Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 9
Possible involvement of ROS generation in vorinostat pretreatment induced enhancement of the antibacterial activity of ciprofloxacin
Authors Masadeh MM, Alzoubi KH , Al-azzam SI, Al-buhairan AM
Received 6 August 2017
Accepted for publication 12 September 2017
Published 17 October 2017 Volume 2017:9 Pages 119—124
DOI https://doi.org/10.2147/CPAA.S148448
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Arthur E. Frankel
Majed M Masadeh,1 Karem H Alzoubi,2 Sayer I Al-azzam,2 Ahlam M Al-buhairan3
1Department of Pharmaceutical Technology, 2Department of Clinical Pharmacy, Jordan University of Science and Technology, Irbid, Jordan; 3Department of Clinical Laboratory Sciences, King Saud University, Riyadh, Saudi Arabia
Abstract: The mechanism underlying ciprofloxacin action involves interference with transcription and replication of bacterial DNA and, thus, the induction of double-strand breaks in DNA. It also involves elevated oxidative stress, which might contribute to bacterial cell death. Vorinostat was shown to induce oxidative DNA damage. The current work investigated a possible interactive effect of vorinostat on ciprofloxacin-induced cytotoxicity against a number of reference bacteria. Standard bacterial strains were Escherichia coli ATCC 35218, Staphylococcus aureus ATCC29213, Pseudomonas aeruginosa ATCC 9027, Staphylococcus epidermidis ATCC 12228, Acinetobacter baumannii ATCC 17978, Proteus mirabilis ATCC 12459, Klebsiella pneumoniae ATCC 13883, methicillin-resistant Staphylococcus aureus (MRSA) (ATCC 43300), and Streptococcus pneumoniae (ATCC 25923). The antibacterial activity of ciprofloxacin, with or without pretreatment of bacterial cells by vorinostat, was examined using the disc diffusion procedure and determination of the minimum inhibitory concentration (MIC) and zones of inhibition of bacterial growth. All tested bacterial strains showed sensitivity to ciprofloxacin. When pretreated with vorinostat, significantly larger zones of inhibition and smaller MIC values were observed in all bacterial strains compared to those treated with ciprofloxacin alone. In correlation, generation of reactive oxygen species (ROS) induced by the antibacterial action of ciprofloxacin was enhanced by treatment of bacterial cells with vorinostat. Results showed the possible agonistic properties of vorinostat when used together with ciprofloxacin. This could be related to the ability of these agents to enhance oxidative stress in bacterial cells.
Keywords: flouroquinolones, MIC, histone deacetylase inhibitor, oxidative stress, antimicrobial susceptibility
Introduction
Ciprofloxacin is a member of the fluoroquinolone antibiotic group and possesses both gram-positive and gram-negative activities. It is commonly used for the treatment of infections, including chronic bacterial prostatitis, urinary tract infections, acute sinusitis, and acute uncomplicated cystitis.1 The mechanism of action for the antibacterial properties of ciprofloxacin is not fully understood. However, the antibacterial action commences by interfering with replication and transcription of DNA through action on bacterial DNA gyrase/topoisomerase II and DNA topoisomerase IV, thus preventing unwinding and duplication of bacterial DNA.2 Eventually, quinolone–enzyme–DNA complexes are formed very rapidly, which leads to the generation of “cellular poisons” and cell death.3,4 Antibiotics including ciprofloxacin were shown to possess antibacterial activity through induction of oxidative stress. For instance, major reactive oxygen species (ROS) including singlet oxygen (1O2) and superoxide anion (O2) were shown to be generated by ciprofloxacin.5–7 Moreover, multiple adverse effects of ciprofloxacin including tendinopathies and phototoxicity were associated with ROS generation.8,9
Vorinostat (suberoylanilide hydroxamic acid) is a derivative of hydroxamic acid that inhibits both histone deacetylase classes I and II,10 and has been approved in the United States for patients with refractory and relapsed cutaneous T-cell lymphoma with persistent, progressive, or recurrent disease on/or following two systemic therapies.11–13 The mechanism of vorinostat’s antiproliferative effect involves inhibition of histone deacetylase activity, leading to the accumulation of acetylated proteins, such as histones.10,14 Additionally, vorinostat was shown to induce DNA damage that is related to generation of oxidative lesions.15–17 We have recently shown that vorinostat induces oxidative chromosomal damage leading to a mutagenic effect in blood lymphocytes.18 Recently, we showed that the antibacterial activity of ciprofloxacin is altered by major antioxidants, such as vitamins E and C,19 tempol, pentoxifylline, and melatonin.20 Given that ciprofloxacin acts by inducing bacterial oxidative damage,21,22 and with the known oxidative cell-damaging activity of vorinostat,18 it is likely that vorinostat pretreatment enhances ciprofloxacin antibacterial activity. Therefore, in this study, the possibility of an interaction between vorinostat and ciprofloxacin was investigated.
