fluvoxamine therapy, revealed that >200 evaluated patients experienced moderate to very substantial clinical improvement with no or limited side effects at days 30 and 90 (Table 2).
  | Table 2 Numbers and proportions of patients assessed by Clinical Global Impression-efficacy index at visit 3 and visit 5 |
Exploratory endpoints
The mean MoCA test score at baseline for the 296 PP patients evaluated was 25.2 (range 7–30). At visit 3 (day 90) and visit 5 (end of follow-up period), the mean score had increased to 27 (range 11–30) and 27.5 (13–30), respectively (P<0.0001 for both comparisons vs baseline).
Data accrued using the ISI instrument indicated that, during the phase of active therapy, >90% of patients in the PP cohort reported an improved quality of sleep versus baseline. Figure 3 illustrates the reduction in mean ISI score – indicative of improved sleep quality – from 13.4 (range 0–28) at baseline to 6.6 (range 0–27) at day 90 and 4.5 (range 0–25) at the end of follow-up (P<0.0001 for both comparisons vs baseline).
  | Figure 3 Mean ISI score responses during the study. |
Safety findings
No deaths or serious ADRs were reported during the study. No laboratory safety evaluation was performed.
A total of 48 clinical ADRs were reported in 22 patients (7.4%). ADRs affecting ≥1% of the ITT population comprised asthenia (n=5), nausea (n=4), dizziness (n=4), somnolence (n=4), anxiety (n=4), and headache (n=3).
The reported ADRs resolved in 21 patients (95.5%). One case of hyperkinesia in a patient who took fluvoxamine and sodium valproate was recorded as “resolved with sequelae” as full resolution of hyperkinesia was not achieved. In 14 patients, no action was taken for the ADRs. Fluvoxamine was discontinued in four patients in the ITT population and the dosage was adjusted in three. Of those patients who underwent dose adjustment, ADRs were managed with nondrug medical intervention in two and with medication in one.
Discussion
The results of this observational study affirm that depression is encountered in a wide range of neurological disorders and that fluvoxamine is effective and well tolerated for the treatment of depression in this context. Effects on exploratory endpoints indicate that fluvoxamine may also have beneficial effects on sleep and cognitive function but those data are in need of substantiation in controlled prospective trials.
Even in the context of a neurological condition, it may be argued that treatment of depression is a valid goal in its own right. Addressing depressive symptoms may also positively influence subjective perceptions of the neurological condition. A similar benefit may attach to the relief of anxiety symptoms. Our data set does not allow us to examine those possibilities directly but the results of our research are, in this context, compatible with the conclusions of Horii et al19 and the findings of Benedetti et al.20
The lifetime prevalence of depression is estimated at 30%–50% in patients with neurological diseases.12 The results of this study agreed with that estimate. Among the neurological diseases associated with depression that were classified according to the ICD, 10th revision, the prevalence of diseases of the nervous system (G00–G99) was highest (≈49%), followed by diseases of the circulatory system (I00–I99) characterized by chronic brain ischemia (31.8%). The demographic profile of this observational study was also comparable to that of a study by Bobak et al,21 who reported that the prevalence of depressive symptoms in the Russian Federation was ≈23% in men and ≈44% in women. Most of our patients had relatively well-characterized neurological diagnoses; observations by Carson et al5 suggest that, in neurology patients with symptoms substantially unexplained by organic disease, an even higher proportion (≈70%) may present with symptoms of depression and/or anxiety. Despite these high estimates of the prevalence of depression, nontreatment of depression in patients with neurological conditions appears, as noted earlier, to be widespread. Failure to identify concomitant depression may be one contributor to this state of affairs, but other considerations may also exert an influence. Medical priority may be given to the neurological condition, with the express intention of deferring any decision on the need for antidepressant interventions until the neurological disease process is stable or controlled. Neurological disease may lead to changes in mood and behavior and it may be appropriate to mitigate such confounding factors before making any decision about whether to take active, specific steps to manage depression. In addition, it should be noted that some neurology patients appear disinclined toward treatment for “emotional disorders”.4 Among a Russian patient population, such views may be influenced by perceptions of depression as an expression of personal weakness.22
Substantial improvements in both depression and anxiety status were noted in this study population (Figures 1 and 2). The scale of the effects on both conditions is consistent with data from controlled trials of fluvoxamine.23–25 In response to fluvoxamine treatment, the HADS score showed a steady improvement in both depression and anxiety score symptoms from moderate (score range 11–15) at baseline to mild (score range 8–10) by day 30 and finally normal (score range 0–7) by day 90. Improvements were registered by ≈97% of patients by the end of 3 months of treatment. The proportion of patients who showed improvement in depression and anxiety symptoms continued to increase gradually after 30 days of treatment and stabilized from 3 months of treatment to the end of the follow-up period. Approximately 2.7% and 1.4% of patients demonstrated worsening in HADS-D and HADS-A scores, respectively, between baseline and day 90.
