Back to Journals » Clinical and Experimental Gastroenterology » Volume 14
Post-Endoscopic Retrograde Cholangiopancreatography Pancreatitis: An Updated Review of Current Preventive Strategies
Authors Bhatt H
Received 19 August 2020
Accepted for publication 13 January 2021
Published 2 February 2021 Volume 2021:14 Pages 27—32
DOI https://doi.org/10.2147/CEG.S276361
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Wing-Kin Syn
Harshil Bhatt1,2
1Goshen Hospital, Goshen, IN, USA; 2Indiana University School of Medicine, South Bend, IN, USA
Correspondence: Harshil Bhatt
Goshen Hospital, 200 High Park Ave, Goshen, IN 46526, USA
Tel +1 574-364-2510
Fax +1 844-850-7282
Email [email protected]
Abstract: Pancreatitis is a serious complication of endoscopic retrograde cholangiopancreatography, with incidence rates as high as 16% in some centers. Recent studies have also shown an upward trend in hospitalization due to endoscopic retrograde cholangiopancreatography-related pancreatitis. Early interventions taken before, during, and after the procedure can significantly reduce the risk of pancreatitis and decrease morbidity and mortality of the patients. To select appropriate patients for endoscopic retrograde cholangiopancreatography, in-depth knowledge of the patient-related and procedure-related risk factors is required. This updated clinical review outlines various pharmacological agents and surgical methods used for the prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis. Current evidence supports the use of rectal non-steroidal anti-inflammatory drugs and pancreatic stent placement as an effective preventive strategy. Further research is needed to compare these preventive modalities to improve patient outcomes after endoscopic retrograde cholangiopancreatography.
Keywords: ERCP, post-ERCP pancreatitis, endoscopic retrograde cholangiopancreatography, rectal non-steroidal anti-inflammatory drugs, pancreatic stent
Introduction
Acute pancreatitis is an acute inflammation involving the pancreas. Acute pancreatitis is diagnosed by the presence of any two of the following three criteria: (a) sudden onset of severe epigastric pain sometimes radiating to the back; (b) elevated levels of serum amylase or lipase (at least three times greater than the upper normal limits); and (c) confirmed findings of pancreatitis on imaging studies (computed tomography scan (CT), magnetic resonance imaging (MRI), or ultrasonography of abdomen).1
Acute pancreatitis is also the most serious complication of endoscopic retrograde cholangiopancreatography (ERCP). In 1991, Cotton et al defined post-ERCP pancreatitis (PEP) as pancreatitis after ERCP associated with epigastric abdominal pain and at least three times increase in serum lipase or amylase activity occurring at 24 hours after the procedure, with the need to require or extend admission to the hospital for at least two days. PEP was graded as mild, moderate, and severe with mild PEP requiring hospitalization for two to three days; moderate PEP requiring hospitalization for up to ten days; and severe PEP needing hospitalization for over ten days with the development of complications such as hemorrhage or pseudocyst (consensus criteria).2 However, the newer revised Atlanta criteria (2012) for the severity of PEP are considered more accurate and reliable than the consensus criteria (Table 1).1
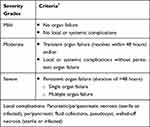 |
Table 1 Revised Atlanta Criteria for Severity of PEP |
Epidemiology
The incidence of PEP is estimated to be anywhere from 2% to 16%, with higher rates observed in high-risk patients.3 A recent retrospective cohort study of over 1.2 million ERCP performed between 2011 and 2017 in the United States published in 2020 by Mutneja et al revealed that the incidence of PEP was around 4.5%. This study also demonstrated upward trends in hospitalization rates (increased by 13.3% from 2011 to 2017) as well as mortality rates related to PEP (2.75% in 2011 to 4.38% of PEP cases in 2017).4
Risk Factors
Many patient-related and procedure-related risk factors have been shown to increase the risk of PEP. The European Society of Gastrointestinal Endoscopy (ESGE) guidelines further divide the risk factors into “definite” and “likely” based on the odd ratios derived from various systemic reviews and meta-analyses. According to ESGE guidelines, patients could be considered high risk for PEP if at least one definite or two likely risk factors are present5–8 (Table 2).