Materials and methods
Microbial growth, culture conditions, and drugs
Activity of the ciprofloxacin–vorinostat combination were investigated across a panel of bacterial reference strains that included Streptococcus pneumoniae ATCC 25923, methicillin-resistant Staphylococcus aureus (MRSA) ATCC 43300, Klebsiella pneumoniae ATCC 13883, Proteus mirabilis ATCC 12459, Acinetobacter baumannii ATCC 17978, Staphylococcus epidermidis ATCC 12228, Pseudomonas aeruginosa ATCC 9027, Staphylococcus aureus ATCC29213, and Escherichia coli ATCC 35218. Microorganisms were stored in 20% glycerol (Sigma-Aldrich, St. Louis, MO, USA) and trypticase soy agar at −70°C (BBL Microbiology Systems, Cockeysville, MD, USA). The minimum inhibitory concentrations (MICs) were evaluated according to the guidelines of the Clinical and Laboratory Standards Institute.23 The ciprofloxacin was a generous gift from Al-HIKMA Pharmaceuticals (Amman, Jordan). Vorinostat was obtained from ABO Swiss Co., Ltd., Fujian, People’s Republic of China.
Testing of antimicrobial susceptibility
Bacterial solutions were constituted on the day of the experiment, and aliquots of 5×104 colony-forming units/drop of each bacterial strain were spread evenly over the face of a sterile plate containing molten BBL Muller-Hinton Gold II agar (BBL Microbiology Systems). Antibiotic solutions were prepared according to the recommendations of the manufacturer. A panel of ciprofloxacin concentrations was used to test for susceptibility of various microorganisms. Twofold serial dilutions were added to a hole at the center of each of the agar plates. The plates were slightly cooled and dried. Thereafter, plates were incubated at 37°C and read 24 hours later. In a proportion of the experiments, a combination mixture of ciprofloxacin 100 µg/mL and vorinostat 100 µM was added to the hole at the center of each of the agar plates.24–26 The zones of growth inhibition around holes containing the antibiotic were measured. Mean values of three independent experiments were recorded.
Determination of MIC
Serial broth dilution method was used for determination of MICs as per the recommendations of the National Committee for Clinical Laboratory Standards.23 Briefly stated, drugs were serially diluted and added to tubes containing Mueller–Hinton broth (BBL Microbiology Systems). Then, tubes were slightly cooled and dried. Thereafter, aliquots containing approximately 5×104 colony-forming units/drop of the tested bacterial strains were placed in each tube. After 18 hours of incubation at 37°C, tubes were read. The MIC was identified as the lowest concentration at which no growth, a faint haze, or fewer than three discrete colonies were detected. Duplicate readings were carried out. The breakpoints shown in the National Committee for CLSI tables were utilized to identify susceptibility versus resistance.23
Measurement of ROS generation
Generation of hydrogen peroxide was used as an indicator of ROS generation. E. coli cells were cultured using nutrient broth (M002; Hi-Media, Mumbai, India) and were then treated with ciprofloxacin (100 µg/mL) for variable periods. These E. coli cells were then incubated with the fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCF-DA) from Sigma-Aldrich for 30 minutes. The intensity of DCF-DA fluorescence was determined by using a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA), with an excitation wavelength of 480 nm and an emission wavelength of 530 nm.
Statistical analysis
Statistical testing was carried out via GraphPad Prism version 4.0 (La Jolla, CA, USA). For statistical analysis, data were evaluated using one-way ANOVA followed by Tukey’s post-test. p<0.05 was considered statistically significant.
Results
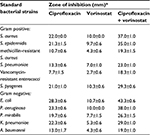
In the current study, the possible interactive effect for vorinostat on ciprofloxacin antibacterial activity was investigated against various species of reference bacteria. Results (Table 1) showed that ciprofloxacin possessed antibacterial activity against several reference bacteria, namely, K. pneumoniae, P. mirabilis, A. baumannii, S. epidermidis, P. aeruginosa, S. aureus, and E. coli. A 15-mm zone of inhibition was selected to indicate bacterial susceptibility to tested agents. Treating bacteria with both vorinostat and ciprofloxacin resulted in significantly larger zones of inhibition than with ciprofloxacin alone in all tested bacterial species (Table 1).