Patients were also assessed using the CGI-efficacy index, which evaluated both therapeutic efficacy and treatment-related adverse events across a range from zero (indicating marked improvement and no side effects) to four (indicating unchanged or worse and with side effects outweighing any therapeutic effect). According to those criteria, the majority of patients had marked improvement with no side effects (Figure 2). No patient recorded a score where the side effects outweighed the therapeutic effect.
The patients in our study were previously treatment-naive, in part perhaps because of the barriers to treatment already identified, but may for those same reasons have been depressed for some time before enrollment. In principle, a longer duration of depression may be linked to a less satisfactory response to therapy,26,27 but no clear indication of such an effect emerges from our data set.
Patients with multiple sclerosis were one of the larger subsets in our population (n=12) and depression is a determinant of quality of life in such patients.10 High-dose fluvoxamine (200 mg/day) has been reported to offer benefits in patients with mild to moderate sclerosis-attributed disability and concomitant depression20 and our own experience suggests that similar gains may be achieved with lower doses which may deliver an improved tolerability experience.28 Patients classified as having tension-type headache (n=7) also benefited from fluvoxamine therapy in our data set. We have no insights into whether fluvoxamine also benefited tension headache per se and the findings of a recent Cochrane review suggest that is unlikely.29 However, there was no indication that the antidepressant effect of fluvoxamine was attenuated in our tension headache patients.
Our investigations into the effects of fluvoxamine on sleep and cognitive function were always intended to be exploratory and should be considered in that light. Nevertheless, findings on both these endpoints are suggestive of additional or ancillary effects from fluvoxamine therapy that may warrant further scrutiny. In our study population, the ISI score improved in 91.5% of patients, indicating enhancement of sleep quality during fluvoxamine treatment. That observation is compatible with earlier observations on sleep quality.30,31 Treatment-related enhancement of melatonin levels may underlie this effect of fluvoxamine32 but direct investigation of that possibility was beyond the scope of our research. It may be noted in general, however, that any effect of fluvoxamine in improving sleep quality might be expected to contribute to the overall antidepressant effect33 and that such an effect may originate from normalization of melatonin secretion and/or enhancement of melatonin levels.34,35
Interest in the possible utility of fluvoxamine in the preservation of cognitive function arises substantially from the drug’s marked efficacy – not shared by others in the SSRI class – as an agonist at sigma-1 receptors.36,37 Fluvoxamine has been reported to improve cognitive outcomes in a preliminary study in patients with depression.38 In our own patients, the MoCA scale scores showed significant improvement from baseline to day 90 and the end of follow-up. The results of our study are thus supportive of the possibility of a favorable impact of fluvoxamine on cognition. However, the limited nature and duration of our investigation mean that our data should only be seen as making a case for further research in this area rather than proving the existence of a pro-cognitive effect of fluvoxamine. Further research in this field needs, among other things, to examine possible long-term effects on cognition and to ascertain whether any such effect is a primary action of fluvoxamine or secondary to its antidepressant actions.
In conclusion, this open-label observational study documented statistically significant improvements in depression and anxiety status in response to 3 months of treatment with fluvoxamine, administered mostly at intermediate dosages (50–100 mg/day). Benefits of fluvoxamine therapy were also demonstrated for exploratory endpoints focused on sleep quality and cognitive function.
Acknowledgments
The authors thank the patients who agreed to take part in this study and their colleagues at the various participating centers.
Permission for use of HADS© in both English and Russian was granted by GL Assessment Ltd, which is part of the Granada Learning Group (389 Chiswick High Road, London W4 4AL, UK), as follows:
- HADS copyright © RP Snaith and AS Zigmond, 1983, 1992, 1994.
- Record form items originally published in Acta Psychiatrica Scandinavica 67: 361–370, copyright © Munksgaard International Publishers Ltd, Copenhagen, 1983.