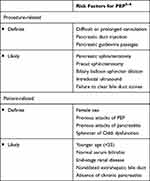 |
Table 2 Risk Factors for Post-ERCP Pancreatitis |
Considering the risks of developing definite or likely procedure-related risk factors per ESGE guidelines could be higher in more complex ERCP-related procedures such as intraductal radiofrequency ablation, pancreatoscopy, and cholangioscopy; the risk of PEP could be high as well.9 In addition to the above, lack of proper training or experience of the endoscopists has also been associated with the development of PEP. A multicentric study (1191 patients) performed by Lee et al showed that less experienced endoscopists (<200 ERCP) was an independent risk factor for PEP (Odds ration 1.63, 95% confidence interval 1.05–2.53, p=0.03).10 A study by Perney et al demonstrated that the use of the drugs (azathioprine, valproic acid) that are known to be toxic to the pancreas before or during ERCP increased the risk of PEP significantly (Odds ratio 3.7, 95% confidence interval [1.1, 12.4], p = 0.04).11
Prevention of PEP
Patient selection is an extremely crucial step in reducing the incidence of PEP. Alternative modalities such as Magnetic Resonance Cholangiopancreatography or Endoscopic ultrasound should be considered for the diagnosis of various pancreaticobiliary disorders whenever possible to avoid ERCP and subsequent risk of developing PEP.
Pharmacological Agents for Prevention of PEP
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
To date, several randomized controlled trials and meta-analyses have been conducted to assess the effectiveness of NSAIDs on the prevention of PEP. A systemic review and meta-analysis by Liu et al of 19 randomized controlled trials involving over 5031 patients revealed that NSAIDs were associated with a reduced risk of PEP and moderate to severe PEP compared with the control group. Particularly, rectal NSAIDs were associated with a lower risk of developing PEP compared with controls.12 Rectal administration of 100 mg of indomethacin or diclofenac immediately before or after ERCP is recommended to reduce the risk of PEP.13 Rectal route has several benefits such as faster onset, better bioavailability due to bypass of the majority of first-pass metabolism, shorter peak and shorter duration of action leading to less adverse effects including gastrointestinal side effects. These benefits also make a rectal route of administration more suitable for procedures like ERCP.
Current literature does not support the nonrectal administration of NSAIDs. A prospective randomized control trial of 170 patients conducted by Kato et al in Japan showed that oral celecoxib had no beneficial effect on the prevention of PEP compared with placebo.14 Another randomized control trial on 430 patients by Ishiwatari et al compared the use of diclofenac (50 mg) before or after ERCP with the placebo. It concluded that there was no benefit of oral diclofenac in the prevention of PEP.15
Intravenous Fluid Hydration
Intravenous fluid hydration with Ringer’s lactate solution has been proven to be quite protective in preventing PEP in several trials. A recent systemic review of three randomized controlled trials showed that hydration with Ringer’s lactate could decrease the incidence of PEP (Odds ratio 0.29; 95% confidence interval 0.16–0.53) as well as severe pancreatitis and hyperamylasemia. Aggressive hydration with 20 mL/kg bolus after ERCP followed by infusion of Ringer’s lactate at 3 mL/kg/hr for 8 hours has been shown to be very effective.16 However, it is important to note that most ERCPs are performed on an outpatient basis and hence, administering 8-hour-long infusion might not be feasible. Data are lacking with regards to evaluating aggressive hydration for outpatient ERCP procedures as well as the added benefit that aggressive hydration brings to the commonly used pharmacological prophylaxis.
Drugs with Uncertain Benefits
Somatostatin and Octreotide
Somatostatin and its analog octreotide inhibit pancreatic enzyme secretion. Their efficacy has been evaluated in several trials. A recent meta-analysis of eleven randomized controlled trials involving 2869 patients concluded that the use of somatostatin is beneficial in PEP when given as a single bolus or long term (more than 12 hours) infusion but lacks any benefit when given as a short-term infusion.17 Another meta-analysis in 2018 carried out by Wang et al showed a significant reduction of PEP risk as well as hyperamylasemia with the use of long-term injection of somatostatin for high-risk patients. However, the use of somatostatin for low-risk patients showed no benefit compared with the placebo group.18 Studies assessing the effect of octreotide have not demonstrated promising results.