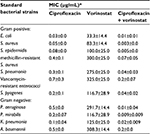
Similar results were obtained with the MICs of ciprofloxacin alone and in combination with vorinostat. Table 2 shows that pretreating bacteria with vorinostat enhanced the antibacterial activity of ciprofloxacin. This is shown by significantly smaller MIC values for the combination at all doses of vorinostat and ciprofloxacin, as compared to either alone (Table 2).
Previous studies from this laboratory showed that induction of antibacterial activity of ciprofloxacin was mediated through ROS generation.19,20,27 To study this possibility, ciprofloxacin 100 μg/mL was used to treat E. coli cells for various periods. Using the fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA), we identified that ciprofloxacin induced an increase in ROS generation of treated cells, and this reached maximal levels at 16 hours (Figure 1A). Pretreatment of E. coli cells with vorinostat 100 µM greatly enhanced ROS generation induced by ciprofloxacin (Figure 1B). Similarly, pretreatment of E.coli cells with vorinostat 100 µM significantly enhanced ciprofloxacin cytotoxicity (Tables 1 and 2).
Discussion
The current study indicates there is enhanced antibacterial activity of ciprofloxacin on pretreating bacteria with vorinostat. The current results were produced using a variety of standard bacterial strains. These results could be important if ciprofloxacin and vorinostat are used concurrently for bacterial infections associated with cancer chemotherapy.
The results indicate the effectiveness of ciprofloxacin on several bacterial strains such as E. coli, S. Aureus, P. aeruginosa, S. epidermidis, A. baumannii, P. mirabilis, and K. pneumonia. This is in agreement with the susceptibility of these bacterial strains to ciprofloxacin as previously shown.19,28,29 Additionally, ROS had an essential role in the antibacterial effect of ciprofloxacin against bacteria such as P. aeruginosa, E. coli, and S. aureus.8,19,21,22 Moreover, common scavengers of ROS, including vitamins E, C, and B12, and other antioxidants such as melatonin, tempol, and pentoxifylline were shown to reduce ciprofloxacin antibacterial activity.19,20,30 During the course of its action against bacterial strains such as E. coli, Enterococcus faecalis, and S. aureus, ciprofloxacin systematically induced the production of ROS.21 Moreover, microorganisms that are sensitive to ciprofloxacin had elevated intracellular levels of superoxide as compared to ones that are resistant.22 Treatment of E. coli with vitamin C or glutathione led to reduced ciprofloxacin antibacterial activity, which was due to scavenging of hydrogen peroxide and superoxide anions species.31
Results show that the combination of ciprofloxacin and vorinostat leads to enhancement of the antibacterial activity of ciprofloxacin against a variety of reference bacteria. As per our information, this study represents the first report of such an effect or drug–drug interaction. Current results could have clinical significance where it suggests that simultaneous use of ciprofloxacin along with vorinostat might positively influence ciprofloxacin antibacterial activity. Thus, combined usage of vorinostat and ciprofloxacin might need to be monitored in patients receiving both drugs.
The mechanism for the observed interactive effect of ciprofloxacin and vorinostat is not known. The bactericidal effect of ciprofloxacin is manifested via inhibition of bacterial DNA gyrase and type II topoisomerase.32,33 However, multiple other effects for ciprofloxacin were reported including inhibition of the growth of various other cell types,34–38 through interference with cell cycle, reduction of cell size,38 inhibition of de novo pyrimidine synthesis,38 and oxidative stress.31,39 Current results showed that the cytotoxicity of ciprofloxacin against bacterial cells was associated with time-dependent ROS generation. This generation of ROS was enhanced via treatment of bacterial cells with vorinostat, which increases oxidative stress. This leads to bacterial cell death and thus enhancement of the antibacterial action of ciprofloxacin. These results are in concordance with our previous reports using ROS scavengers, namely, vitamins C and E, where ROS scavengers were shown to prevent ROS generation induced by ciprofloxacin in bacterial cells simultaneously as attenuation of the antibacterial activity of ciprofloxacin.19 Therefore, it is likely that vorinostat enhances the antibacterial activity of ciprofloxacin via boosting ROS generation inside bacterial cells, thus facilitating the death of bacterial cells when ciprofloxacin is applied.