- This edition first published in 1994 by NFER-Nelson Publishing Company Ltd (now GL Assessment Ltd).
Permission for use of MoCA© in both English and Russian was granted by Dr Ziad Nasreddine, MD ([email protected]).
Permission for use of ISI© in both English and Russian was granted by Charles M Morin, PhD (Professeur Titulaire, Université Laval École de Psychologie, 2325, Rue des Bibliothèques, Québec, Québec, Canada G1V 0A6).
Hughes associates, Oxford, UK, provided writing and editorial assistance in the preparation of this report.
Disclosure
The authors report no conflicts of interest in this work.
References
Voznesenskaya TG. Depression in neurological practice. Difficult Patients. 2003;1:26–30. | ||
Hellmann-Regen J, Piber D, Hinkelmann K, et al. Depressive syndromes in neurological disorders. Eur Arch Psychiatry Clin Neurosci. 2013;263(Suppl 2):S123–S136. | ||
Rickards H. Depression in neurological disorders: an update. Curr Opin Psychiatry. 2006;19:294–298. | ||
Carson AJ, Ringbauer B, MacKenzie L, Warlow C, Sharpe M. Neurological disease, emotional disorder, and disability: they are related: a study of 300 consecutive new referrals to a neurology outpatient department. J Neurol Neurosurg Psychiatry. 2000;68:202–206. | ||
Carson AJ, Ringbauer B, Stone J, McKenzie L, Warlow C, Sharpe M. Do medically unexplained symptoms matter? A prospective cohort study of 300 new referrals to neurology outpatient clinics. J Neurol Neurosurg Psychiatry. 2000;68:207–210. | ||
Price A, Rayner L, Okon-Rocha E, et al. Antidepressants for the treatment of depression in neurological disorders: a systematic review and meta-analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry. 2011;82:914–923. | ||
Kutlubaev MA, Hackett ML. Part II: predictors of depression after stroke and impact of depression on stroke outcome: an updated systematic review of observational studies. Int J Stroke. 2014;9:1026–1036. | ||
Melikyan E, Guekht A, Milchakova L, Lebedeva A, Bondareva I, Gusev E. Health-related quality of life in Russian adults with epilepsy: the effect of socio-demographic and clinical factors. Epilepsy Behav. 2012;25:670–675. | ||
Winter Y, von Campenhausen S, Popov G, et al. Social and clinical determinants of quality of life in Parkinson’s disease in a Russian cohort study. Parkinsonism Relat Disord. 2010;16:243–248. | ||
Yamout B, Issa Z, Herlopian A, et al. Predictors of quality of life among multiple sclerosis patients: a comprehensive analysis. Eur J Neurol. 2013;20:756–764. | ||
Winter Y, Epifanova-Bertschi N, Sankowski R, et al. Health-related quality of life and its determinants in the urban Russian population with major depressive disorder: a cross-sectional study. Int J Psychiatry Med. 2012;43:35–49. | ||
Kapfhammer HP. [Coexistent depressive and anxiety disorders in neurological diseases: from a perspective of multimorbidity]. Nervenarzt. 2014;85:437. German. | ||
Kanner AM. Should neurologists be trained to recognize and treat comorbid depression of neurologic disorders? Yes. Epilepsy Behav. 2005;6:303–311. | ||
Lukmanji S, Tram Pham T, Blaikie L, et al. Online tools for individuals with depression and neurologic conditions. Neurol Clin Pract. 2017;7:344–353. | ||
Schröder J, Brückner K, Fischer A, et al. Efficacy of a psychological online intervention for depression in people with epilepsy: a randomized controlled trial. Epilepsia. 2014;55:2069–2076. | ||
Shal’nova SA, Evstifeeva SE, Deev AD, et al. [The prevalence of anxiety and depression in different regions of the Russian Federation and its association with sociodemographic factors (according to the data of the ESSE-RF study)]. Ter Arkh. 2014;86:53–60. Russian. | ||
Brommet E, Andrade LH, Hwang I, et al. Cross-national epidemiology of DSM-IV depressive episode. BMC Med. 2011;9:90. | ||
Kanner AM, editor. Depression in Neurologic Disorders: Diagnosis and Management. Chichester, UK: Wiley-Blackwell; 2012. | ||