Nitrates
Glyceryl trinitrate helps in the drainage of the pancreatic duct after ERCP and may relax the sphincter of Oddi.19 Recent data suggest that combining nitrates with rectal NSAIDs might be beneficial over rectal NSAIDs alone. A double-blind randomized controlled trial (2014, 300 patients) by Sotoudehmanesh et al revealed that the group that received a combination of rectal indomethacin (100 mg) and sublingual nitrate (5 mg) had a significantly lower risk of development of PEP compared to the group that received rectal indomethacin with sublingual placebo (Risk ratio- 0.39, 95% confidence interval- 0.18–0.86, p = 0.016).20 A randomized control superiority trial conducted by Tomoda et al published in 2019 discovered that use of rectal diclofenac 50 mg along with sublingual isosorbide dinitrate 5 mg significantly reduced the incidence of PEP compared with rectal diclofenac alone (relative risk 0.59; 95% confidence interval 0.37–0.95; p-value = 0.03).21 Even though the earlier data is promising, large multicenter trials are needed to validate these data.
Protease Inhibitors
Current evidence suggests that protease inhibitors (ulinastatin, nafamostat mesylate, gabexate mesylate) are not effective in preventing PEP, and they are not widely recommended for this purpose. A recent epidemiological analysis of health insurance claims database conducted by Seta et al in Japan revealed that the rates of prescription of protease inhibitors for the prevention of PEP increased from 72.3% in 2005–07 to 83.6% in 2010–15 (p < 0.001) despite the contrary evidence.22
A recent network meta-analysis by Lyu et al examined 86 randomized control trials involving over 25,000 patients to compare the individual efficacy of nine major drugs – allopurinol, diclofenac, nitrate, gabexate, nafamostat, octreotide, somatostatin, ulinastatin and indomethacin. The analysis showed that allopurinol, nafamostat, and octreotide had similar efficacy as placebo in reducing the risk of PEP. However, diclofenac, gabexate, nitrate, indomethacin, ulinastatin, and somatostatin were more effective than placebo. However, the analysis concluded that there was a need for better high-quality trials to compare the drugs head-to-head.23
Endoscopic Interventions for the Prevention of PEP
Placement of prophylactic pancreatic stent may be advantageous in high-risk patients such as those with difficult cannulation, inadvertent pancreatic duct (PD) cannulation, pancreatic duct injection or pancreatic sphincterotomy (Table 2). On the basis of higher rates of PEP, difficult cannulation is defined as one in which there are more than five attempts at cannulation or prolonged duration before cannulation (more than 5–10 minutes).24 The stent mainly reduces the intraductal pressure when there is papillary edema. A meta-analysis of 14 studies with over 1500 patients concluded that pancreatic stent placement was associated with a reduction of PEP (relative risk 0.39; 95% confidence interval 0.29–0.53; p < 0.001) compared with no stent placement.25 The pancreatic stent can also reduce the severity of PEP.26 The size of the pancreatic stent has also been studied. As per the meta-analysis (6 randomized controlled trials with 561 patients) carried out by Afghani et al, size 5-Fr (French) stent was found to be superior to the size 3-Fr stent for high-risk patients.27 The study also showed that 5-Fr single-pigtail, unflanged pancreatic stent and 5-Fr straight flanged stent performed similarly and better than 3-Fr single-pigtail unflanged stents.
It is also advised that the stent should not be removed immediately after the procedure. A single-center randomized prospective study (151 patients) by Cha et al showed thatthe patient group where the stent was left for 7 to 10 days had a significantly lower rate of PEP compared to the group where the stent was immediately removed (4.3% versus 21.3%, p= 0.027). The severity of PEP was also lower in the group where the stent was left in place for 7 to 10 days.28
Pancreatic stents could also lead to complications such as stent occlusion that sometimes can lead to pancreatitis, stent migration that may need surgical procedure, perforation of duct, duodenal erosions, stent-related ductal changes and infections. Moreover, a failed pancreatic stent procedure itself is considered an independent risk factor for the development of PEP. A secondary analysis of randomized controlled data by Choksi et al showed that the rate of PEP was higher after a failed attempt at stent placement compared with the rate of PEP without the stent placement attempt (35% versus 12%).29 Another drawback is the need for another endoscopy for stent removal. Biodegradable stents, which dissolve spontaneously could be beneficial and cost-effective for eliminating the need for a repeat endoscopy. Larger scale studies comparing the usefulness of biodegradable stents in preventing PEP are required to provide more input.