Vorinostat was shown to induce DNA damage, which is related to the generation of oxidative lesions.15–17 We have recently shown that vorinostat induces oxidative chromosomal damage, leading to a mutagenic effect in blood lymphocytes.18 Given the importance of ROS, energy metabolism, and mitochondrial functions for the antibacterial action of floroquinolones,8,19,21,22 it is probable that vorinostat-induced DNA damage has a role in the observed enhancement of ciprofloxacin antibacterial activity by vorinostat. Thus, a drug–drug interaction between vorinostat and ciprofloxacin is a possibility. More studies are required to identify the exact mechanism whereby vorinostat interacts with fluoroquinolone action.
Conclusion
The antibacterial activity of ciprofloxacin is enhanced when it is combined with vorinostat. The importance of such an observation is related to the wide usage of quinolone antibiotics and their great therapeutic value. Thus, studying the clinical consequences of simultaneous use of vorinostat and ciprofloxacin in patients being treated against bacterial infections is recommended.
Acknowledgment
Deanship of Research, JUST/Irbid/Jordan.
Disclosure
The authors report no conflicts of interest in this work.
References
Al-Soud YA, Al-Masoudi NA. A new class of dihaloquinolones bearing N′-aldehydoglycosylhydrazides, mercapto-1,2,4- triazole, oxadiazoline and a-amino ester precursors: synthesis and antimicrobial activity. J Braz Chem Soc. 2003;14(5):790–796. | ||
Oliphant CM, Green GM. Quinolones: a comprehensive review. Am Fam Physician. 2002;65(3):455–464. | ||
Chen CR, Malik M, Snyder M, Drlica K. DNA gyrase and topoisomerase IV on the bacterial chromosome: quinolone-induced DNA cleavage. J Mol Biol. 1996;258(4):627–637. | ||
Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61(3):377–392. | ||
Dwyer DJ, Collins JJ, Walker GC. Unraveling the physiological complexities of antibiotic lethality. Annu Rev Pharmacol Toxicol. 2015;55:313–332. | ||
Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130(5):797–810. | ||
Zhao X, Drlica K. Reactive oxygen species and the bacterial response to lethal stress. Curr Opin Microbiol. 2014;21:1–6. | ||
Umezawa N, Arakane K, Ryu A, Mashiko S, Hirobe M, Nagano T. Participation of reactive oxygen species in phototoxicity induced by quinolone antibacterial agents. Arch Biochem Biophys. 1997;342(2):275–281. | ||
Pouzaud F, Bernard-Beaubois K, Thevenin M, Warnet JM, Hayem G, Rat P. In vitro discrimination of fluoroquinolones toxicity on tendon cells: involvement of oxidative stress. J Pharmacol Exp Ther. 2004;308(1):394–402. | ||
Richon VM. Targeting histone deacetylases: development of vorinostat for the treatment of cancer. Epigenomics. 2010;2(3):457–465. | ||
Prebet T, Vey N. Vorinostat in acute myeloid leukemia and myelodysplastic syndromes. Expert Opin Investig Drugs. 2011;20(2):287–295. | ||
Mann BS, Johnson JR, He K, et al. Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin Cancer Res. 2007;13(8):2318–2322. | ||
Siegel D, Hussein M, Belani C, et al. Vorinostat in solid and hematologic malignancies. J Hematol Oncol. 2009;2:31. | ||
Marks PA, Richon VM, Miller T, Kelly WK. Histone deacetylase inhibitors. Adv Cancer Res. 2004;91:137–168. | ||
Namdar M, Perez G, Ngo L, Marks PA. Selective inhibition of histone deacetylase 6 (HDAC6) induces DNA damage and sensitizes transformed cells to anticancer agents. Proc Natl Acad Sci U S A. 2010;107(46):20003–20008. | ||
Petruccelli LA, Dupéré-Richer D, Pettersson F, Retrouvey H, Skoulikas S, Miller WH Jr. Vorinostat induces reactive oxygen species and DNA damage in acute myeloid leukemia cells. PLoS One. 2011;6(6):e20987. | ||
Lee JH, Choy ML, Ngo L, Foster SS, Marks PA. Histone deacetylase inhibitor induces DNA damage, which normal but not transformed cells can repair. Proc Natl Acad Sci U S A. 2010;107(33):14639–14644. | ||