Horii A, Uno A, Kitahara T, et al. Effects of fluvoxamine on anxiety, depression, and subjective handicaps of chronic dizziness patients with or without neuro-otologic diseases. J Vestib Res. 2007;17:1–8. | ||
Benedetti F, Campori E, Colombo C, Smeraldi E. Fluvoxamine treatment of major depression associated with multiple sclerosis. J Neuropsychiatry Clin Neurosci. 2004;16:364–366. | ||
Bobak M, Pikhart H, Pajak A, et al. Depressive symptoms in urban population samples in Russia, Poland and the Czech Republic. Br J Psychiatry. 2006;188:359–365. | ||
Turvey CL, Jogerst G, Kim MY, Frolova E. Cultural differences in depression-related stigma in late-life: a comparison between the USA, Russia, and South Korea. Int Psychogeriatr. 2012;24:1642–1647. | ||
Haffmans PM, Timmerman L, Hoogduin CA. Efficacy and tolerability of citalopram in comparison with fluvoxamine in depressed outpatients: a double-blind, multicentre study. The LUCIFER Group. Int Clin Psychopharmacol. 1996;11:157–164. | ||
Kiev A, Feiger A. A double-blind comparison of fluvoxamine and paroxetine in the treatment of depressed outpatients. J Clin Psychiatry. 1997;58:146–152. | ||
Walczak DD, Apter JT, Halikas JA, et al. The oral dose-effect relationship for fluvoxamine: a fixed-dose comparison against placebo in depressed outpatients. Ann Clin Psychiatry. 1996;8:139–151. | ||
Dotoli D, Spagnolo C, Bongiorno F, et al. Relapse during a 6-month continuation treatment with fluvoxamine in an Italian population: the role of clinical, psychosocial and genetic variables. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:442–448. | ||
Okuda A, Suzuki T, Kishi T, et al. Duration of untreated illness and antidepressant fluvoxamine response in major depressive disorder. Psychiatry Clin Neurosci. 2010;64:268–273. | ||
Pérez LP, González RS, Lázaro EB. Treatment of mood disorders in multiple sclerosis. Curr Treat Options Neurol. 2015;17:323. | ||
Banzi R, Cusi C, Randazzo C, Sterzi R, Tedesco D, Moja L. Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) for the prevention of tension-type headache in adults. Cochrane Database Syst Rev. 2015;5:CD011681. | ||
Neylan TC, Metzler TJ, Schoenfeld FB, et al. Fluvoxamine and sleep disturbances in posttraumatic stress disorder. J Trauma Stress. 2001;14:461–467. | ||
Wilson SJ, Bell C, Coupland NJ, Nutt DJ. Sleep changes during long-term treatment of depression with fluvoxamine – a home-based study. Psychopharmacology. 2000;149:360–365. | ||
Sunami E, Usuda K, Nishiyama Y, Otori T, Katsura K, Katayama Y. A preliminary study of fluvoxamine maleate on depressive state and serum melatonin levels in patients after cerebral infarction. Intern Med. 2012;51:1187–1193. | ||
Tsuno N, Besset A, Ritchie K. Sleep and depression. J Clin Psychiatry. 2005;66:1254–1269. | ||
Crasson M, Kjiri S, Colin A, et al. Serum melatonin and urinary 6-sulfatoxymelatonin in major depression. Psychoneuroendocrinology. 2004;29:1–12. | ||
von Bahr C, Ursing C, Yasui N, Tybring G, Bertilsson L, Röjdmark S. Fluvoxamine but not citalopram increases serum melatonin in healthy subjects: an indication that cytochrome P450 CYP1A2 and CYP2C19 hydroxylate melatonin. Eur J Clin Pharmacol. 2000;56:123–127. | ||
van Waarde A, Ramakrishnan NK, Rybczynska AA, et al. The cholinergic system, sigma-1 receptors and cognition. Behav Brain Res. 2011;221:543–554. | ||
Niitsu T, Iyo M, Hashimoto K. Sigma-1 receptor agonists as therapeutic drugs for cognitive impairment in neuropsychiatric diseases. Curr Pharm Des. 2012;18(7):875–883. | ||
Mandelli L, Serretti A, Colombo C, et al. Improvement of cognitive functioning in mood disorder patients with depressive symptomatic recovery during treatment: an exploratory analysis. Psychiatry Clin Neurosci. 2006;60(5):598–604. |
Supplementary materials
  | Table S1 Participating investigators and centers, all of which are in the Russian Federation |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