Several studies have also looked at the cannulation procedures, but the outcome has been quite variable. Guidewire-assisted cannulation has been suggested to reduce the incidence of PEP.30 A systemic review and meta-analysis of over 12 randomized controlled trials including 3450 patients by Tse et al found that the risk of PEP was significantly reduced with guidewire cannulation compared with contrast-material assisted procedures (RR 0.51, 95% confidence interval 0.32–0.82). In the study, the guidewire technique also led to higher primary cannulation success rates as well as it reduced the need for precut sphincterotomy.31 In case of difficulties such as anatomical variations, malignancy or when more than two or three unsuccessful attempts have been made with guide-wire techniques, alternative methods such as double-guide wire techniques, precut-sphincterotomy, wire cannulation along stent, or trans pancreatic precut papillotomy are performed. These techniques have been associated with increased risk of PEP and prophylactic stent placement and rectal NSAIDs should be considered to reduce the incidence of PEP. A recent meta-analysis showed that the use of the double-guidewire technique was not superior to other methods (precut sphincterotomy, wire cannulation along the pancreatic stent) for patients with difficult cannulation.32 There has also not been any concrete evidence of using a particular type of electrocautery to reduce PEP risk.33
There have been few studies comparing rectal NSAIDs, pancreatic stent placement, or a combination of rectal NSAIDs with stent placement for the prevention of PEP, but the results again are quite inconsistent. A study published in 2019 to compare pharmacological agents (rectal indomethacin, sublingual nitrate, lactated ringer solution) plus stenting versus pharmacological agents alone failed to show inferiority or noninferiority of prophylactic use of pharmacological agents alone.34 Another trial by Elmunzer et al is currently underway to compare rectal indomethacin with a combination of stenting plus rectal indomethacin for preventing PEP.35
Conclusion
PEP can lead to more complications if proper preventive measures are not undertaken before, during, or after the procedure. Identification of high-risk individuals and consideration of alternative approaches other than ERCP remains critical to reducing the incidence of PEP. When ERCP is unavoidable, rectal NSAIDs are the preferred mode of intervention compared to other modalities due to their low cost, ease of administration, and better side-effect profile. Large-scale studies comparing pharmacological agents with the surgical techniques or their combination are much-needed to delineate the superiority of one approach over the other.
Abbreviations
ERCP, endoscopic retrograde cholangiopancreatography; PEP, post-endoscopic retrograde cholangiopancreatography pancreatitis; NSAIDs, non-steroidal anti-inflammatory drugs; CT, computed tomography scan; MRI, magnetic resonance imaging; mL, milliliters; kg, kilogram; hr, hour.
Data Sharing Statement
The author declares that data supporting the findings of this study are available within the article.
Author Contributions
The sole author HB made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
None to report.
Disclosure
The author reports no conflicts of interest for this work.