Alzoubi KH, Khabour OF, Jaber AG, Al-Azzam SI, Mhaidat NM, Masadeh MM. Tempol prevents genotoxicity induced by vorinostat: role of oxidative DNA damage. Cytotechnology. 2014;66(3):449–455. | ||
Masadeh MM, Mhaidat NM, Alzoubi KH, Al-Azzam SI, Shaweesh AI. Ciprofloxacin-induced antibacterial activity is reversed by vitamin E and vitamin C. Curr Microbiol. 2012;64(5):457–462. | ||
Masadeh MM, Alzoubi KH, Al-Azzam SI, Khabour OF, Al-Buhairan AM. Ciprofloxacin-induced antibacterial activity is atteneuated by pretreatment with antioxidant agents. Pathogens. 2016;5(1). pii: E28. | ||
Albesa I, Becerra MC, Battán PC, Páez PL. Oxidative stress involved in the antibacterial action of different antibiotics. Biochem Biophys Res Commun. 2004;317(2):605–609. | ||
Becerra MC, Albesa I. Oxidative stress induced by ciprofloxacin in Staphylococcus aureus. Biochem Biophys Res Commun. 2002;297(4):1003–1007. | ||
CLSI. Methods for Dilution Antimicrobial Susceptibility Test for Bacteria that Grow Aerobically; Approved Standard – Tenth Edition. Wayne, PA, USA: CLSI; 2015. Available from: https://clsi.org/media/1632/m07a10_sample.pdf. Accessed 06 October, 2017. | ||
Solovieva ME, Soloviev VV, Akatov VS. Vitamin B12b increases the cytotoxicity of short-time exposure to ascorbic acid, inducing oxidative burst and iron-dependent DNA damage. Eur J Pharmacol. 2007;566(1–3):206–214. | ||
Solovieva ME, Solovyev VV, Kudryavtsev AA, Trizna YA, Akatov VS. Vitamin B12b enhances the cytotoxicity of dithiothreitol. Free Radic Biol Med. 2008;44(10):1846–1856. | ||
Saito M, Sasaki T, Matsuoka H. Vitamin B(12) promotes Cx40 and HCN4 gene expression at an early stage of cardiomyocyte differentiation. Exp Anim. 2009;58(1):57–60. | ||
Masadeh MM, Alzoubi KH, Al-azzam SI. Flouroquinolones-induced antibacterial activity atteneuation by pretreatment with Vitamin B12. Int J Pharmacol. 2015;11(1):67–71. | ||
Furqan S, Paracha SA. Frequency of Streptococcus pneumonia and Haemophilus influenza in acute exacerbation of chronic obstructive airway disease and their sensitivity to levofloxacin. J Pak Med Assoc. 2014;64(4):399–402. | ||
Campoli-Richards DM, Monk JP, Price A, Benfield P, Todd PA, Ward A. Ciprofloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1988;35(4):373–447. | ||
Masadeh MM, Alzoubi KH, Khabour OF, Al-Azzam SI. Ciprofloxacin-induced antibacterial activity is attenuated by phosphodiesterase inhibitors. Curr Ther Res Clin Exp. 2014;77:14–17. | ||
Goswami M, Mangoli SH, Jawali N. Involvement of reactive oxygen species in the action of ciprofloxacin against Escherichia coli. Antimicrob Agents Chemother. 2006;50(3):949–954. | ||
Gootz TD, Barrett JF, Sutcliffe JA. Inhibitory effects of quinolone antibacterial agents on eucaryotic topoisomerases and related test systems. Antimicrob Agents Chemother. 1990;34(1):8–12. | ||
Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. | ||
Lawrence JW, Darkin-Rattray S, Xie F, Neims AH, Rowe TC. 4-Quinolones cause a selective loss of mitochondrial DNA from mouse L1210 leukemia cells. J Cell Biochem. 1993;51(2):165–174. | ||
Lawrence JW, Claire DC, Weissig V, Rowe TC. Delayed cytotoxicity and cleavage of mitochondrial DNA in ciprofloxacin-treated mammalian cells. Mol Pharmacol. 1996;50(5):1178–1188. | ||
Nordmann P, Pechinot A, Kazmierczak A. Cytotoxicity and uptake of pefloxacin, ciprofloxacin, and ofloxacin in primary cultures of rat hepatocytes. J Antimicrob Chemother. 1989;24(3):355–363. | ||
Oomori Y, Yasue T, Aoyama H, Hirai K, Suzue S, Yokota T. Effects of fleroxacin on HeLa cell functions and topoisomerase II. J Antimicrob Chemother. 1988;22(Suppl D):91–97. | ||
Forsgren A, Bredberg A, Pardee AB, Schlossman SF, Tedder TF. Effects of ciprofloxacin on eucaryotic pyrimidine nucleotide biosynthesis and cell growth. Antimicrob Agents Chemother. 1987;31(5):774–779. | ||
Gürbay A, Hincal F. Ciprofloxacin-induced glutathione redox status alterations in rat tissues. Drug Chem Toxicol. 2004;27(3):233–242. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.