References
1. Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. doi:10.1136/gutjnl-2012-302779
2. Cotton PB, Lehman G, Vennes J, et al. Endoscopic sphincterotomy complications and their management: an attempt at consensus. Gastrointest Endosc. 1991;37(3):383–393. doi:10.1016/s0016-5107(91)70740-2
3. Kochar B, Akshintala VS, Afghani E, et al. Incidence, severity, and mortality of post-ERCP pancreatitis: a systematic review by using randomized, controlled trials. Gastrointest Endosc. 2015;81(1):143–149.e9. doi:10.1016/j.gie.2014.06.045
4. Mutneja H, Vohra I, Go A, et al. Temporal trends and mortality of post-endoscopic retrograde cholangiopancreatography pancreatitis in the United States: a nationwide analysis [published online ahead of print, 2020 Jul 15]. Endoscopy. 2020. doi:10.1055/a-1220-2242
5. Maitin-Casalis N, Neeman T, Thomson A. Protective effect of advanced age on post-ERCP pancreatitis and unplanned hospitalisation. Intern Med J. 2015;45(10):1020–1025. doi:10.1111/imj.12844
6. Chen JJ, Wang XM, Liu XQ, et al. Risk factors for post-ERCP pancreatitis: a systematic review of clinical trials with a large sample size in the past 10 years. Eur J Med Res. 2014;19(1):26. doi:10.1186/2047-783X-19-26
7. Vandervoort J, Soetikno RM, Tham TC, et al. Risk factors for complications after performance of ERCP. Gastrointest Endosc. 2002;56(5):652–656. doi:10.1067/mge.2002.129086
8. Dumonceau JM, Kapral C, Aabakken L, et al. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2020;52(2):127–149. doi:10.1055/a-1075-4080
9. Suarez AL, Coté GA, Elmunzer BJ. Adjunctive radiofrequency ablation for the endoscopic treatment of ampullary lesions with intraductal extension (with video). Endosc Int Open. 2016;4(7):E748–E751. doi:10.1055/s-0042-107665
10. Lee HJ, Cho CM, Heo J, et al. Impact of hospital volume and the experience of endoscopist on adverse events related to endoscopic retrograde cholangiopancreatography: a prospective observational study. Gut Liver. 2020;14(2):257–264. doi:10.5009/gnl18537
11. Perney P, Berthier E, Pageaux GP, et al. Are drugs a risk factor of post-ERCP pancreatitis? Gastrointest Endosc. 2003;58(5):696–700. doi:10.1016/s0016-5107(03)02019-4
12. Liu L, Li C, Huang Y, Jin H. Nonsteroidal anti-inflammatory drugs for endoscopic retrograde cholangiopancreatography postoperative pancreatitis prevention: a systematic review and meta-analysis. J Gastrointest Surg. 2019;23(10):1991–2001. doi:10.1007/s11605-018-3967-7
13. Dumonceau JM, Andriulli A, Elmunzer BJ, et al. Prophylaxis of post-ERCP pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) guideline - updated June 2014. Endoscopy. 2014;46(9):799–815. doi:10.1055/s-0034-1377875
14. Kato K, Shiba M, Kakiya Y, et al. Celecoxib oral administration for prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis: a randomized prospective trial. Pancreas. 2017;46(7):880–886. doi:10.1097/MPA.0000000000000852
15. Ishiwatari H, Urata T, Yasuda I, et al. No benefit of oral diclofenac on post-endoscopic retrograde cholangiopancreatography pancreatitis. Dig Dis Sci. 2016;61(11):3292–3301. doi:10.1007/s10620-016-4251-x
16. Wu D, Wan J, Xia L, et al. The efficiency of aggressive hydration with lactated ringer solution for the prevention of post-ERCP pancreatitis: a systematic review and meta-analysis. J Clin Gastroenterol. 2017;51(8):e68–e76. doi:10.1097/MCG.0000000000000856
17. Qin X, Lei WS, Xing ZX, et al. Prophylactic effect of somatostatin in preventing Post-ERCP pancreatitis: an updated meta-analysis. Saudi J Gastroenterol. 2015;21(6):372–378. doi:10.4103/1319-3767.167187
18. Wang G, Xiao G, Xu L, et al. Effect of somatostatin on prevention of post-endoscopic retrograde cholangiopancreatography pancreatitis and hyperamylasemia: a systematic review and meta-analysis. Pancreatology. 2018;18(4):370–378. doi:10.1016/j.pan.2018.03.002
19. Bhatia V, Ahuja V, Acharya SK, et al. A randomized controlled trial of valdecoxib and glyceryl trinitrate for the prevention of post-ERCP pancreatitis. J Clin Gastroenterol. 2011;45(2):170–176. doi:10.1097/MCG.0b013e3181eb600e
20. Sotoudehmanesh R, Eloubeidi MA, Asgari AA, Farsinejad M, Khatibian M. A randomized trial of rectal indomethacin and sublingual nitrates to prevent post-ERCP pancreatitis. Am J Gastroenterol. 2014;109(6):903–909. doi:10.1038/ajg.2014.9
21. Tomoda T, Kato H, Ueki T, et al. Combination of diclofenac and sublingual nitrates is superior to diclofenac alone in preventing pancreatitis after endoscopic retrograde cholangiopancreatography. Gastroenterology. 2019;156(6):1753–1760.e1. doi:10.1053/j.gastro.2019.01.267
22. Seta T, Takahashi Y, Yamashita Y, et al. Status of use of protease inhibitors for the prevention and treatment of pancreatitis after endoscopic retrograde cholangiopancreatography: an epidemiologic analysis of the evidence-practice gap using a health insurance claims database. Drug Discov Ther. 2019;13(3):137–144. doi:10.5582/ddt.2019.01029
23. Lyu Y, Wang B, Cheng Y, et al. Comparative efficacy of 9 major drugs for postendoscopic retrograde cholangiopancreatography pancreatitis: a network meta-analysis. Surg Laparosc Endosc Percutan Tech. 2019;29(6):426–432. doi:10.1097/SLE.0000000000000707
24. Halttunen J, Meisner S, Aabakken L, et al. Difficult cannulation as defined by a prospective study of the Scandinavian Association for Digestive Endoscopy (SADE) in 907 ERCPs. Scand J Gastroenterol. 2014;49(6):752–758. doi:10.3109/00365521.2014.894120
25. Mazaki T, Mado K, Masuda H, et al. Prophylactic pancreatic stent placement and post-ERCP pancreatitis: an updated meta-analysis. J Gastroenterol. 2014;49(2):343–355. doi:10.1007/s00535-013-0806-1
26. Shi QQ, Ning XY, Zhan LL, et al. Placement of prophylactic pancreatic stents to prevent post-endoscopic retrograde cholangiopancreatography pancreatitis in high-risk patients: a meta-analysis. World J Gastroenterol. 2014;20(22):7040–7048. doi:10.3748/wjg.v20.i22.7040
27. Afghani E, Akshintala VS, Khashab MA, et al. 5-Fr vs. 3-Fr pancreatic stents for the prevention of post-ERCP pancreatitis in high-risk patients: a systematic review and network meta-analysis. Endoscopy. 2014;46(7):573–580. doi:10.1055/s-0034-1365701
28. Cha SW, Leung WD, Lehman GA, et al. Does leaving a main pancreatic duct stent in place reduce the incidence of precut biliary sphincterotomy-associated pancreatitis? A randomized, prospective study. Gastrointest Endosc. 2013;77(2):209–216. doi:10.1016/j.gie.2012.08.022
29. Choksi NS, Fogel EL, Cote GA, et al. The risk of post-ERCP pancreatitis and the protective effect of rectal indomethacin in cases of attempted but unsuccessful prophylactic pancreatic stent placement. Gastrointest Endosc. 2015;81(1):150–155. doi:10.1016/j.gie.2014.07.033
30. Testoni PA, Mariani A, Aabakken L, et al. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2016;48(7):657–683. doi:10.1055/s-0042-108641
31. Tse F, Yuan Y, Moayyedi P, Leontiadis GI. Guide wire-assisted cannulation for the prevention of post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy. 2013;45(8):605–618. doi:10.1055/s-0032-1326640
32. Tse F, Yuan Y, Moayyedi P, et al. Double-guidewire technique in difficult biliary cannulation for the prevention of post-ERCP pancreatitis: a systematic review and meta-analysis. Endoscopy. 2017;49(1):15–26. doi:10.1055/s-0042-119035
33. Macintosh DG, Love J, Abraham NS. Endoscopic sphincterotomy by using pure-cut electrosurgical current and the risk of post-ERCP pancreatitis: a prospective randomized trial. Gastrointest Endosc. 2004;60(4):551–556. doi:10.1016/s0016-5107(04)01917-0
34. Sotoudehmanesh R, Ali-Asgari A, Khatibian M, et al. Pharmacological prophylaxis versus pancreatic duct stenting plus pharmacological prophylaxis for prevention of post-ERCP pancreatitis in high risk patients: a randomized trial. Endoscopy. 2019;51(10):915–921. doi:10.1055/a-0977-3119
35. Elmunzer BJ, Serrano J, Chak A, et al. Correction to: rectal indomethacin alone versus indomethacin and prophylactic pancreatic stent placement for preventing pancreatitis after ERCP: study protocol for a randomized controlled trial. Trials. 2020;21(1):471. doi:10.1186/s13063-020-04458-0
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
